Carotenoids from Starfish Patiria pectinifera: Therapeutic Activity in Models of Inflammatory Diseases
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Safety of Carotenoid mixture of Starfish P. pectinifera (MC)
2.2. The MC Effectiveness in Treatment of Carcinogenic and Allergic Skin Pathologies, and Systemic Inflammation (SI)
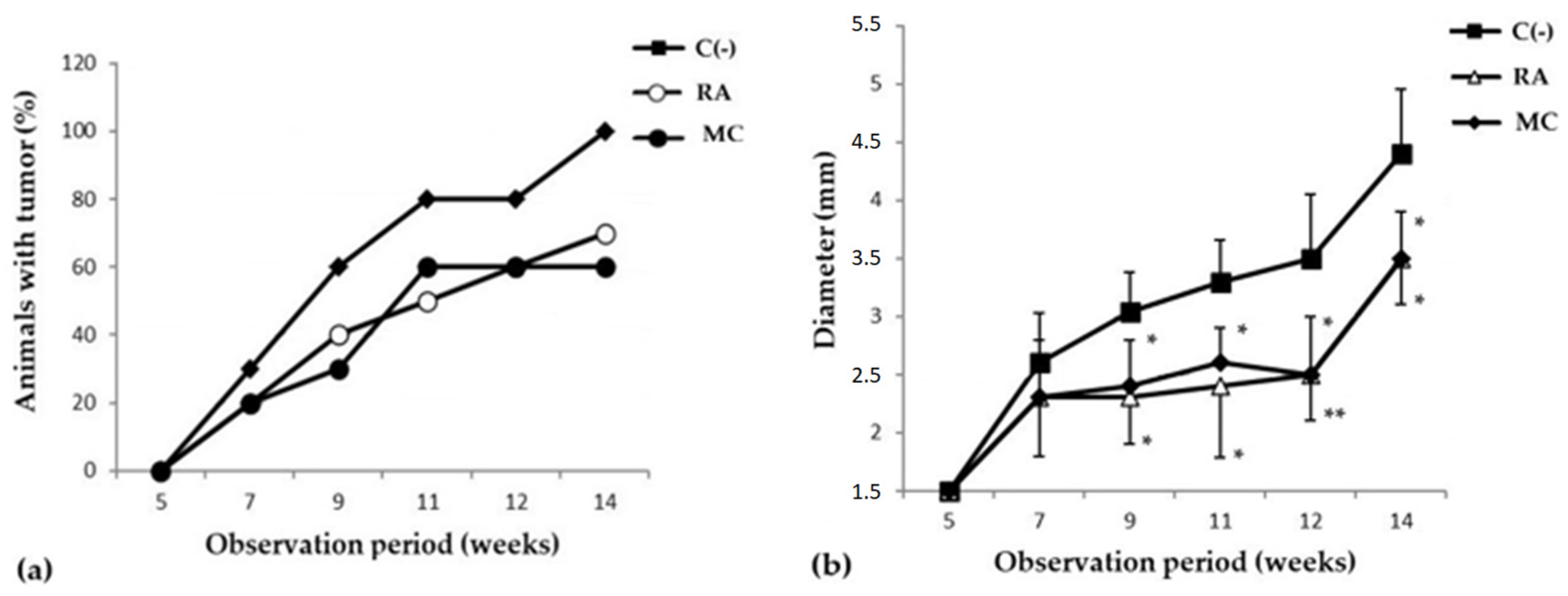
2.2.1. Evaluation of Cancer-Preventive Activity of MC
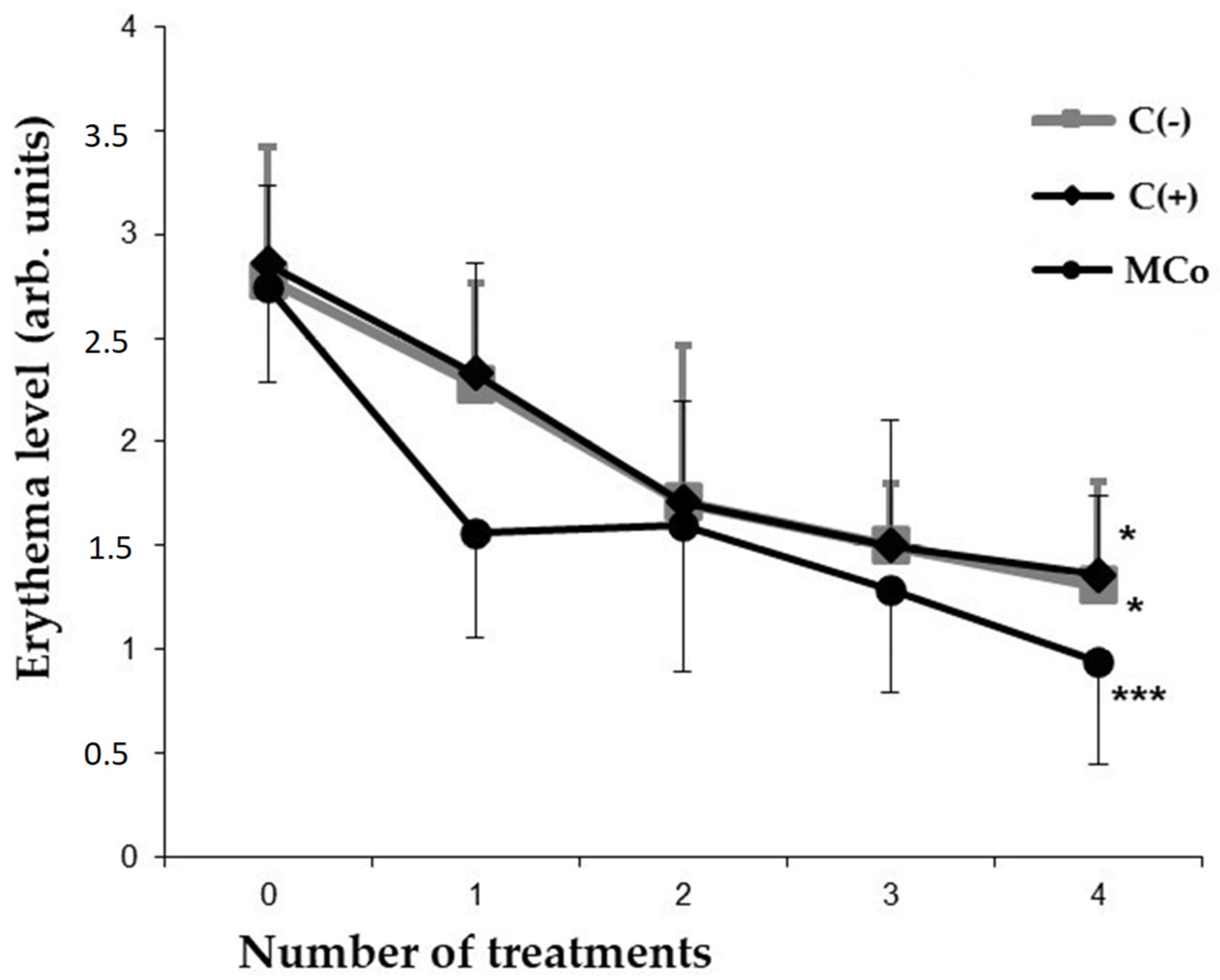
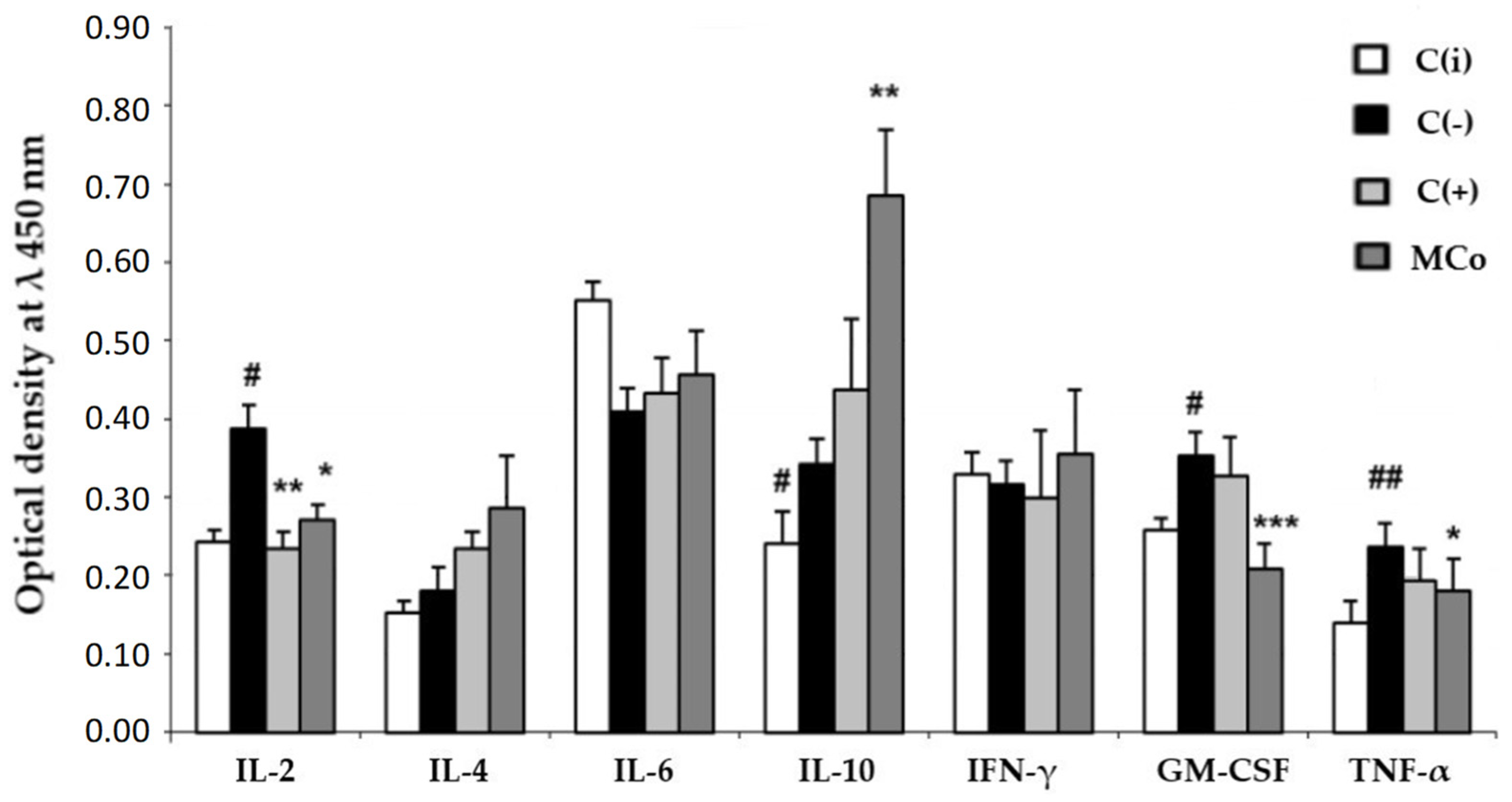
2.2.2. Evaluation of the MC Ointment Form in Allergic Contact Dermatitis (ACD)
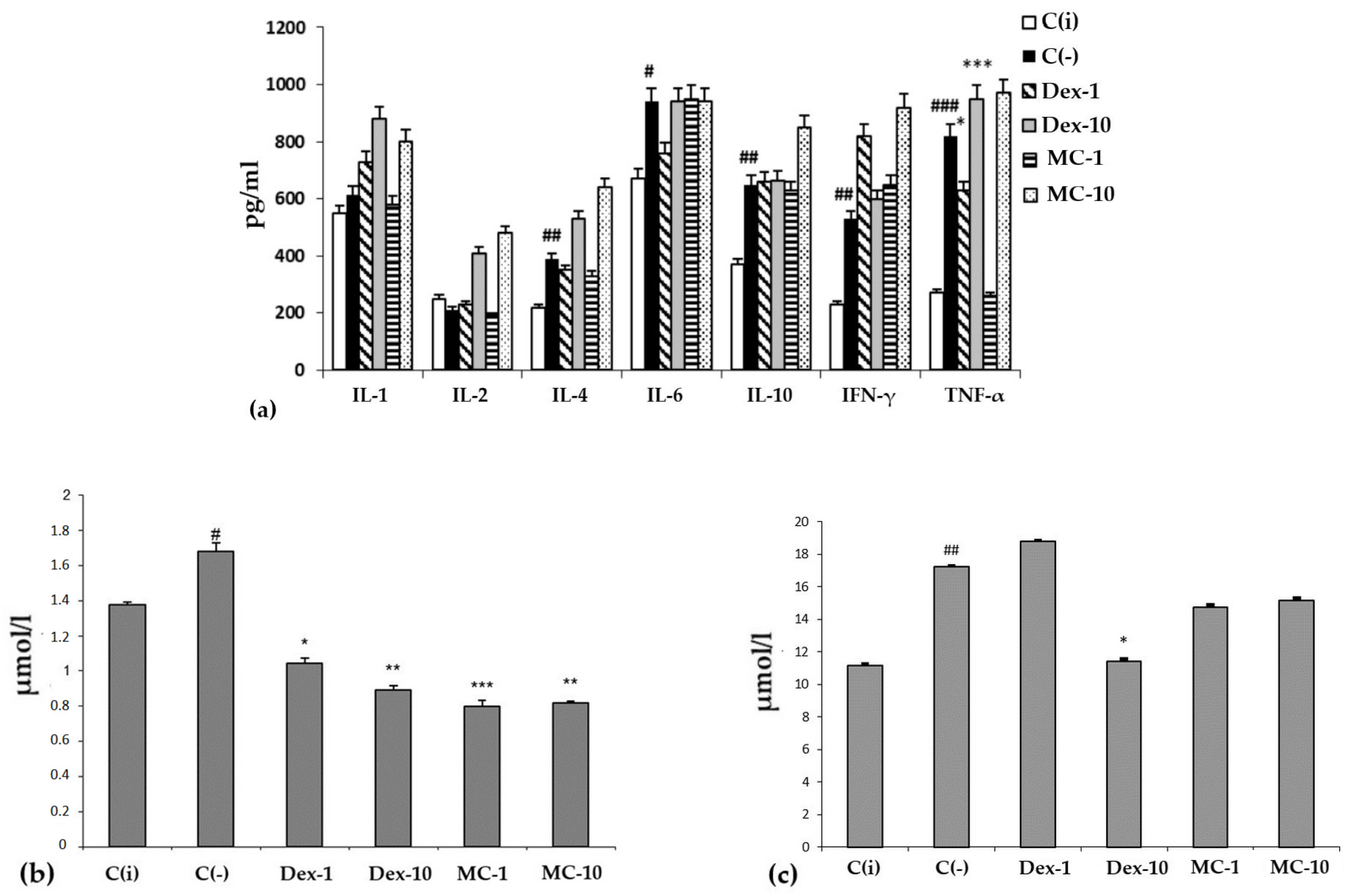
2.2.3. Anti-Inflammatory Activity of MC in SI Model
3. Discussion
4. Materials and Methods
4.1. Preparations
4.2. Animals
4.3. Safety Assessment of MC
4.4. Murine Model of Skin Carcinogenesis
4.5. Murine model of Experimental Dermatitis
4.6. Murine Model of Inflammation
4.7. Immunological and Biochemical Parameters Studies
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jahns, L.; Conrad, Z.; Johnson, L.K.; Whigham, L.D.; Wu, D.; Claycombe-Larson, K.J. A diet high in carotenoid-rich vegetables and fruits favorably impacts inflammation status by increasing plasma concentrations of IFN-α2 and decreasing MIP-1β and TNF-α in healthy individuals during a controlled feeding trial. Nutr. Res. 2018, 52, 98–104. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef] [PubMed]
- Kowsalya, K.; Vidya, N.; Vijayalakshmi, V.; Arun, M. Super nutritive marine astaxanthin, an effectual dietary carotenoid for neurodegenerative diseases. Int. Res. J. Multidiscip. Technovation 2019, 1, 115–124. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Role of natural antioxidants from functional foods in neurodegenerative and metabolic disorders. Oxidative Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Mullan, K.; Williams, M.A.; Cardwell, C.R.; McGuinness, B.; Passmore, P.; Silvestri, G.; Woodside, J.V.; McKay, G.J. Serum concentrations of vitamin E and carotenoids are altered in Alzheimer’s disease: A case-control study. Alzheimers Dement. 2017, 3, 432–439. [Google Scholar] [CrossRef]
- Guest, J.; Grant, R. Carotenoids and neurobiological health. Adv. Neurobiol. 2016, 12, 199–228. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, N.M.; Saedisomeolia, A.; Abdolahi, M.; Shayeganrad, A.; Sangsari, G.T.; Rad, B.H.; Muench, G. Molecular anti-inflammatory mechanisms of retinoids and carotenoids in Alzheimer’s disease: A review of current Evidence. J. Mol. Neurosci. 2017, 61, 289–304. [Google Scholar] [CrossRef]
- Giordano, P.; Scicchitano, P.; Locorotondo, M.; Mandurino, C.; Ricci, G.; Carbonara, S.; Gesualdo, M.; Zito, A.; Dachille, A.; Caputo, P.; et al. Carotenoids and cardiovascular risk. Curr. Pharm. Des. 2012, 18, 5577–5589. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease. Current state of knowledge. J. Funct. Foods 2017, 38, 45–65. [Google Scholar] [CrossRef]
- Wolak, T.; Paran, E. Can carotenoids attenuate vascular aging? Vascul. Pharmacol. 2013, 59, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; d’Orazio, N. Prevention of cardiovascular diseases with carotenoids. Front. Biosci. 2017, 9, 165–171. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S.; Daglia, M.; Rengasamy, K.R.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, M.S.; Fang, Y.J.; Xu, M.; Huang, W.Q.; Pan, Z.Z.; Chen, Y.M.; Zhang, C.X. Serum carotenoids and colorectal cancer risk: A case-control study in Guangdong, China. Mol. Nutr. Food Res. 2017, 61, 1700267. [Google Scholar] [CrossRef]
- Butalla, A.C.; Crane, T.F.; Patil, B.; Wertheim, B.C.; Thomson, P.; Thomson, C.A. Effect of a carrot juice intervention on plasma carotenoids, oxidative stress, and inflammation in overweigth breast cancer survivors. Nutr. Cancer. 2012, 64, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Zhai, K.; Abotaleb, M.; Ashrafizadeh, M.; Brockmueller, A.; Shakibaei, M.; Biringer, K.; Bugos, O.; et al. Carotenoids in cancer metastasis—Status quo and outlook. Biomolecules 2020, 10, 1653. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef]
- McClinton, K.J.; Aliani, M.; Kuny, S.; Sauvé, Y.; Suh, M. Differential effect of a carotenoid-rich diet on retina function in non-diabetic and diabetic rats. Nutr. Neurosci. 2020, 23, 838–848. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid. Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, C.; Wu, J. Astaxanthin in liver health and disease: A potential therapeutic agent. Drug Des. Devel. Ther. 2020, 14, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on human subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Milito, A.; Castellano, I.; Damiani, E. From Sea to Skin: Is There a Future for Natural Photoprotectants? Mar. Drugs 2021, 19, 379. [Google Scholar] [CrossRef]
- Roark, M.W.; Stringham, J.M. Visual performance in the “Real World”: Contrast sensitivity, visual acuity, and effects of macular carotenoids. Mol. Nutr. Food Res. 2019, 63, e1801053. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Mahmoud, A. The role of food supplements in the treatment of the infertile man. Reprod. BioMed. Online 2003, 7, 385–391. [Google Scholar] [CrossRef]
- Kurashova, N.A.; Dashiev, B.G.; Kolesnikov, S.I.; Dmitrenok, P.S.; Kozlovskaya, E.P.; Kasyanov, S.P.; Epur, N.V.; Usov, N.G.; Kolesnikova, L.I. Processes, and antioxidant protection in men with pathozoospermia after COVID-19. The effectiveness of correction with a promising antioxidant complex. Bull. Exp. Biol. Med. 2022, 173, 606–610. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Pivina, L.; Srinath, S.; Gasmi Benahmed, A.; Semenova, Y.; Menzel, A.; Dadar, M.; Bjorklund, G. Interrelations between COVID-19 and other disorders. Clin. Immunol. 2021, 224, 108651. [Google Scholar] [CrossRef]
- Talukdar, J.; Dasgupta, S.; Nagle, V.; Bhadra, B. COVID-19: Potential of Microalgae Derived Natural Astaxanthin as Adjunctive Supplement in Alleviating Cytokine Storm. 2020. Available online: https://dx.doi.org/10.2139/ssrn.3579738 (accessed on 20 August 2023).
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Farzaei, M.H. Astaxanthin, COVID -19 and immune response: Focus on oxidative stress, apoptosis and autophagy. Phytother. Res. 2020, 34, 2790–2792. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.J.; Martínez-Espinosa, R.M. Carotenoids from Haloarchaea and Their Potential in Biotechnology. Mar. Drugs 2015, 13, 5508–5532. [Google Scholar] [CrossRef] [PubMed]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From plants to food and feed industries. In Microbial Carotenoids. Methods in Molecular Biology; Barreiro, C., Barredo, J.L., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1852. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Chapter Carotenoids and photosynthesis. In Carotenoids in Nature. Subcellular Biochemistry 79; Stange, C., Ed.; Springer International Publishing: New York, NY, USA, 2016; pp. 111–139. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; De Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A Treasure from the Sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158617. [Google Scholar] [CrossRef]
- Genç, Y.; Bardakci, H.; Yücel, Ç.; Karatoprak, G.Ş.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E. Oxidative Stress and Marine Carotenoids: Application by Using Nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef]
- Barros, M.P.; Rodrigo, M.J.; Zacarias, L. Dietary carotenoid roles in redox homeostasis and human health. J. Agric. Food Chem. 2018, 66, 5733–5740. [Google Scholar] [CrossRef]
- Davinelli, S.; Saso, L.; D’Angeli, F.; Calabrese, V.; Intrieri, M.; Scapagnini, G. Astaxanthin as a Modulator of Nrf2, NF-κB, and Their Crosstalk: Molecular Mechanisms and Possible Clinical Applications. Molecules 2022, 27, 502. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Huang, F.; Zhong, S.; Ding, R.; Su, J.; Li, X. Astaxanthin attenuates ferroptosis via Keap1-Nrf2/HO-1 signaling pathways in LPS-induced acute lung injury. Life Sci. 2022, 311 Pt A, 121091. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.L.; Artyukov, A.A.; Drozdov, K.A. Mass Species of the Sea Star Asterina Pectinifera as a Potential Object of Mariculture. Int. J. Oceanogr. Aquac. 2020, 4, 000195. [Google Scholar] [CrossRef]
- Dai, Y.; Prithiviraj, N.; Gan, J.; Zhang, X.A.; Yan, J. Tissue Extract Fractions from Starfish Undergoing Regeneration Promote Wound Healing and Lower Jaw Blastema Regeneration of Zebrafish. Sci. Rep. 2016, 6, 38693. [Google Scholar] [CrossRef] [PubMed]
- World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=459559 (accessed on 20 August 2023).
- Artyukov, A.A.; Rutskova, T.A.; Kupera, E.V.; Makhankov, V.V.; Glazunov, V.P.; Kozlovskaya, E.P. Method for Obtaining a Carotenoid Complex from Starfish. Russian Patent 2469732 C1, 20 December 2012. [Google Scholar]
- Artyukov, A.A.; Kupera, E.V.; Rutskova, T.A.; Makhankov, V.V.; Glazunov, V.P.; Klimovich, A.A.; Popov, A.M.; Kozlovskaya, E.P. An Agent Based on Biologically Active Compounds of Marine Hydrobionts, Which Has a Cancer-Preventive Effect and Increases the Therapeutic Activity of Antitumor Antibiotics. Russian Patent 2659682 C1, 3 July 2018. [Google Scholar]
- Maoka, T.; Tsushima, M.; Matsuno, T. New acetylenic carotenoids starfishes from the Asterina pectinifera and Asterias amurensis. Comp. Biochem. Physiol. 1989, 93, 829–834. [Google Scholar] [CrossRef]
- Popov, A.M.; Krivoshapko, O.N.; Artyukov, A.A. Prospects for the clinical use of astaxanthin and another oxygenated carotenoides. Biopharm. J. 2013, 5, 13–30. [Google Scholar]
- Wang, T.; Gavin, H.M.; Arlt, V.M.; Lawrence, B.P.; Fenton, S.E.; Medina, D.; Vorderstrasse, B.A. Aryl hydrocarbon receptor activation during pregnancy, and in adult nulliparous mice, delays the subsequent development of DMBA-induced mammary tumors. Int. J. Cancer 2010, 128, 1509–1523. [Google Scholar] [CrossRef]
- Vorontsova, J.E.; Cherezov, R.O.; Kuzin, B.A.; Simonova, O.B. Aryl-hydrocarbon receptor as a potential target for anticancer therapy. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2019, 13, 36–54. [Google Scholar] [CrossRef]
- Aleksandrova, A.V. The role of matrix metalloproteinases in the healing of burn wounds. Eur. Stud. Sci. J. 2013, 2, 40. [Google Scholar]
- Meephansan, J.; Rungjang, A.; Yingmema, W.; Deenonpoe, R.; Ponnikorn, S. Effect of astaxanthin on cutaneous wound healing. Clin. Cosmet. Investig. Dermatol. 2017, 10, 259–265. [Google Scholar] [CrossRef]
- Chou, H.Y.; Lee, C.; Pan, J.L.; Wen, Z.H.; Huang, S.H.; Lan, C.W.; Liu, W.T.; Hour, T.C.; Hseu, Y.C.; Hwang, B.H.; et al. Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2016, 17, 955. [Google Scholar] [CrossRef] [PubMed]
- Berezovskaya, I.V. Classification of chemicals according to the parameters of acute toxicity in parenteral routes of administration. Rus. Khim. Pharm. J. 2003, 37, 32–34. [Google Scholar]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.N.; Bunyatyan, N.D.; Vasiliev, A.N.; Verstakova, O.L.; Zhuravleva, M.V.; Lepakhin, V.K.; Korobov, N.V.; Merkulov, V.A.; Orekhov, S.N.; Sakaeva, I.V.; et al. Guidelines for Conducting Preclinical Studies of Drugs; Grif and K: Tula, Russia, 2012; 944p, ISBN 9785812514663. [Google Scholar]
- Dorozhkin, V.I.; Biryukova, N.P.; Bakhmutova, T.V. Modern Requirements to the Study of General Toxic Effect of Pharmacological Substances. Probl. Vet. Sanit. Hyg. Ecol. 2019, 2, 205–215. [Google Scholar] [CrossRef]


| No., Gender | Intact Animals | Water-Alcohol Solution (Solvent) in a Volume of 0.5 mL | MC at a Dose of 500 mg/kg. | |||
|---|---|---|---|---|---|---|
| First Day | On the 14th Day | Before Administration | On the 14th Day | Before Administration | On the 14th Day | |
| IP injection | ||||||
| 1♂ | 23.5 | 30.2 | 22.6 | 30.6 | 23.0 | 31.1 |
| 2♂ | 23.4 | 31.1 | 23.4 | 30.1 | 23.8 | 31.8 |
| 3♂ | 22.8 | 31.6 | 22.8 | 31.1 | 22.3 | 31.2 |
| 1♀ | 21.3 | 30.8 | 22.1 | 30.9 | 22.4 | 29.8 |
| 2♀ | 23.4 | 31.1 | 23.2 | 32.1 | 23.0 | 31.1 |
| 3♀ | 23.2 | 31.6 | 22.4 | 31.3 | 22.6 | 30.3 |
| Oral administration | ||||||
| 1♂ | 22.4 | 30.9 | 22.2 | 31.3 | 23.6 | 29.9 |
| 2♂ | 22.9 | 31.1 | 23.4 | 31.1 | 23.2 | 30.4 |
| 3♂ | 23.3 | 31.1 | 23.8 | 31.8 | 22.3 | 30.1 |
| 1♀ | 22.9 | 30.4 | 22.3 | 30.4 | 23.3 | 30.4 |
| 2♀ | 23.5 | 31.8 | 23.8 | 32.1 | 23.8 | 32.1 |
| 3♀ | 22.4 | 33.1 | 23.4 | 32.3 | 22.8 | 31.3 |
| Group | Number of Animals with Tumor, % | Number of Tumors | Average Tumor Diameter, mm | Latent Period, Week |
|---|---|---|---|---|
| Intact | - | - | - | - |
| C(−) | 100 | 27 | 4.4 ± 1.6 | 4.2 ± 2.9 |
| RA | 70 | 15 | 3.5 ± 0.9 | 4.6 ± 1.1 |
| MC | 60 | 13 | 3.5 ± 1.01 | 4.3 ± 2.7 |
| Group Animals | 1 Days | 2 Days | 4 Days |
|---|---|---|---|
| Healing, %, (m ± σ) | |||
| C(−) | - | - | - |
| Fucidin | 8 ± 2.5 | 20 ± 3.5 | 24 ± 2.3 |
| MC, 1% | 43 ± 2.9 | 40 ± 2.6 | 80 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, A.M.; Kozlovskaya, E.P.; Klimovich, A.A.; Rutckova, T.A.; Vakhrushev, A.I.; Hushpulian, D.M.; Gazaryan, I.G.; Makhankov, V.V.; Son, O.M.; Tekutyeva, L.A. Carotenoids from Starfish Patiria pectinifera: Therapeutic Activity in Models of Inflammatory Diseases. Mar. Drugs 2023, 21, 470. https://doi.org/10.3390/md21090470
Popov AM, Kozlovskaya EP, Klimovich AA, Rutckova TA, Vakhrushev AI, Hushpulian DM, Gazaryan IG, Makhankov VV, Son OM, Tekutyeva LA. Carotenoids from Starfish Patiria pectinifera: Therapeutic Activity in Models of Inflammatory Diseases. Marine Drugs. 2023; 21(9):470. https://doi.org/10.3390/md21090470
Chicago/Turabian StylePopov, Aleksandr M., Emma P. Kozlovskaya, Anna A. Klimovich, Tatyana A. Rutckova, Aleksey I. Vakhrushev, Dmitry M. Hushpulian, Irina G. Gazaryan, Vyacheslav V. Makhankov, Oksana M. Son, and Liudmila A. Tekutyeva. 2023. "Carotenoids from Starfish Patiria pectinifera: Therapeutic Activity in Models of Inflammatory Diseases" Marine Drugs 21, no. 9: 470. https://doi.org/10.3390/md21090470
APA StylePopov, A. M., Kozlovskaya, E. P., Klimovich, A. A., Rutckova, T. A., Vakhrushev, A. I., Hushpulian, D. M., Gazaryan, I. G., Makhankov, V. V., Son, O. M., & Tekutyeva, L. A. (2023). Carotenoids from Starfish Patiria pectinifera: Therapeutic Activity in Models of Inflammatory Diseases. Marine Drugs, 21(9), 470. https://doi.org/10.3390/md21090470









