In Search of the Role of Three-Finger Starfish Proteins
Abstract
:1. Introduction
2. Results
2.1. Identification of Proteins Containing the LU Domains in Asterias Rubens Genome
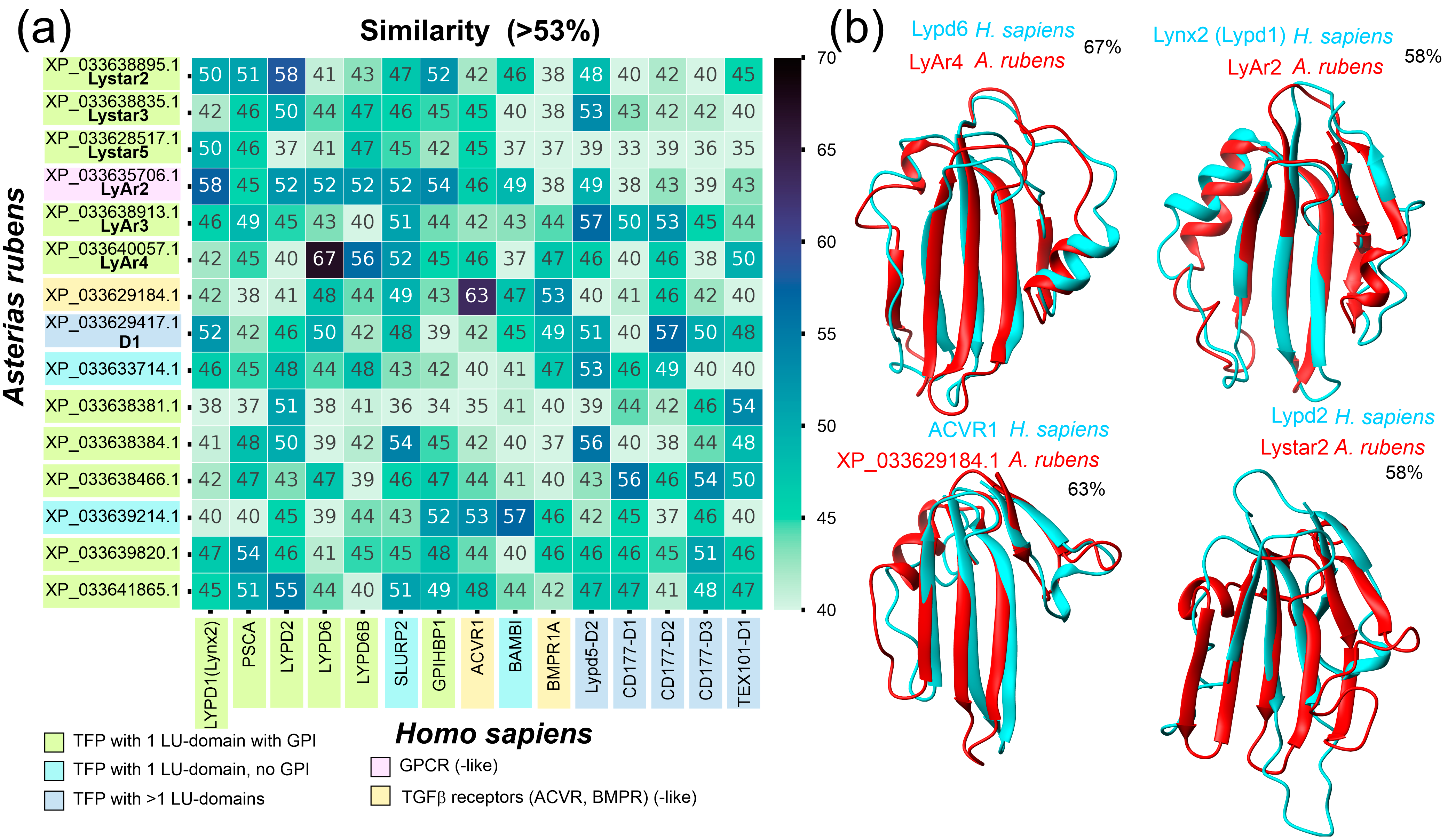
2.2. Comparison of A. rubens TFPs with Known Human TFPs
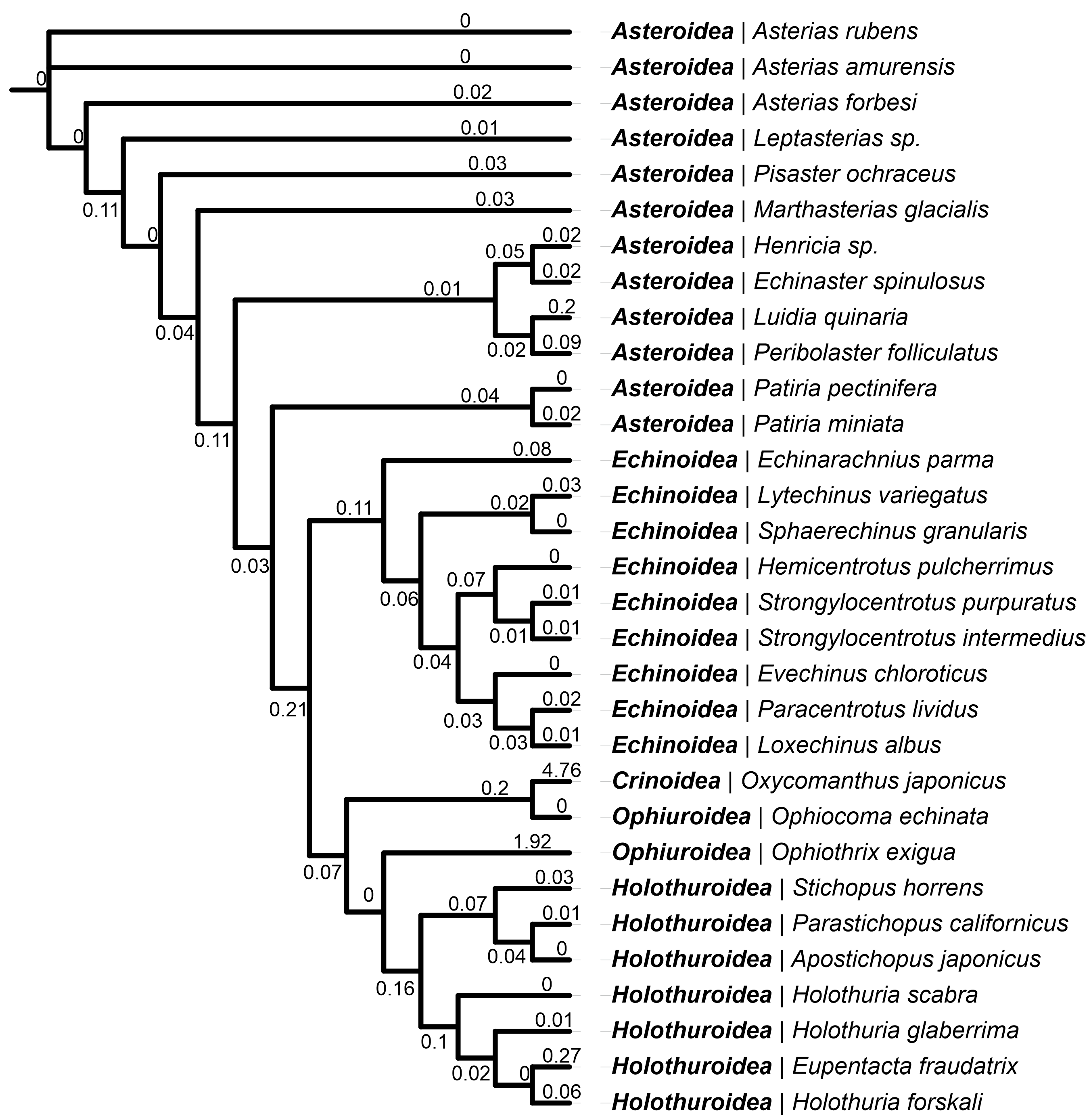
2.3. Lystar5 Homologues in Echinoderm Species
2.4. Expression of the TFPs Genes in Different Tissues of A. rubens on a mRNA Level
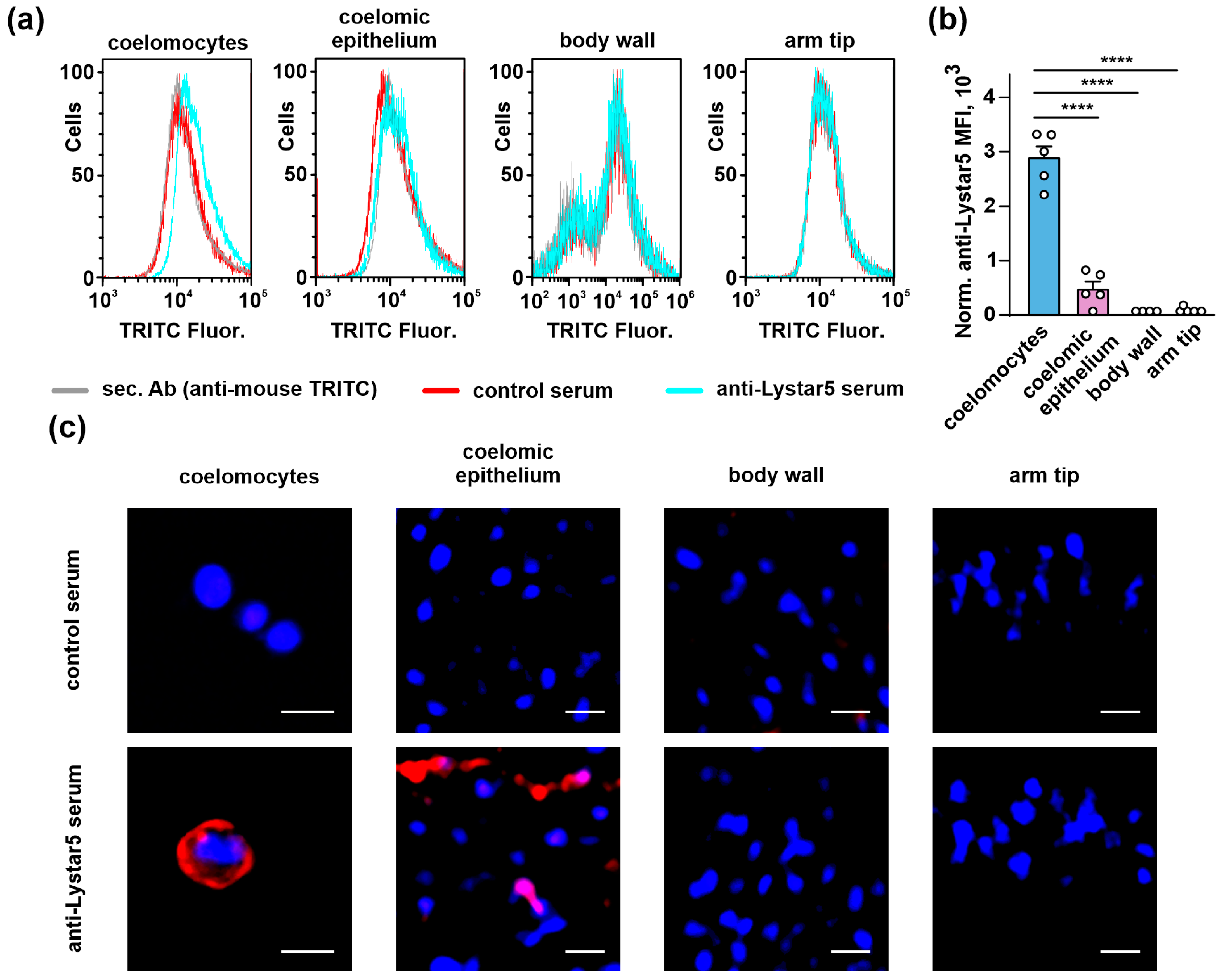
2.5. Lystar5 Is Expressed in Coelomocytes and Coelomic Epithelium on a Protein Level
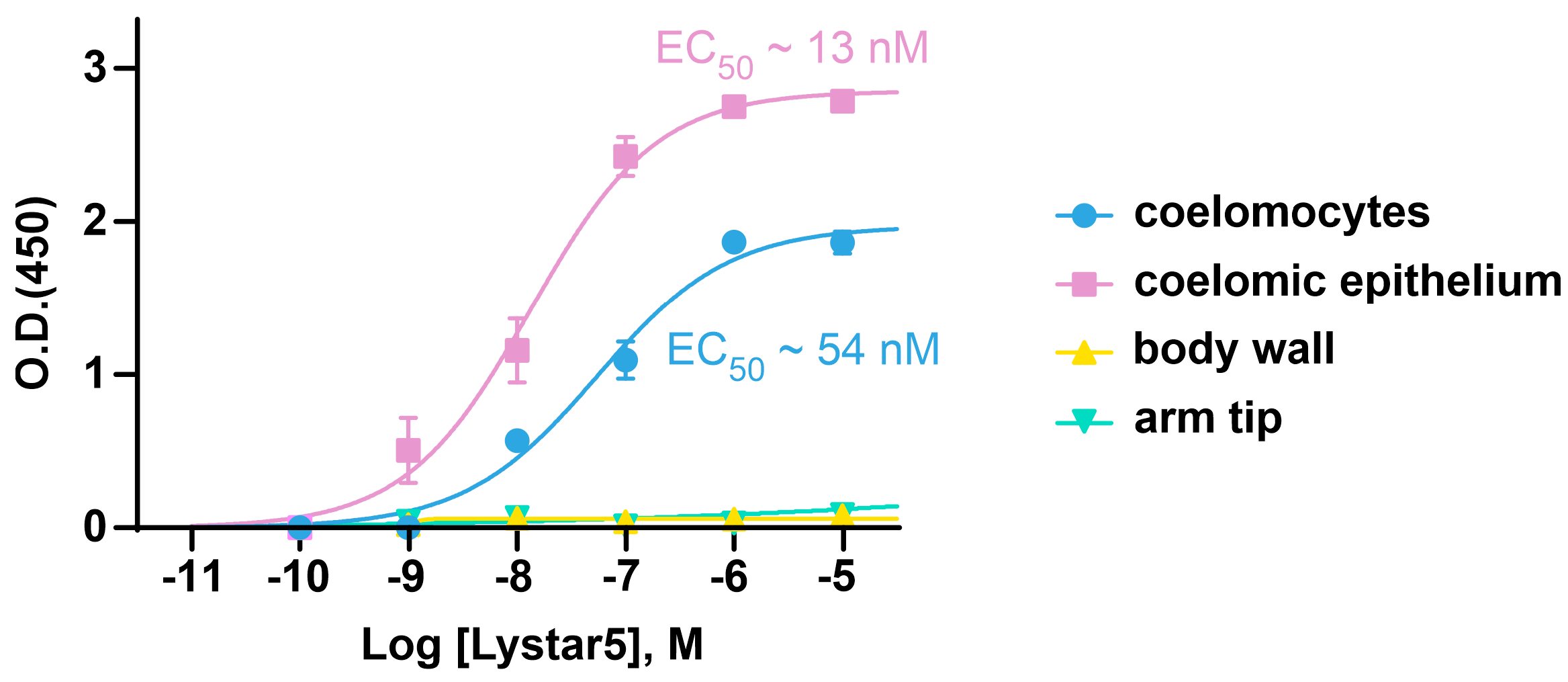
2.6. Lystar5 Targets the Membrane Protein in Coelomocytes and Coelomic Epithelium
2.7. Integrin α-8-like Protein Is Suggested Lystar5 Target in Coelomocytes and Coelomic Epithelium
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis
4.2. Animals and Tissue Collection
4.3. Real-Time PCR Analysis of Expression of TFP Genes in Different Tissues of A. rubens
4.4. Recombinant Lystar5 Expression and Purification
4.5. Mice Immunization
4.6. Flow Cytometry
4.7. Confocal Microscopy
4.8. ELISA
4.9. Affinity Extraction
4.10. Protein Digestion and Peptide Fractionation
4.11. MALDI TOF/TOF Mass Spectrometry
4.12. Protein Identification
4.13. Bio-Layer Interferometry Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galat, A.; Gross, G.; Drevet, P.; Sato, A.; Ménez, A. Conserved Structural Determinants in Three-Fingered Protein Domains. FEBS J. 2008, 275, 3207–3225. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, N.A.; Loktyushov, E.V.; Bychkov, M.L.; Shenkarev, Z.O.; Lyukmanova, E.N. Three-Finger Proteins from the Ly6/uPAR Family: Functional Diversity within One Structural Motif. Biochemistry 2017, 82, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Loughner, C.L.; Bruford, E.A.; McAndrews, M.S.; Delp, E.E.; Swamynathan, S.; Swamynathan, S.K. Organization, Evolution and Functions of the Human and Mouse Ly6/uPAR Family Genes. Hum. Genom. 2016, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Onichtchouk, D.; Chen, Y.G.; Dosch, R.; Gawantka, V.; Delius, H.; Massagué, J.; Niehrs, C. Silencing of TGF-Beta Signalling by the Pseudoreceptor BAMBI. Nature 1999, 401, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef]
- Sachs, U.J.H.; Andrei-Selmer, C.L.; Maniar, A.; Weiss, T.; Paddock, C.; Orlova, V.V.; Choi, E.Y.; Newman, P.J.; Preissner, K.T.; Chavakis, T.; et al. The Neutrophil-Specific Antigen CD177 Is a Counter-Receptor for Platelet Endothelial Cell Adhesion Molecule-1 (CD31) *. J. Biol. Chem. 2007, 282, 23603–23612. [Google Scholar] [CrossRef]
- Miyazono, K.; Maeda, S.; Imamura, T. BMP Receptor Signaling: Transcriptional Targets, Regulation of Signals, and Signaling Cross-Talk. Cytokine Growth Factor Rev. 2005, 16, 251–263. [Google Scholar] [CrossRef]
- Greenwald, J.; Fischer, W.H.; Vale, W.W.; Choe, S. Three-Finger Toxin Fold for the Extracellular Ligand-Binding Domain of the Type II Activin Receptor Serine Kinase. Nat. Struct. Biol. 1999, 6, 18–22. [Google Scholar] [CrossRef]
- Klages, J.; Kotzsch, A.; Coles, M.; Sebald, W.; Nickel, J.; Müller, T.; Kessler, H. The Solution Structure of BMPR-IA Reveals a Local Disorder-to-Order Transition upon BMP-2 Binding. Biochemistry 2008, 47, 11930–11939. [Google Scholar] [CrossRef]
- Antil-Delbeke, S.; Gaillard, C.; Tamiya, T.; Corringer, P.-J.; Changeux, J.-P.; Servent, D.; Ménez, A. Molecular Determinants by Which a Long Chain Toxin from Snake Venom Interacts with the Neuronal A7-Nicotinic Acetylcholine Receptor. J. Biol. Chem. 2000, 275, 29594–29601. [Google Scholar] [CrossRef]
- Bocharov, E.V.; Lyukmanova, E.N.; Ermolyuk, Y.S.; Schulga, A.A.; Pluzhnikov, K.A.; Dolgikh, D.A.; Kirpichnikov, M.P.; Arseniev, A.S. Resonance Assignment of C-13-N-15-Labeled Snake Neurotoxin II from Naja Oxiana. Appl. Magn. Reson. 2003, 24, 247–254. [Google Scholar] [CrossRef]
- Gilquin, B.; Roumestand, C.; Zinn-Justin, S.; Ménez, A.; Toma, F. Refined Three-Dimensional Solution Structure of a Snake Cardiotoxin: Analysis of the Side-Chain Organization Suggests the Existence of a Possible Phospholipid Binding Site. Biopolymers 1993, 33, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Kessler, P.; Marchot, P.; Silva, M.; Servent, D. The Three-Finger Toxin Fold: A Multifunctional Structural Scaffold Able to Modulate Cholinergic Functions. J. Neurochem. 2017, 142, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Koludarov, I.; Senoner, T.; Jackson, T.N.W.; Dashevsky, D.; Heinzinger, M.; Aird, S.D.; Rost, B. Domain Loss Enabled Evolution of Novel Functions in the Snake Three-Finger Toxin Gene Superfamily. Nat. Commun. 2023, 14, 4861. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wüster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular Evolution and Phylogeny of Elapid Snake Venom Three-Finger Toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Wang, J.-X.; Parisini, E.; Dascher, C.C.; Nigrovic, P.A. Ly6 Family Proteins in Neutrophil Biology. J. Leukoc. Biol. 2013, 94, 585–594. [Google Scholar] [CrossRef]
- Fletcher, C.M.; Harrison, R.A.; Lachmann, P.J.; Neuhaus, D. Structure of a Soluble, Glycosylated Form of the Human Complement Regulatory Protein CD59. Structure 1994, 2, 185–199. [Google Scholar] [CrossRef]
- Yu, J.; Murthy, V.; Liu, S.-L. Relating GPI-Anchored Ly6 Proteins uPAR and CD59 to Viral Infection. Viruses 2019, 11, 1060. [Google Scholar] [CrossRef]
- Mar, K.B.; Rinkenberger, N.R.; Boys, I.N.; Eitson, J.L.; McDougal, M.B.; Richardson, R.B.; Schoggins, J.W. LY6E Mediates an Evolutionarily Conserved Enhancement of Virus Infection by Targeting a Late Entry Step. Nat. Commun. 2018, 9, 3603. [Google Scholar] [CrossRef]
- Arredondo, J.; Chernyavsky, A.I.; Grando, S.A. SLURP-1 and -2 in Normal, Immortalized and Malignant Oral Keratinocytes. Life Sci. 2007, 80, 2243–2247. [Google Scholar] [CrossRef]
- Arvaniti, M.; Jensen, M.M.; Soni, N.; Wang, H.; Klein, A.B.; Thiriet, N.; Pinborg, L.H.; Muldoon, P.P.; Wienecke, J.; Imad Damaj, M.; et al. Functional Interaction between Lypd6 and Nicotinic Acetylcholine Receptors. J. Neurochem. 2016, 138, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Ibañez-Tallon, I.; Miwa, J.M.; Wang, H.L.; Adams, N.C.; Crabtree, G.W.; Sine, S.M.; Heintz, N. Novel Modulation of Neuronal Nicotinic Acetylcholine Receptors by Association with the Endogenous Prototoxin Lynx1. Neuron 2002, 33, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, V.; George, A.A.; Nishi, R.; Whiteaker, P. The Prototoxin LYPD6B Modulates Heteromeric A3β4-Containing Nicotinic Acetylcholine Receptors, but Not A7 Homomers. FASEB J. 2016, 30, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Tekinay, A.B.; Nong, Y.; Miwa, J.M.; Lieberam, I.; Ibanez-Tallon, I.; Greengard, P.; Heintz, N. A Role for LYNX2 in Anxiety-Related Behavior. Proc. Natl. Acad. Sci. USA 2009, 106, 4477–4482. [Google Scholar] [CrossRef] [PubMed]
- Shlepova, O.V.; Shulepko, M.A.; Shipunova, V.O.; Bychkov, M.L.; Kukushkin, I.D.; Chulina, I.A.; Azev, V.N.; Shramova, E.I.; Kazakov, V.A.; Ismailova, A.M.; et al. Selective Targeting of A7 Nicotinic Acetylcholine Receptor by Synthetic Peptide Mimicking Loop I of Human SLURP-1 Provides Efficient and Prolonged Therapy of Epidermoid Carcinoma in Vivo. Front. Cell Dev. Biol. 2023, 11, 1256716. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Shulepko, M.A.; Bychkov, M.L.; Kulbatskii, D.S.; Shlepova, O.V.; Vasilyeva, N.A.; Andreev-Andrievskiy, A.A.; Popova, A.S.; Lagereva, E.A.; Loktyushov, E.V.; et al. Water-Soluble Variant of Human Lynx1 Positively Modulates Synaptic Plasticity and Ameliorates Cognitive Impairment Associated with A7-nAChR Dysfunction. J. Neurochem. 2020, 155, 45–61. [Google Scholar] [CrossRef]
- Herberg, S.; Gert, K.R.; Schleiffer, A.; Pauli, A. The Ly6/uPAR Protein Bouncer Is Necessary and Sufficient for Species-Specific Fertilization. Science 2018, 361, 1029–1033. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Guo, Q.; Zhang, S.; Ji, D.; Li, H. Identification and Expression of a New Ly6 Gene Cluster in Zebrafish Danio rerio, with Implications of Being Involved in Embryonic Immunity. Fish Shellfish Immunol. 2016, 54, 230–240. [Google Scholar] [CrossRef]
- Da Silva, S.M.; Gates, P.B.; Brockes, J.P. The Newt Ortholog of CD59 Is Implicated in Proximodistal Identity during Amphibian Limb Regeneration. Dev. Cell 2002, 3, 547–555. [Google Scholar] [CrossRef]
- Garza-Garcia, A.; Harris, R.; Esposito, D.; Gates, P.B.; Driscoll, P.C. Solution Structure and Phylogenetics of Prod1, a Member of the Three-Finger Protein Superfamily Implicated in Salamander Limb Regeneration. PLoS ONE 2009, 4, e7123. [Google Scholar] [CrossRef]
- Hijazi, A.; Haenlin, M.; Waltzer, L.; Roch, F. The Ly6 Protein Coiled Is Required for Septate Junction and Blood Brain Barrier Organisation in Drosophila. PLoS ONE 2011, 6, e17763. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Joiner, W.J.; Wu, M.N.; Yue, Z.; Smith, C.J.; Sehgal, A. Identification of SLEEPLESS, a Sleep-Promoting Factor. Science 2008, 321, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Edgecombe, G.D.; Giribet, G.; Dunn, C.W.; Hejnol, A.; Kristensen, R.M.; Neves, R.C.; Rouse, G.W.; Worsaae, K.; Sørensen, M.V. Higher-Level Metazoan Relationships: Recent Progress and Remaining Questions. Org. Divers. Evol. 2011, 11, 151–172. [Google Scholar] [CrossRef]
- Shabelnikov, S.; Bobkov, D.; Sharlaimova, N.; Petukhova, O. Injury Affects Coelomic Fluid Proteome of the Common Starfish Asterias rubens. J. Exp. Biol. 2019, 222, jeb198556. [Google Scholar] [CrossRef]
- Paramonov, A.S.; Shulepko, M.A.; Makhonin, A.M.; Bychkov, M.L.; Kulbatskii, D.S.; Chernikov, A.M.; Myshkin, M.Y.; Shabelnikov, S.V.; Shenkarev, Z.O.; Kirpichnikov, M.P.; et al. New Three-Finger Protein from Starfish Asteria Rubens Shares Structure and Pharmacology with Human Brain Neuromodulator Lynx2. Mar. Drugs 2022, 20, 503. [Google Scholar] [CrossRef]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-Scale Prediction of Atomic-Level Protein Structure with a Language Model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef]
- Telford, M.J.; Lowe, C.J.; Cameron, C.B.; Ortega-Martinez, O.; Aronowicz, J.; Oliveri, P.; Copley, R.R. Phylogenomic Analysis of Echinoderm Class Relationships Supports Asterozoa. Proc. R. Soc. B. 2014, 281, 20140479. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Q.; Zeng, X.; Ni, G. High-Quality Chromosome-Level Genome Assembly of the Northern Pacific Sea Star Asterias amurensis. DNA Res. 2024, 31, dsae007. [Google Scholar] [CrossRef]
- Farias, L.P.; Tararam, C.A.; Miyasato, P.A.; Nishiyama, M.Y.; Oliveira, K.C.; Kawano, T.; Verjovski-Almeida, S.; Leite, L.C.d.C. Screening the Schistosoma Mansoni Transcriptome for Genes Differentially Expressed in the Schistosomulum Stage in Search for Vaccine Candidates. Parasitol. Res. 2011, 108, 123–135. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Cinar, B.; Jensen, M.M.; Lyukmanova, E.N.; Shulepko, M.A.; Tsetlin, V.; Klein, A.B.; Mikkelsen, J.D. Expression of the Ly-6 Family Proteins Lynx1 and Ly6H in the Rat Brain Is Compartmentalized, Cell-Type Specific, and Developmentally Regulated. Brain Struct. Funct. 2014, 219, 1923–1934. [Google Scholar] [CrossRef]
- Bychkov, M.L.; Isaev, A.B.; Andreev-Andrievskiy, A.A.; Petrov, K.; Paramonov, A.S.; Kirpichnikov, M.P.; Lyukmanova, E.N. Aβ1-42 Accumulation Accompanies Changed Expression of Ly6/uPAR Proteins, Dysregulation of the Cholinergic System, and Degeneration of Astrocytes in the Cerebellum of Mouse Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2023, 24, 14852. [Google Scholar] [CrossRef] [PubMed]
- Hruska, M.; Keefe, J.; Wert, D.; Tekinay, A.B.; Hulce, J.J.; Ibañez-Tallon, I.; Nishi, R. Prostate Stem Cell Antigen Is an Endogenous Lynx1-like Prototoxin That Antagonizes Alpha7-Containing Nicotinic Receptors and Prevents Programmed Cell Death of Parasympathetic Neurons. J. Neurosci. 2009, 29, 14847–14854. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.M.; Arvaniti, M.; Mikkelsen, J.D.; Michalski, D.; Pinborg, L.H.; Härtig, W.; Thomsen, M.S. Prostate Stem Cell Antigen Interacts with Nicotinic Acetylcholine Receptors and Is Affected in Alzheimer’s Disease. Neurobiol. Aging 2015, 36, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Thibaud-Nissen, F.; Souvorov, A.; Murphy, T.; DiCuccio, M.; Kitts, P. Eukaryotic Genome Annotation Pipeline. In The NCBI Handbook [Internet], 2nd ed.; National Center for Biotechnology Information: Bethesda, MD, USA, 2013. [Google Scholar]
- Özhan, G.; Sezgin, E.; Wehner, D.; Pfister, A.S.; Kühl, S.J.; Kagermeier-Schenk, B.; Kühl, M.; Schwille, P.; Weidinger, G. Lypd6 Enhances Wnt/β-Catenin Signaling by Promoting Lrp6 Phosphorylation in Raft Plasma Membrane Domains. Dev. Cell 2013, 26, 331–345. [Google Scholar] [CrossRef]
- Kulbatskii, D.; Shenkarev, Z.; Bychkov, M.; Loktyushov, E.; Shulepko, M.; Koshelev, S.; Povarov, I.; Popov, A.; Peigneur, S.; Chugunov, A.; et al. Human Three-Finger Protein Lypd6 Is a Negative Modulator of the Cholinergic System in the Brain. Front. Cell Dev. Biol. 2021, 9, 2593. [Google Scholar] [CrossRef]
- Huminiecki, L.; Goldovsky, L.; Freilich, S.; Moustakas, A.; Ouzounis, C.; Heldin, C.-H. Emergence, Development and Diversification of the TGF-β Signalling Pathway within the Animal Kingdom. BMC Evol. Biol. 2009, 9, 28. [Google Scholar] [CrossRef]
- Hamann, J.; Aust, G.; Araç, D.; Engel, F.B.; Formstone, C.; Fredriksson, R.; Hall, R.A.; Harty, B.L.; Kirchhoff, C.; Knapp, B.; et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G Protein—Coupled Receptors. Pharmacol. Rev. 2015, 67, 338–367. [Google Scholar] [CrossRef]
- Liebscher, I.; Cevheroğlu, O.; Hsiao, C.; Maia, A.F.; Schihada, H.; Scholz, N.; Soave, M.; Spiess, K.; Trajković, K.; Kosloff, M.; et al. A Guide to Adhesion GPCR Research. FEBS J. 2022, 289, 7610–7630. [Google Scholar] [CrossRef]
- Kanungo, K. The Coelomocytes of Asteroid Echinoderms. In Invertebrate Blood: Cells and Serum Factors; Cheng, T.C., Ed.; Springer: Boston, MA, USA, 1984; pp. 7–39. ISBN 978-1-4684-4766-8. [Google Scholar]
- Guatelli, S.; Ferrario, C.; Bonasoro, F.; Anjo, S.I.; Manadas, B.; Candia Carnevali, M.D.; Varela Coelho, A.; Sugni, M. More than a Simple Epithelial Layer: Multifunctional Role of Echinoderm Coelomic Epithelium. Cell Tissue Res. 2022, 390, 207–227. [Google Scholar] [CrossRef]
- Sharlaimova, N.; Shabelnikov, S.; Bobkov, D.; Martynova, M.; Bystrova, O.; Petukhova, O. Coelomocyte Replenishment in Adult Asterias rubens: The Possible Ways. Cell Tissue Res. 2021, 383, 1043–1060. [Google Scholar] [CrossRef]
- Zaigraev, M.M.; Lyukmanova, E.N.; Paramonov, A.S.; Shenkarev, Z.O.; Chugunov, A.O. Orientational Preferences of GPI-Anchored Ly6/uPAR Proteins. Int. J. Mol. Sci. 2022, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, T.B.; Miwa, J.M. Lynx1 and the Family of Endogenous Mammalian Neurotoxin-like Proteins and Their Roles in Modulating nAChR Function. Pharmacol. Res. 2023, 194, 106845. [Google Scholar] [CrossRef] [PubMed]
- Marek, I.; Hilgers, K.F.; Rascher, W.; Woelfle, J.; Hartner, A. A Role for the Alpha-8 Integrin Chain (Itga8) in Glomerular Homeostasis of the Kidney. Mol. Cell. Pediatr. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Steinegger, M.; Meier, M.; Mirdita, M.; Vöhringer, H.; Haunsberger, S.J.; Söding, J. HH-Suite3 for Fast Remote Homology Detection and Deep Protein Annotation. BMC Bioinform. 2019, 20, 473. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Pierleoni, A.; Martelli, P.L.; Casadio, R. PredGPI: A GPI-Anchor Predictor. BMC Bioinform. 2008, 9, 392. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. A Combined Transmembrane Topology and Signal Peptide Prediction Method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef]
- UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Kunzmann, P.; Müller, T.D.; Greil, M.; Krumbach, J.H.; Anter, J.M.; Bauer, D.; Islam, F.; Hamacher, K. Biotite: New Tools for a Versatile Python Bioinformatics Library. BMC Bioinform. 2023, 24, 236. [Google Scholar] [CrossRef] [PubMed]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Antao, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely Available Python Tools for Computational Molecular Biology and Bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef]
- Kanungo, K. In Vitro Studies on the Effects of Cell-Free Coelomic Fluid, Calcium, and/or Magnesium on Clumping of Coelomocytes of the Sea Star Asterias Forbesi (Echinodermata: Asteroidea). Biol. Bull. 1982, 163, 438–452. [Google Scholar] [CrossRef]
- Sluchanko, N.N.; Kapitonova, A.A.; Shulepko, M.A.; Kukushkin, I.D.; Kulbatskii, D.S.; Tugaeva, K.V.; Varfolomeeva, L.A.; Minyaev, M.E.; Boyko, K.M.; Popov, V.O.; et al. Crystal Structure Reveals Canonical Recognition of the Phosphorylated Cytoplasmic Loop of Human Alpha7 Nicotinic Acetylcholine Receptor by 14-3-3 Protein. Biochem. Biophys. Res. Commun. 2023, 682, 91–96. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyukmanova, E.N.; Bychkov, M.L.; Chernikov, A.M.; Kukushkin, I.D.; Kulbatskii, D.S.; Shabelnikov, S.V.; Shulepko, M.A.; Zhao, R.; Guo, W.; Kirpichnikov, M.P.; et al. In Search of the Role of Three-Finger Starfish Proteins. Mar. Drugs 2024, 22, 488. https://doi.org/10.3390/md22110488
Lyukmanova EN, Bychkov ML, Chernikov AM, Kukushkin ID, Kulbatskii DS, Shabelnikov SV, Shulepko MA, Zhao R, Guo W, Kirpichnikov MP, et al. In Search of the Role of Three-Finger Starfish Proteins. Marine Drugs. 2024; 22(11):488. https://doi.org/10.3390/md22110488
Chicago/Turabian StyleLyukmanova, Ekaterina N., Maxim L. Bychkov, Andrei M. Chernikov, Ilya D. Kukushkin, Dmitrii S. Kulbatskii, Sergey V. Shabelnikov, Mikhail A. Shulepko, Ran Zhao, Wenxiao Guo, Mikhail P. Kirpichnikov, and et al. 2024. "In Search of the Role of Three-Finger Starfish Proteins" Marine Drugs 22, no. 11: 488. https://doi.org/10.3390/md22110488
APA StyleLyukmanova, E. N., Bychkov, M. L., Chernikov, A. M., Kukushkin, I. D., Kulbatskii, D. S., Shabelnikov, S. V., Shulepko, M. A., Zhao, R., Guo, W., Kirpichnikov, M. P., Shenkarev, Z. O., & Paramonov, A. S. (2024). In Search of the Role of Three-Finger Starfish Proteins. Marine Drugs, 22(11), 488. https://doi.org/10.3390/md22110488









