Design, Synthesis, Antifungal Activity, and Molecular Docking of Streptochlorin Derivatives Containing the Nitrile Group
Abstract
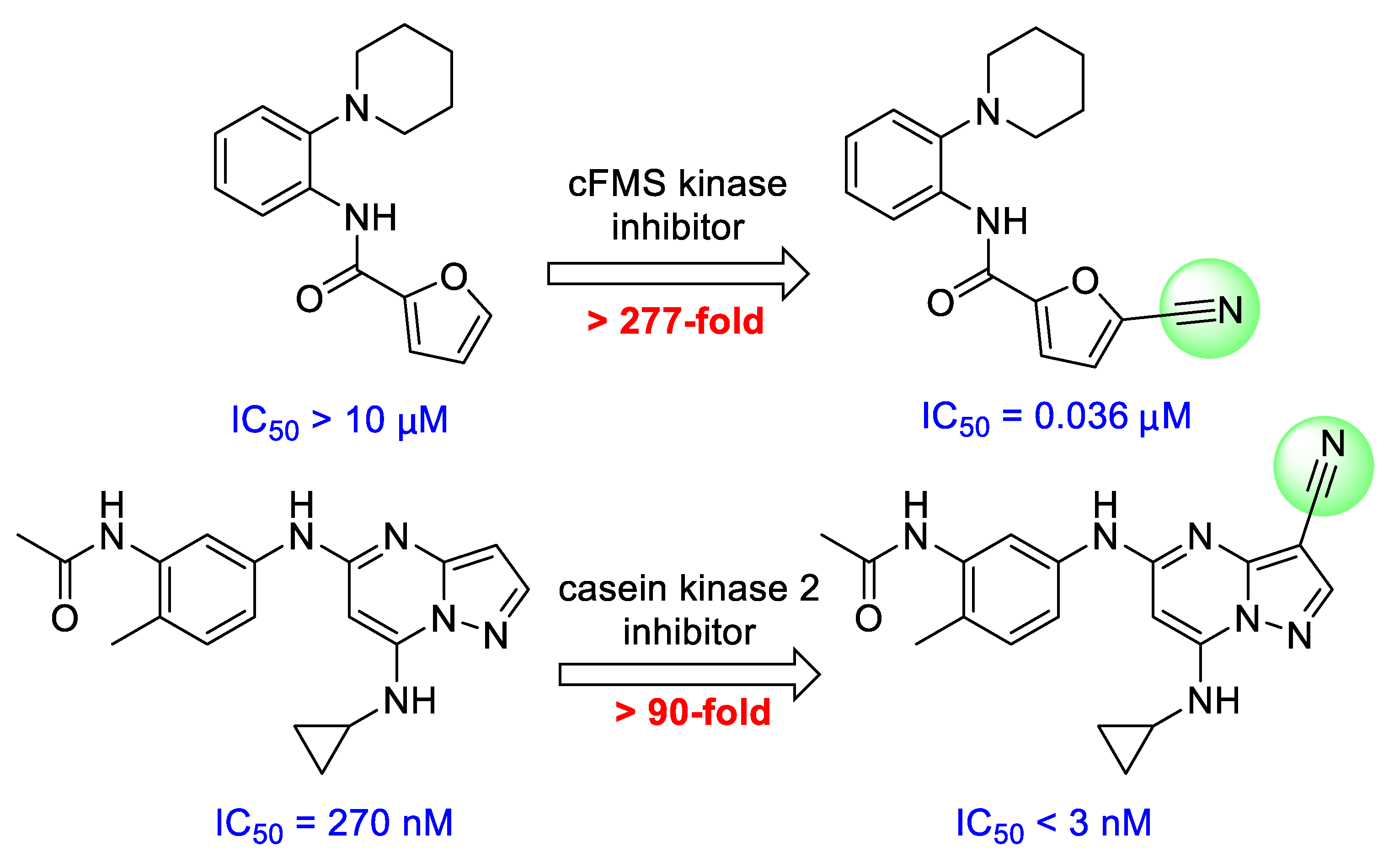
:1. Introduction
2. Results and Discussion
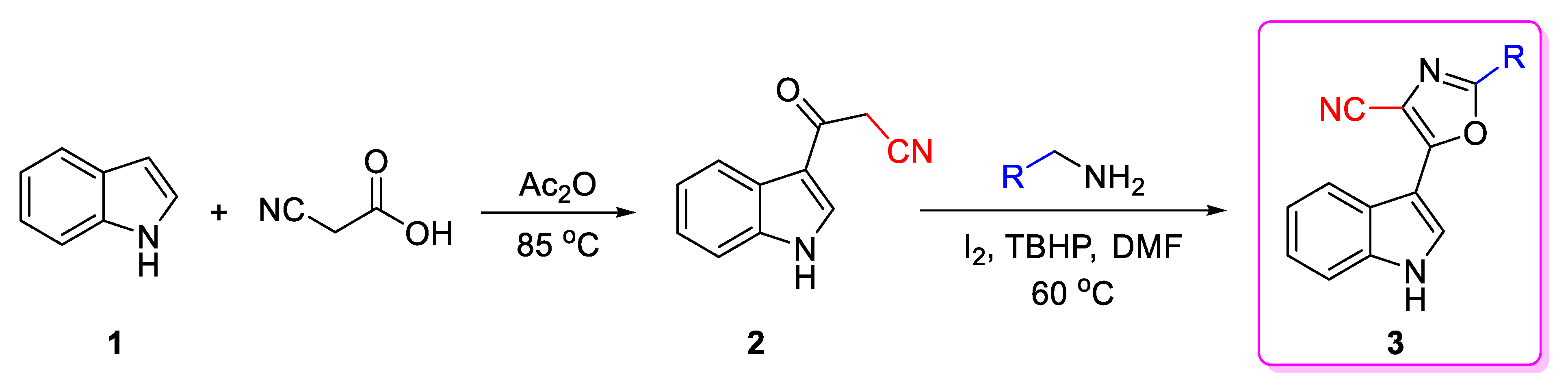
2.1. Synthetic Chemistry
2.2. Antifungal Activity and Structure–Activity Relationships (SARs)
2.3. Molecular Docking
3. Materials and Methods
3.1. Chemicals
3.1.1. Preparation of 3-(1H-indol-3-yl)-3-oxopropanenitrile (2)
3.1.2. General Procedure for the Synthesis of 2-substituted-4-cyano-5-(1H-indol-3-yl)oxazole (3)
3.2. Compound Data
3.2.1. 5-(1H-indol-3-yl)oxazole-4-carbonitrile (3a)
3.2.2. 5-(1H-indol-3-yl)-2-methyloxazole-4-carbonitrile (3b)
3.2.3. 2-ethyl-5-(1H-indol-3-yl)oxazole-4-carbonitrile (3c)
3.2.4. 5-(1H-indol-3-yl)-2-propyloxazole-4-carbonitrile (3d)
3.2.5. 2-butyl-5-(1H-indol-3-yl)oxazole-4-carbonitrile (3e)
3.2.6. 5-(1H-indol-3-yl)-2-pentyloxazole-4-carbonitrile (3f)
3.2.7. 5-(1H-indol-3-yl)-2-isobutyloxazole-4-carbonitrile (3g)
3.2.8. 2-benzyl-5-(1H-indol-3-yl) oxazole-4-carbonitrile (3h)
3.2.9. 5-(1H-indol-3-yl)-2-phenyloxazole-4-carbonitrile (3i)
3.2.10. 5-(1H-indol-3-yl)-2-(o-tolyl) oxazole-4-carbonitrile (3j)
3.2.11. 2-(2-fluorophenyl)-5-(1H-indol-3-yl) oxazole-4-carbonitrile (3k)
3.2.12. 5-(1H-indol-3-yl)-2-(m-tolyl) oxazole-4-carbonitrile (3l)
3.2.13. 5-(1H-indol-3-yl)-2-(3-methoxyphenyl) oxazole-4-carbonitrile (3m)
3.2.14. 2-(3-fluorophenyl)-5-(1H-indol-3-yl) oxazole-4-carbonitrile (3n)
3.2.15. 2-(3-bromophenyl)-5-(1H-indol-3-yl) oxazole-4-carbonitrile (3o)
3.2.16. 5-(1H-indol-3-yl)-2-(p-tolyl) oxazole-4-carbonitrile (3p)
3.2.17. 2-(4-fluorophenyl)-5-(1H-indol-3-yl) oxazole-4-carbonitrile (3q)
3.2.18. 2-(4-chlorophenyl)-5-(1H-indol-3-yl) oxazole-4-carbonitrile (3r)
3.2.19. 5-(1H-indol-3-yl)-2-(4-(trifluoromethyl)phenyl)oxazole-4-carbonitrile (3s)
3.2.20. 5-(1H-indol-3-yl)-2-(thiophen-2-yl)oxazole-4-carbonitrile (3t)
3.3. Biological Assays
3.4. Molecular Docking Strategy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TLC | thin layer chromatography |
| Ac2O | acetic anhydride |
| MeOH | methanol |
| TBHP | tert-Butyl hydroperoxide |
| DMF | N, N-dimethylformamide |
| m.p. | melting point |
| PDA | Potato Dextrose Agar (Medium) |
| EC50 | 50% effective concentration |
| tLeuRS | Thermus thermophiles leucyl-tRNA synthetase |
References
- Kwak, T.W.; Shin, H.J.; Jeong, Y.I.; Han, M.E.; Oh, S.O.; Kim, H.J.; Kim, D.H.; Kang, D.H. Anticancer activity of streptochlorin, a novel antineoplastic agent, in cholangiocarcinoma. Drug Des. Dev. Ther. 2015, 9, 2201–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Shin, H.J.; Kim, D.Y.; Shim, D.W.; Kim, T.J.; Ye, S.K.; Won, H.S.; Koppula, S.; Kang, T.B.; Lee, K.H. Streptochlorin suppresses allergic dermatitis and mast cell activation via regulation of Lyn/Fyn and Syk signaling pathways in cellular and mouse models. PLoS ONE 2013, 8, e74194. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.K.; Shin, H.J.; Lee, H.S.; Kwon, H.J. Streptochlorin, a marine natural product, inhibits NF-κB activation and suppresses angiogenesis in vitro. J. Microbiol. Biotechnol. 2007, 17, 1338–1343. [Google Scholar] [PubMed]
- Shim, D.W.; Shin, H.J.; Han, J.W.; Shin, W.Y.; Sun, X.; Shim, E.J.; Kim, T.J.; Kang, T.B.; Lee, K.H. Anti-inflammatory effect of Streptochlorin via TRIF-dependent signaling pathways in cellular and mouse models. Int. J. Mol. Sci. 2015, 16, 6902–6910. [Google Scholar] [CrossRef] [Green Version]
- Joshi, B.S.; Taylor, W.I.; Bhate, D.S.; Karmarkar, S.S. The structure and synthesis of pimprinine. Tetrahedron 1963, 19, 1437–1439. [Google Scholar] [CrossRef]
- Naik, S.R.; Harindran, J.; Varde, A.B. Pimprinine, an extracellular alkaloid produced by Streptomyces CDRIL-312: Fermentation, isolation and pharmacological activity. J. Biotechnol. 2001, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Kroiss, J.; Kaltenpoth, M.; Schneider, B.; Schwinger, M.G.; Hertweck, C.; Maddula, R.K.; Strohm, E.; Svatos, A. Symbiotic Streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 2010, 6, 261–263. [Google Scholar] [CrossRef]
- Park, C.; Shin, H.J.; Kim, G.Y.; Kwon, T.K.; Nam, T.J.; Kim, S.K.; Cheong, J.; Choi, I.W.; Choi, Y.H. Induction of apoptosis by streptochlorin isolated from Streptomyces sp. in human leukemic U937 cells. Toxicol. Vitr. 2008, 22, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.C.; Wang, J.G.; Wang, M.; Meng, X.G.; Wu, A.X. One-pot total synthesis: The first total synthesis of chiral alkaloid pimprinol A and the facile construction of its natural congeners from amino acids. Tetrahedron 2014, 70, 7470–7475. [Google Scholar] [CrossRef]
- Wei, Y.; Fang, W.; Wan, Z.; Wang, K.; Yang, Q.; Cai, X.; Shi, L.; Yang, Z. Antiviral effects against EV71 of pimprinine and its derivatives isolated from Streptomyces sp. Virol. J. 2014, 11, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Li, R.; Li, Y.; Li, S.; Yu, J.; Zhao, B.; Liao, A.; Wang, Y.; Wang, Z.; Lu, A.; et al. Discovery of Pimprinine Alkaloids as Novel Agents against a Plant Virus. J. Agric. Food Chem. 2019, 67, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Chen, Q.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y.C.; Yang, G.F.; Clough, J. Synthesis and fungicidal activity of novel pimprinine analogues. Eur. J. Med. Chem. 2012, 53, 283–291. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Chen, Q.; Xie, C.H.; Mulholland, N.; Turner, S.; Irwin, D.; Gu, Y.C.; Yang, G.F.; Clough, J. Synthesis and antifungal activity of novel streptochlorin analogues. Eur. J. Med. Chem. 2015, 92, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Jia, C.Y.; Gu, Y.C.; Mulholland, N.; Turner, S.; Beattie, D.; Zhang, W.H.; Yang, G.F.; Clough, J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017, 126, 669–674. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, D.C.; Liu, C.; Song, Z.L.; Liu, J.R.; Guo, S.K.; Tan, J.Y.; Qiu, R.L.; Jin, B.; Zhang, H.; et al. Streptochlorin analogues as potential antifungal agents: Design, synthesis, antifungal activity and molecular docking study. Bioorg. Med. Chem. 2021, 35, 116073. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Huang, N. A survey of the role of nitrile groups in protein-ligand interactions. Future Med. Chem. 2018, 10, 2713–2728. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Fleming, F.F.; Yao, L.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-containing pharmaceuticals: Efficacious roles of the nitrile pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourounakis, P.N.; Rekka, E.; Demopoulos, V.J.; Retsas, S. Effect of the position of the cyano-group of cyanopregnenolones on their drug metabolic inducing activity. Eur. J. Drug Metab. Pharmacokinet. 1991, 16, 9–13. [Google Scholar] [CrossRef]
- Ha, Y.S.; Kim, I.Y. Enzalutamide: Looking back at its preclinical discovery. Expert Opin. Drug Discov. 2014, 9, 837–845. [Google Scholar] [CrossRef]
- Xue, W.; Fu, T.; Deng, S.; Yang, F.; Yang, J.; Zhu, F. Molecular Mechanism for the Allosteric Inhibition of the Human Serotonin Transporter by Antidepressant Escitalopram. ACS Chem. Neurosci. 2022, 13, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; He, L.; Shao, M.; Yang, Z.; Ran, Y.; Li, D.; Zhou, Y.; Tang, M.; Wang, T.; Gong, Y.; et al. Discovery of a highly selective JAK3 inhibitor for the treatment of rheumatoid arthritis. Sci. Rep. 2018, 8, 5273. [Google Scholar] [CrossRef] [Green Version]
- Godfraind, T. Discovery and Development of Calcium Channel Blockers. Front. Pharmacol. 2017, 8, 286. [Google Scholar]
- Janssen, P.A.J.; Lewi, P.J.; Arnold, E.; Daeyaert, F.; de Jonge, M.; Heeres, J.; Koymans, L.; Vinkers, M.; Guillemont, J.; Pasquier, E.; et al. In search of a novel anti-HIV drug: Multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6dimethylphenyl]amino]-2-pyrimidinyl]amino]-benzonitrile (R278474, rilpivirine). J. Med. Chem. 2005, 48, 1901–1909. [Google Scholar]
- Keating, G.M. Vildagliptin A Review of its Use in Type 2 Diabetes Mellitus. Drugs 2010, 70, 2089–2112. [Google Scholar]
- Mitani, S.; Araki, S.; Yamaguchi, T.; Takii, Y.; Ohshima, T.; Matsuo, N. Biological properties of the novel fungicide cyazofamid against Phytophthora infestans on tomato and Pseudoperonospora cubensis on cucumber. Pest Manage. Sci. 2002, 58, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Clough, J.M. The strobilurins, oudemansins, and myxothiazols, fungicidal derivatives of beta-methoxyacrylic acid. Nat. Prod. Rep. 1993, 10, 565–574. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manage. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Wollenberg, R.D.; Taft, M.H.; Giese, S.; Thiel, C.; Balazs, Z.; Giese, H.; Manstein, D.J.; Sondergaard, T.E. Phenamacril is a reversible and noncompetitive inhibitor of Fusarium class I myosin. J. Biol. Chem. 2019, 294, 1328–1337. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhou, X.E.; Gong, Y.; Zhu, Y.; Cao, X.; Brunzelle, J.S.; Xu, H.E.; Zhou, M.; Melcher, K.; Zhang, F. Structural basis of Fusarium myosin I inhibition by phenamacril. PLoS Path. 2020, 16, e1008323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarleton, M.; Dyson, L.; Gilbert, J.; Sakoff, J.A.; McCluskey, A. Focused library development of 2-phenylacrylamides as broad spectrum cytotoxic agents. Bioorg. Med. Chem. 2013, 21, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Zhou, Y.X.; Chen, G.J.; Yang, Z.Q.; Li, Q.; Liu, P.J. Iodine-catalyzed oxidative annulation of 3-cyanoacetylindoles with benzylamines: Facile access to 5-(3-indolyl)oxazoles. Org. Biomol. Chem. 2018, 16, 3572–3575. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-R.; Liu, J.-M.; Gao, Y.; Shi, Z.; Nie, K.-R.; Guo, D.; Deng, F.; Zhang, H.-F.; Ali, A.S.; Zhang, M.-Z.; et al. Discovery of Novel Pimprinine and Streptochlorin Derivatives as Potential Antifungal Agents. Mar. Drugs 2022, 20, 740. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Ng, T.B.; Zhang, J.; Zhou, M.; Song, F.; Lu, F.; Liu, Y. Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides 2007, 28, 553–559. [Google Scholar] [CrossRef]







| No. | R= | Growth Inhibition (%) | |||||
|---|---|---|---|---|---|---|---|
| BOT a | ALS | GIB | RHI | COL | ALL | ||
| 3a | H | 100.0 b | 45.6 | 85.4 | 51.3 | 88.9 | 79.3 |
| 3b | CH3 | 40.4 | 27.3 | 40.9 | 64.2 | 47.0 | 47.3 |
| 3c | C2H5 | 21.8 | 14.1 | 24.8 | 39.3 | 28.3 | 26.7 |
| 3d | n-C3H7 | 26.1 | 16.7 | 11.0 | 44.4 | 17.8 | 26.1 |
| 3e | n-C4H9 | 23.9 | 0.0 | 3.1 | 32.9 | 14.4 | 24.2 |
| 3f | n-C5H11 | 26.4 | 4.2 | 10.3 | 58.8 | 20.1 | 35.8 |
| 3g | iso-butyl | 35.3 | 43.3 | 43.3 | 63.0 | 53.7 | 51.4 |
| 3h | Bn | 33.8 | 21.7 | 39.7 | 67.5 | 28.4 | 37.6 |
| 3i | Ph | 20.4 | 6.7 | 11.4 | 0.0 | 17.6 | 15.0 |
| 3j | (2-CH3)-Ph | 21.1 | 4.5 | 9.0 | 40.2 | 17.0 | 23.3 |
| 3k | (2-F)-Ph | 17.2 | 31.5 | 12.2 | 45.9 | 14.8 | 15.6 |
| 3l | (3-CH3)-Ph | 18.3 | 4.5 | 12.1 | 33.8 | 18.7 | 22.5 |
| 3m | (3-OCH3)-Ph | 19.4 | 3.9 | 8.2 | 16.2 | 0.9 | 13.2 |
| 3n | (3-F)-Ph | 10.8 | 2.9 | 3.9 | 21.6 | 8.6 | 7.9 |
| 3o | (3-Br)-Ph | 10.8 | 5.8 | 4.2 | 20.5 | 8.2 | 17.5 |
| 3p | (4-CH3)-Ph | 33.9 | 5.8 | 4.1 | 13.0 | 21.3 | 22.2 |
| 3q | (4-F)-Ph | 16.4 | 7.7 | 17.8 | 23.4 | 4.4 | 17.5 |
| 3r | (4-Cl)-Ph | 40.0 | 15.0 | 13.8 | 25.2 | 12.7 | 18.4 |
| 3s | (4-CF3)-Ph | 7.4 | 7.9 | 0.0 | 8.8 | 3.6 | 12.2 |
| 3t | 2-Thienyl | 8.5 | 0.0 | 5.8 | 24.0 | 5.2 | 16.9 |
| Osthole | / | 70.4 | 61.2 | 57.0 | 66.5 | 92.3 | 31.3 |
| Boscalid | / | 100.0 | 57.6 | 40.9 | 87.3 | 25.5 | 92.8 |
| Flutriafol | / | 93.9 | 81.9 | 91.8 | 79.9 | 98.4 | 100.0 |
| Pathogen | Compound | Toxic Regression | R | EC50 (μg/mL) | 95% Confidence Interval |
|---|---|---|---|---|---|
| Rhizoctonia solani | 3b | Y = 3.7265 + 1.0627X | 0.9974 | 15.79 | 14.6091~17.0648 |
| 3g | Y = 4.8431 + 0.2153X | 0.9901 | 5.36 | 3.6186~7.9249 | |
| 3h | Y = 4.9980 + 0.1754X | 0.9779 | 1.03 | 0.3249~3.2444 | |
| Boscalid | Y = 5.4550 + 0.9056X | 0.9853 | 0.31 | 0.2214~0.4326 | |
| Botrytis cinerea | 3a | Y = 4.6025 + 1.1407X | 0.9973 | 2.23 | 1.7204~2.8914 |
| Boscalid | Y = 5.4374 + 1.1977X | 0.9934 | 0.43 | 0.3585~0.5190 | |
| Gibberella zeae | 3a | Y = 4.1054 + 1.1956X | 0.9953 | 5.60 | 4.1288~7.5968 |
| Boscalid | Y = 5.3462 + 1.6505X | 0.9889 | 0.62 | 0.5023~0.7576 | |
| Colletotrichum lagenarium | 3a | Y = 3.5588 + 1.3771X | 0.9889 | 11.13 | 8.3162~14.8955 |
| Carbendazim | Y = 4.2332 + 2.3002X | 0.9800 | 2.15 | 1.5600~4.6000 | |
| Boscalid | Y = 2.9242 + 1.3510X | 0.9673 | 34.39 | 18.9576~62.3960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-R.; Gao, Y.; Jin, B.; Guo, D.; Deng, F.; Bian, Q.; Zhang, H.-F.; Han, X.-Y.; Ali, A.S.; Zhang, M.-Z.; et al. Design, Synthesis, Antifungal Activity, and Molecular Docking of Streptochlorin Derivatives Containing the Nitrile Group. Mar. Drugs 2023, 21, 103. https://doi.org/10.3390/md21020103
Liu J-R, Gao Y, Jin B, Guo D, Deng F, Bian Q, Zhang H-F, Han X-Y, Ali AS, Zhang M-Z, et al. Design, Synthesis, Antifungal Activity, and Molecular Docking of Streptochlorin Derivatives Containing the Nitrile Group. Marine Drugs. 2023; 21(2):103. https://doi.org/10.3390/md21020103
Chicago/Turabian StyleLiu, Jing-Rui, Ya Gao, Bing Jin, Dale Guo, Fang Deng, Qiang Bian, Hai-Feng Zhang, Xin-Ya Han, Abdallah S. Ali, Ming-Zhi Zhang, and et al. 2023. "Design, Synthesis, Antifungal Activity, and Molecular Docking of Streptochlorin Derivatives Containing the Nitrile Group" Marine Drugs 21, no. 2: 103. https://doi.org/10.3390/md21020103
APA StyleLiu, J.-R., Gao, Y., Jin, B., Guo, D., Deng, F., Bian, Q., Zhang, H.-F., Han, X.-Y., Ali, A. S., Zhang, M.-Z., Zhang, W.-H., & Gu, Y.-C. (2023). Design, Synthesis, Antifungal Activity, and Molecular Docking of Streptochlorin Derivatives Containing the Nitrile Group. Marine Drugs, 21(2), 103. https://doi.org/10.3390/md21020103









