Sea Anemone Kunitz Peptide HCIQ2c1: Structure, Modulation of TRPA1 Channel, and Suppression of Nociceptive Reaction In Vivo
Abstract
1. Introduction
2. Results
2.1. Expression and Purification of HCIQ2c1 and 15N-HCIQ2c1 Analogue
2.2. HCIQ2c1 Does Not Influence Motor and Orienting-Exploratory Activities in the Open Field Test
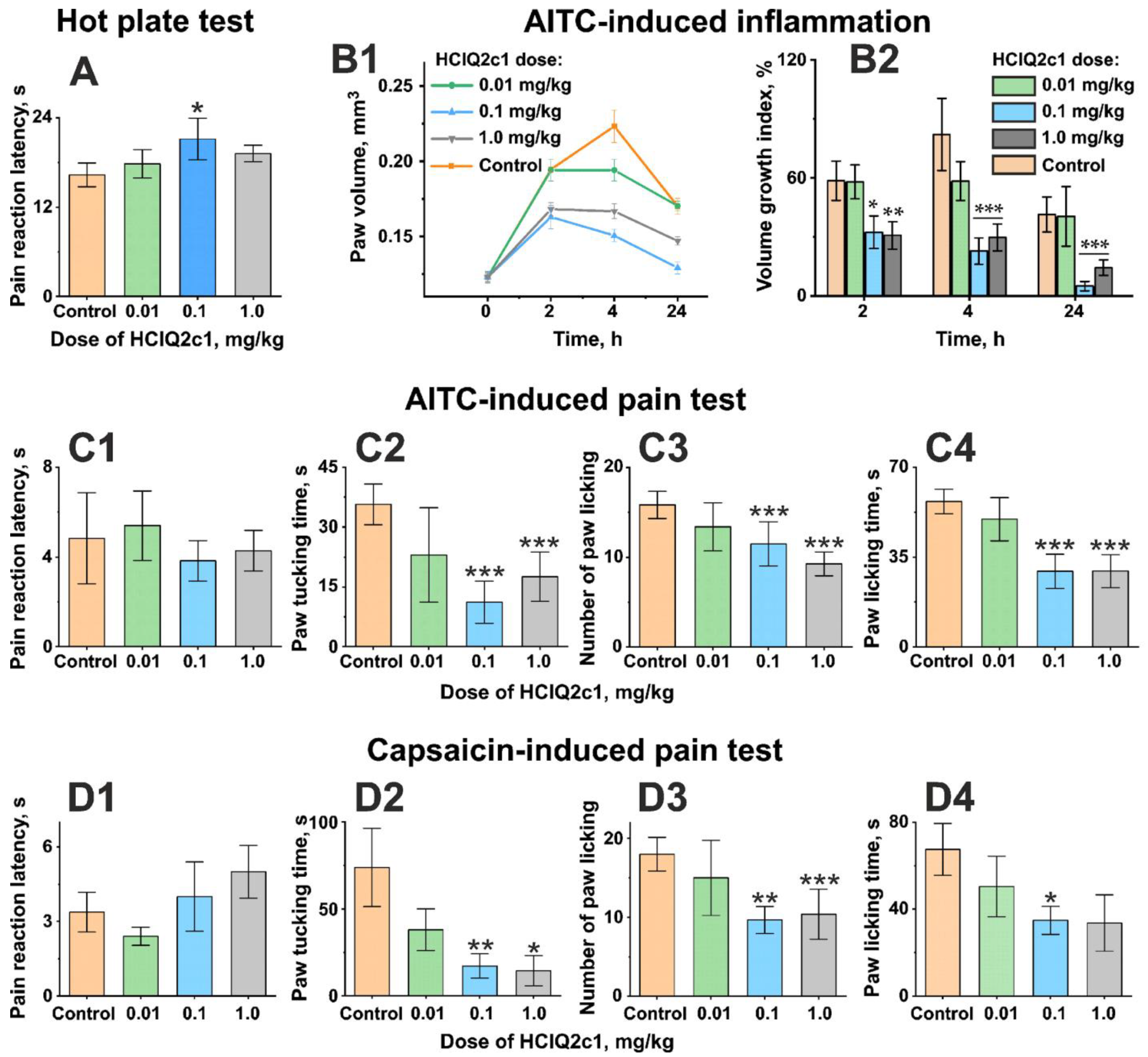
2.3. HCIQ2c1 Influences Acute Pain Sensibility in the Hot Plate Test
2.4. Analgesic and Anti-Inflammatory Activities of HCIQ2c1 in the AITC-Induced Nociceptive Behavior Test
2.5. Analgesic Activity of HCIQ2c1 in the Capsaicin-Induced Pain Test
2.6. HCIQ2c1 Is an Allosteric Modulator of Rat TRPA1
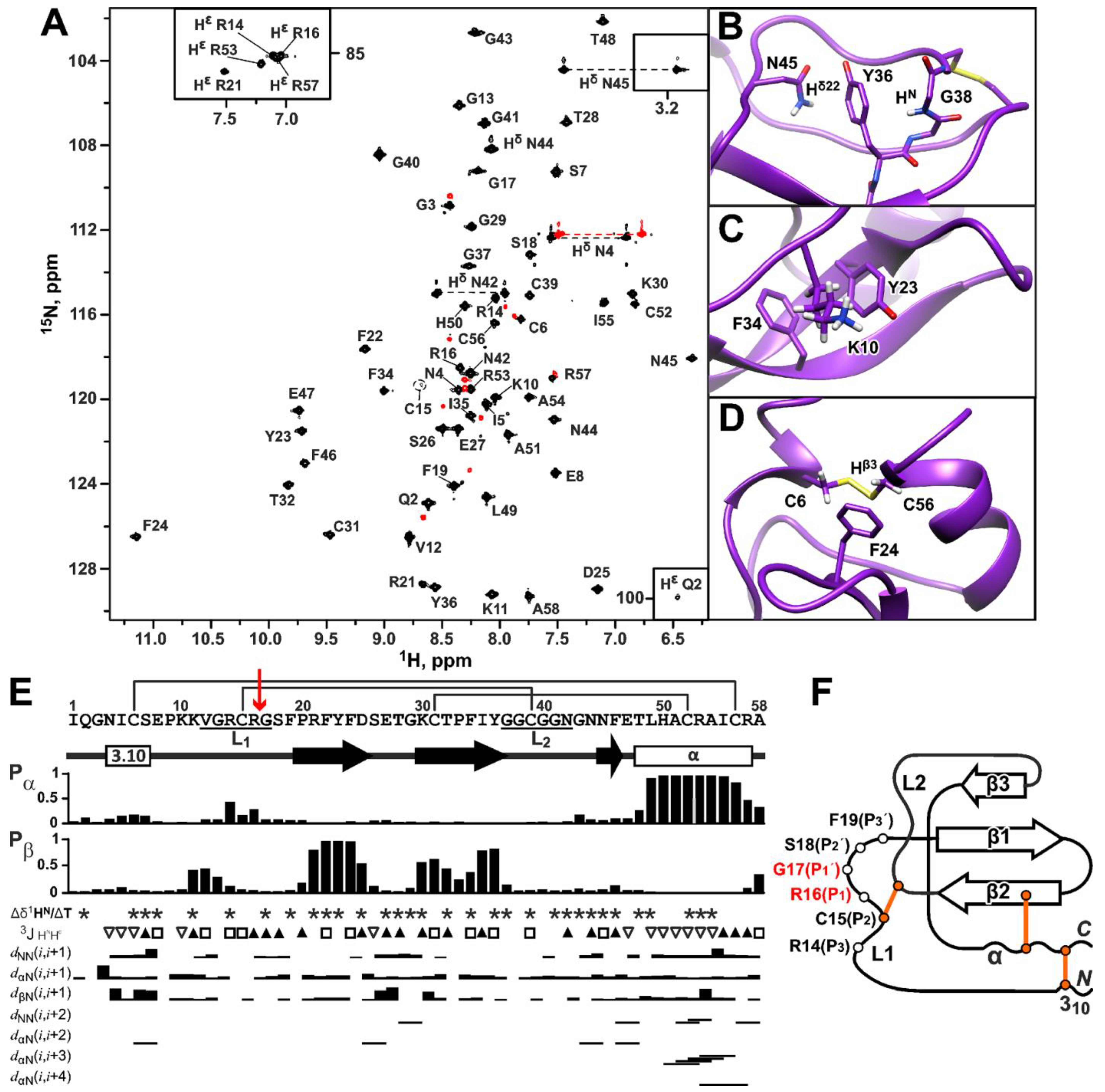
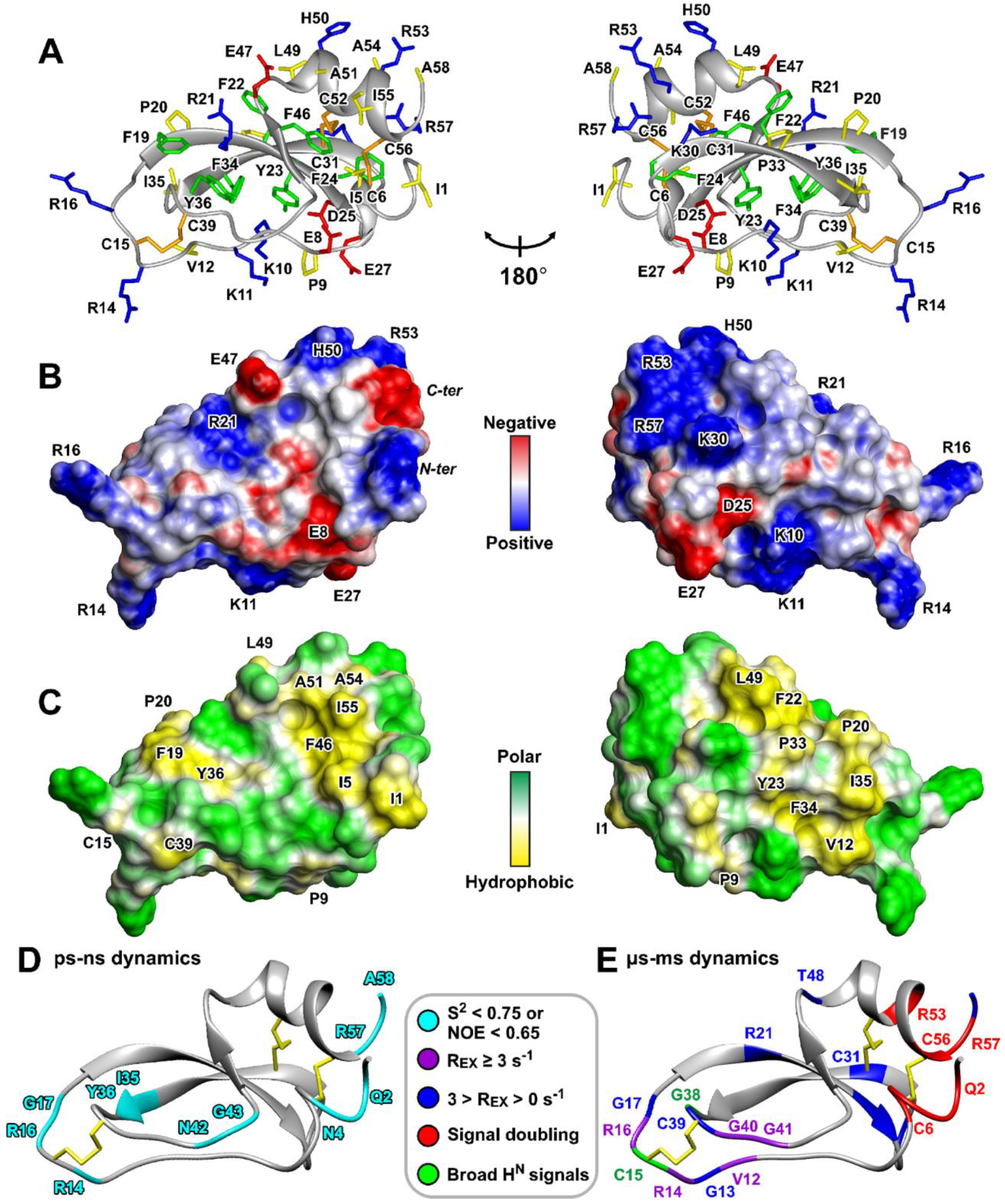
2.7. Kunitz-Type 3D Structure of HCIQ2c1
2.8. Backbone Dynamics of HCIQ2c1
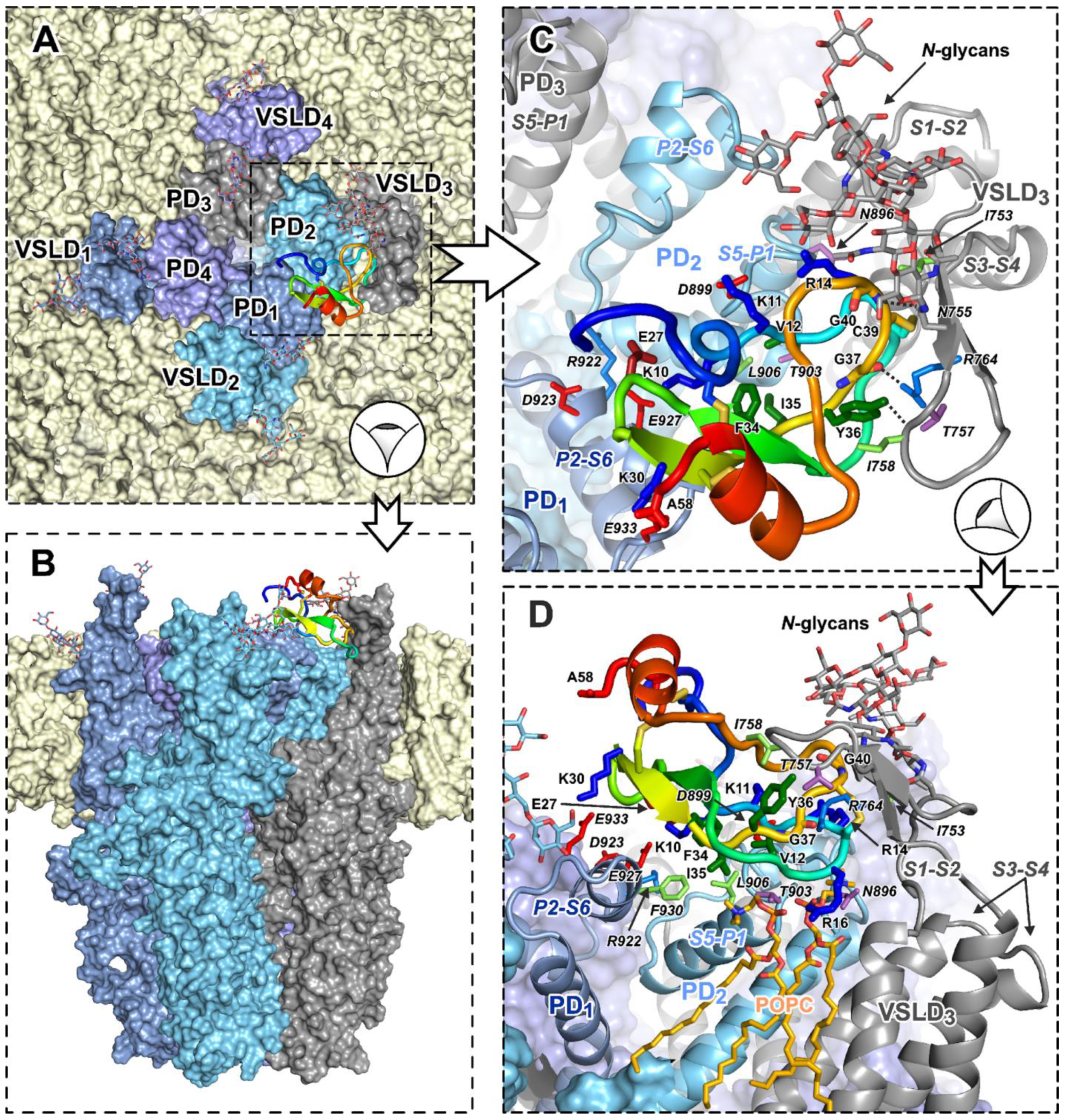
2.9. Computer Modeling of the TRPA1/HCIQ2c1 Complex
2.9.1. TRPA1–HCIQ2c1 Ensemble Docking
- Conformational clustering of the HCIQ2c1 NMR ensemble yielded four diverse structures, which underwent MD simulations in aqueous solution with 0.15 M NaCl for 500 ns each. The four trajectories were combined into an aggregate 2000 ns trajectory. Repeated clustering resulted in 49 peptide conformations used for docking.
- The open state of the rat TRPA1 channel was modeled based on the previously resolved cryo-electron microscopy structure of the human orthologue (PDB code 6V9X [6]). A 500 ns MD simulation in an explicit 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) lipid bilayer (aqueous solution, 0.15 M NaCl) was performed. Clustering of this trajectory by conformations of extracellular residues yielded 131 TRPA1 conformations. Glycans were removed from TRPA1 before clustering, and glycan-free structures were used for subsequent docking.
- Ensemble protein–protein docking was initiated as a series of 131 × 49 = 6419 independent docking runs, each yielding the top 100 solutions, resulting in a total ensemble of 641,900 probable structures of the TRPA1/HCIQ2c1 complex.
- This vast ensemble was filtered according to certain criteria (Table S3), including the area of the interaction interface (S), the complementarity of hydrophobic/hydrophilic properties at the interface (Cp) [39], and the total number of specific intermolecular contacts (interactions) that stabilize the complex, including ionic (at least six), H-bonds, stacking, and π–cation interactions. Based on these criteria, just four dissimilar docking solutions were selected (Figure 6 and Figure S6) for further assessment.
2.9.2. Molecular Dynamics of the TRPA1/HCIQ2c1 Complexes Points the Most Probable Binding Mode
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Expression of HCIQ2c1
5.2. MALDI TOF/MS Analysis
5.3. Animal Studies
5.3.1. Open Field Test
5.3.2. Hot Plate Test
5.3.3. Capsaicin Test
5.3.4. Allylisothiocyanate-Induced Pain and Paw Edema Test
5.4. Electrophysiological Recordings
5.5. NMR Experiments and Spatial Structure Calculation
5.6. Computer Modeling
5.6.1. Construction of the TRPA1 Channel Model
5.6.2. Molecular Dynamics (MD) Simulations
5.6.3. Clustering of Trajectories and Initial NMR Structures
5.6.4. Ensemble Docking
5.6.5. Docking Analysis and Solution Filtering
5.6.6. Analysis of Trajectories
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (Transient Receptor Potential) Ion Channel Family: Structures, Biological Functions and Therapeutic Interventions for Diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-J.; Sweet, T.-B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current Progress in the Mammalian TRP Ion Channel Family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.-P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 Ion Channel Suggests Regulatory Mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Landini, L.; Souza Monteiro De Araujo, D.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? Int. J. Mol. Sci. 2022, 23, 4529. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Wang, Z.; Zubcevic, L.; Hsu, A.L.; He, Q.; Borgnia, M.J.; Ji, R.-R.; Lee, S.-Y. Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Neuron 2020, 105, 882–894.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin King, J.V.; Paulsen, C.E.; Cheng, Y.; Julius, D. Irritant-Evoked Activation and Calcium Modulation of the TRPA1 Receptor. Nature 2020, 585, 141–145. [Google Scholar] [CrossRef]
- Liu, C.; Reese, R.; Vu, S.; Rougé, L.; Shields, S.D.; Kakiuchi-Kiyota, S.; Chen, H.; Johnson, K.; Shi, Y.P.; Chernov-Rogan, T.; et al. A Non-Covalent Ligand Reveals Biased Agonism of the TRPA1 Ion Channel. Neuron 2021, 109, 273–284.e4. [Google Scholar] [CrossRef]
- Gupta, R.; Saito, S.; Mori, Y.; Itoh, S.G.; Okumura, H.; Tominaga, M. Structural Basis of TRPA1 Inhibition by HC-030031 Utilizing Species-Specific Differences. Sci. Rep. 2016, 6, 37460. [Google Scholar] [CrossRef]
- Terrett, J.A.; Chen, H.; Shore, D.G.; Villemure, E.; Larouche-Gauthier, R.; Déry, M.; Beaumier, F.; Constantineau-Forget, L.; Grand-Maître, C.; Lépissier, L.; et al. Tetrahydrofuran-Based Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonists: Ligand-Based Discovery, Activity in a Rodent Asthma Model, and Mechanism-of-Action via Cryogenic Electron Microscopy. J. Med. Chem. 2021, 64, 3843–3869. [Google Scholar] [CrossRef]
- Gui, J.; Liu, B.; Cao, G.; Lipchik, A.M.; Perez, M.; Dekan, Z.; Mobli, M.; Daly, N.L.; Alewood, P.F.; Parker, L.L.; et al. A Tarantula-Venom Peptide Antagonizes the TRPA1 Nociceptor Ion Channel by Binding to the S1–S4 Gating Domain. Curr. Biol. 2014, 24, 473–483. [Google Scholar] [CrossRef]
- Jain, A.; Brönneke, S.; Kolbe, L.; Stäb, F.; Wenck, H.; Neufang, G. TRP-Channel-Specific Cutaneous Eicosanoid Release Patterns. Pain 2011, 152, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, I.; Gomes, P.; Aranake, S.; Shetty, M.; Karnik, P.; Damle, M.; Kuruganti, S.; Thorat, S.; Khairatkar-Joshi, N. Expression of Functional TRPA1 Receptor on Human Lung Fibroblast and Epithelial Cells. J. Recept. Signal Transduct. 2011, 31, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.; Xu, M.; Zhang, H.; Chen, Y.; Chung, K.F.; Adcock, I.M.; Li, F. Roles of TRPA1 and TRPV1 in Cigarette Smoke-Induced Airway Epithelial Cell Injury Model. Free Radic. Biol. Med. 2019, 134, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Naert, R.; López-Requena, A.; Talavera, K. TRPA1 Expression and Pathophysiology in Immune Cells. Int. J. Mol. Sci. 2021, 22, 11460. [Google Scholar] [CrossRef] [PubMed]
- Staruschenko, A.; Jeske, N.A.; Akopian, A.N. Contribution of TRPV1-TRPA1 Interaction to the Single Channel Properties of the TRPA1 Channel. J. Biol. Chem. 2010, 285, 15167–15177. [Google Scholar] [CrossRef]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a Therapeutic Target for Nociceptive Pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef]
- Menezes, C.; Thakur, N.L. Sea Anemone Venom: Ecological Interactions and Bioactive Potential. Toxicon 2022, 208, 31–46. [Google Scholar] [CrossRef]
- Norton, R.S. Structures of Sea Anemone Toxins. Toxicon 2009, 54, 1075–1088. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Mosharova, I.V.; Korolkova, Y.V.; Shelukhina, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Murashev, A.N.; Kozlov, S.A.; Stensvåg, K.; et al. Peptide from Sea Anemone Metridium Senile Affects Transient Receptor Potential Ankyrin-Repeat 1 (TRPA1) Function and Produces Analgesic Effect. J. Biol. Chem. 2017, 292, 2992–3004. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Solstad, R.G.; Mineev, K.S.; Korolkova, Y.V.; Mosharova, I.V.; Dyachenko, I.A.; Palikov, V.A.; Palikova, Y.A.; Murashev, A.N.; Arseniev, A.S.; et al. New Disulfide-Stabilized Fold Provides Sea Anemone Peptide to Exhibit Both Antimicrobial and TRPA1 Potentiating Properties. Toxins 2017, 9, 154. [Google Scholar] [CrossRef]
- Kvetkina, A.; Leychenko, E.; Chausova, V.; Zelepuga, E.; Chernysheva, N.; Guzev, K.; Pislyagin, E.; Yurchenko, E.; Menchinskaya, E.; Aminin, D.; et al. A New Multigene HCIQ Subfamily from the Sea Anemone Heteractis Crispa Encodes Kunitz-Peptides Exhibiting Neuroprotective Activity against 6-Hydroxydopamine. Sci. Rep. 2020, 10, 4205. [Google Scholar] [CrossRef] [PubMed]
- Kvetkina, A.; Pislyagin, E.; Menchinskaya, E.; Yurchenko, E.; Kalina, R.; Kozlovskiy, S.; Kaluzhskiy, L.; Menshov, A.; Kim, N.; Peigneur, S.; et al. Kunitz-Type Peptides from Sea Anemones Protect Neuronal Cells against Parkinson’s Disease Inductors via Inhibition of ROS Production and ATP-Induced P2X7 Receptor Activation. Int. J. Mol. Sci. 2022, 23, 5115. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, J.; Alvarez, P.; Joseph, E.K.; Ferrari, L.F.; Chen, X.; Levine, J.D. In Vivo and in Vitro Comparison of Female and Male Nociceptors. J. Pain 2012, 13, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.-H.; Wu, K.-Y.; Su, N.C.; Edwards, A.; Huang, G.-J. The Influence of Sex Difference on Behavior and Adult Hippocampal Neurogenesis in C57BL/6 Mice. Sci. Rep. 2023, 13, 17297. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Animal Models of Pain: Progress and Challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Lo Vecchio, S.; Gazerani, P.; Arendt-Nielsen, L. Dose–Response Study of Topical Allyl Isothiocyanate (Mustard Oil) as a Human Surrogate Model of Pain, Hyperalgesia, and Neurogenic Inflammation. Pain 2017, 158, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Mironov, P.A.; Kulbatskii, D.S.; Shulepko, M.A.; Paramonov, A.S.; Chernaya, E.M.; Logashina, Y.A.; Andreev, Y.A.; Kirpichnikov, M.P.; Shenkarev, Z.O. Recombinant Production, NMR Solution Structure, and Membrane Interaction of the Phα1β Toxin, a TRPA1 Modulator from the Brazilian Armed Spider Phoneutria Nigriventer. Toxins 2023, 15, 378. [Google Scholar] [CrossRef] [PubMed]
- Frishman, D.; Argos, P. Knowledge-based Protein Secondary Structure Assignment. Proteins Struct. Funct. Bioinform. 1995, 23, 566–579. [Google Scholar] [CrossRef]
- Shen, Y.; Bax, A. Protein Backbone and Sidechain Torsion Angles Predicted from NMR Chemical Shifts Using Artificial Neural Networks. J. Biomol. NMR 2013, 56, 227–241. [Google Scholar] [CrossRef]
- Schubert, M.; Labudde, D.; Oschkinat, H.; Schmieder, P. Software Tool for the Prediction of Xaa-Pro Peptide Bond Conformations in Proteins Based on 13C Chemical Shift Statistics. J. Biomol. NMR 2002, 24, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Tuchsen, E.; Woodward, C. Assignment of Asparagine-44 Side-Chain Primary Amide Proton NMR Resonances and the Peptide Amide N1H Resonance of Glycine-37 in Basic Pancreatic Trypsin Inhibitor. Biochemistry 1987, 26, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Güntert, P. Automated NMR Structure Calculation with CYANA. In Protein NMR Techniques; Humana Press: Totowa, NJ, USA, 2004; Volume 278, pp. 353–378. ISBN 978-1-59259-809-0. [Google Scholar]
- Pyrkov, T.V.; Chugunov, A.O.; Krylov, N.A.; Nolde, D.E.; Efremov, R.G. PLATINUM: A Web Tool for Analysis of Hydrophobic/Hydrophilic Organization of Biomolecular Complexes. Bioinformatics 2009, 25, 1201–1202. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; McManus, D.P. Structure and Function of Invertebrate Kunitz Serine Protease Inhibitors. Dev. Comp. Immunol. 2013, 39, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Krowarsch, D.; Cierpicki, T.; Jelen, F.; Otlewski, J. Canonical Protein Inhibitors of Serine Proteases. Cell. Mol. Life Sci. CMLS 2003, 60, 2427–2444. [Google Scholar] [CrossRef] [PubMed]
- Chand, H.S.; Schmidt, A.E.; Bajaj, S.P.; Kisiel, W. Structure-Function Analysis of the Reactive Site in the First Kunitz-Type Domain of Human Tissue Factor Pathway Inhibitor-2. J. Biol. Chem. 2004, 279, 17500–17507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kneller, J.M.; Lu, M.; Bracken, C. An Effective Method for the Discrimination of Motional Anisotropy and Chemical Exchange. J. Am. Chem. Soc. 2002, 124, 1852–1853. [Google Scholar] [CrossRef]
- Pyrkov, T.V.; Chugunov, A.O.; Krylov, N.A.; Nolde, D.E.; Efremov, R.G. Complementarity of Hydrophobic/Hydrophilic Properties In Protein—Ligand Complexes: A New Tool to Improve Docking Results. In Biophysics and the Challenges of Emerging Threats; Puglisi, J.D., Ed.; NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Dordrecht, The Netherlands, 2009; pp. 21–41. ISBN 978-90-481-2367-4. [Google Scholar]
- Mourão, C.; Schwartz, E. Protease Inhibitors from Marine Venomous Animals and Their Counterparts in Terrestrial Venomous Animals. Mar. Drugs 2013, 11, 2069–2112. [Google Scholar] [CrossRef]
- Isaeva, M.P.; Chausova, V.E.; Zelepuga, E.A.; Guzev, K.V.; Tabakmakher, V.M.; Monastyrnaya, M.M.; Kozlovskaya, E.P. A New Multigene Superfamily of Kunitz-Type Protease Inhibitors from Sea Anemone Heteractis Crispa. Peptides 2012, 34, 88–97. [Google Scholar] [CrossRef]
- Kozlov, S.; Grishin, E. The Mining of Toxin-like Polypeptides from EST Database by Single Residue Distribution Analysis. BMC Genom. 2011, 12, 88. [Google Scholar] [CrossRef]
- Antuch, W.; Berndt, K.D.; Chávez, M.A.; Delfín, J.; Wüthrich, K. The NMR Solution Structure of a Kunitz-type Proteinase Inhibitor from the Sea Anemone Stichodactyla helianthus. Eur. J. Biochem. 1993, 212, 675–684. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, R.; Peigneur, S.; Pons, T.; Alvarez, C.; González, L.; Chávez, M.; Tytgat, J. The Kunitz-Type Protein ShPI-1 Inhibits Serine Proteases and Voltage-Gated Potassium Channels. Toxins 2016, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Billen, B.; Derua, R.; Waelkens, E.; Debaveye, S.; Béress, L.; Tytgat, J. A Bifunctional Sea Anemone Peptide with Kunitz Type Protease and Potassium Channel Inhibiting Properties. Biochem. Pharmacol. 2011, 82, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Bruhn, T.; Guillemare, E.; Moinier, D.; Lancelin, J.-M.; Béress, L.; Lazdunski, M. Kalicludines and Kaliseptine. J. Biol. Chem. 1995, 270, 25121–25126. [Google Scholar] [CrossRef]
- Andreev, Y.A.; Kozlov, S.A.; Koshelev, S.G.; Ivanova, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. Analgesic Compound from Sea Anemone Heteractis Crispa Is the First Polypeptide Inhibitor of Vanilloid Receptor 1 (TRPV1). J. Biol. Chem. 2008, 283, 23914–23921. [Google Scholar] [CrossRef]
- Nikolaev, M.V.; Dorofeeva, N.A.; Komarova, M.S.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Grishin, E.V.; Tikhonov, D.B.; Kozlov, S.A. TRPV1 Activation Power Can Switch an Action Mode for Its Polypeptide Ligands. PLoS ONE 2017, 12, e0177077. [Google Scholar] [CrossRef] [PubMed]
- Sintsova, O.V.; Palikov, V.A.; Palikova, Y.A.; Klimovich, A.A.; Gladkikh, I.N.; Andreev, Y.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Dyachenko, I.A.; Kozlov, S.A.; et al. Peptide Blocker of Ion Channel TRPV1 Exhibits a Long Analgesic Effect in the Heat Stimulation Model. Dokl. Biochem. Biophys. 2020, 493, 215–217. [Google Scholar] [CrossRef]
- Monastyrnaya, M.; Peigneur, S.; Zelepuga, E.; Sintsova, O.; Gladkikh, I.; Leychenko, E.; Isaeva, M.; Tytgat, J.; Kozlovskaya, E. Kunitz-Type Peptide HCRG21 from the Sea Anemone Heteractis Crispa Is a Full Antagonist of the TRPV1 Receptor. Mar. Drugs 2016, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.; Monastyrnaya, M.; Zelepuga, E.; Sintsova, O.; Tabakmakher, V.; Gnedenko, O.; Ivanov, A.; Hua, K.-F.; Kozlovskaya, E. New Kunitz-Type HCRG Polypeptides from the Sea Anemone Heteractis Crispa. Mar. Drugs 2015, 13, 6038–6063. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, I.; Peigneur, S.; Sintsova, O.; Lopes Pinheiro-Junior, E.; Klimovich, A.; Menshov, A.; Kalinovsky, A.; Isaeva, M.; Monastyrnaya, M.; Kozlovskaya, E.; et al. Kunitz-Type Peptides from the Sea Anemone Heteractis Crispa Demonstrate Potassium Channel Blocking and Anti-Inflammatory Activities. Biomedicines 2020, 8, 473. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Hu, Y.-T.; Yang, W.-S.; He, Y.-W.; Feng, J.; Wang, B.; Zhao, R.-M.; Ding, J.-P.; Cao, Z.-J.; Li, W.-X.; et al. Hg1, Novel Peptide Inhibitor Specific for Kv1.3 Channels from First Scorpion Kunitz-Type Potassium Channel Toxin Family. J. Biol. Chem. 2012, 287, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Liang, S. An Overview of Peptide Toxins from the Venom of the Chinese Bird Spider Selenocosmia Huwena Wang [=Ornithoctonus Huwena (Wang)]. Toxicon 2004, 43, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.-H.; He, Q.-Y.; Peng, K.; Diao, J.-B.; Jiang, L.-P.; Tang, X.; Liang, S.-P. Discovery of a Distinct Superfamily of Kunitz-Type Toxin (KTT) from Tarantulas. PLoS ONE 2008, 3, e3414. [Google Scholar] [CrossRef]
- Droctové, L.; Ciolek, J.; Mendre, C.; Chorfa, A.; Huerta, P.; Carvalho, C.; Gouin, C.; Lancien, M.; Stanajic-Petrovic, G.; Braco, L.; et al. A New Kunitz-type Snake Toxin Family Associated with an Original Mode of Interaction with the Vasopressin 2 Receptor. Br. J. Pharmacol. 2022, 179, 3470–3481. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, L.; Peterson, B.Z. Calcicludine Binding to the Outer Pore of L-Type Calcium Channels Is Allosterically Coupled to Dihydropyridine Binding. Biochemistry 2007, 46, 7590–7598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harvey, A.L. Twenty Years of Dendrotoxins. Toxicon 2001, 39, 15–26. [Google Scholar] [CrossRef]
- Báez, A.; Salceda, E.; Fló, M.; Graña, M.; Fernández, C.; Vega, R.; Soto, E. α-Dendrotoxin Inhibits the ASIC Current in Dorsal Root Ganglion Neurons from Rat. Neurosci. Lett. 2015, 606, 42–47. [Google Scholar] [CrossRef]
- Fló, M.; Margenat, M.; Pellizza, L.; Graña, M.; Durán, R.; Báez, A.; Salceda, E.; Soto, E.; Alvarez, B.; Fernández, C. Functional Diversity of Secreted Cestode Kunitz Proteins: Inhibition of Serine Peptidases and Blockade of Cation Channels. PLOS Pathog. 2017, 13, e1006169. [Google Scholar] [CrossRef]
- Karczewski, J.; Connolly, T.M. The Interaction of Disagregin with the Platelet Fibrinogen Receptor, Glycoprotein IIb-IIIa. Biochem. Biophys. Res. Commun. 1997, 241, 744–748. [Google Scholar] [CrossRef]
- Mans, B.J.; Louw, A.I.; Neitz, A.W.H. Savignygrin, a Platelet Aggregation Inhibitor from the Soft Tick Ornithodoros Savignyi, Presents the RGD Integrin Recognition Motif on the Kunitz-BPTI Fold. J. Biol. Chem. 2002, 277, 21371–21378. [Google Scholar] [CrossRef]
- Egan, T.J.; Acuña, M.A.; Zenobi-Wong, M.; Zeilhofer, H.U.; Urech, D. Effects of N-Glycosylation of the Human Cation Channel TRPA1 on Agonist-Sensitivity. Biosci. Rep. 2016, 36, e00390. [Google Scholar] [CrossRef] [PubMed]
- Aubdool, A.A.; Kodji, X.; Abdul-Kader, N.; Heads, R.; Fernandes, E.S.; Bevan, S.; Brain, S.D. TRPA1 Activation Leads to Neurogenic Vasodilatation: Involvement of Reactive Oxygen Nitrogen Species in Addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. [Google Scholar] [CrossRef] [PubMed]
- Maleeva, E.E.; Palikova, Y.A.; Palikov, V.A.; Kazakov, V.A.; Simonova, M.A.; Logashina, Y.A.; Tarasova, N.V.; Dyachenko, I.A.; Andreev, Y.A. Potentiating TRPA1 by Sea Anemone Peptide Ms 9a-1 Reduces Pain and Inflammation in a Model of Osteoarthritis. Mar. Drugs 2023, 21, 617. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Salas, M.; Bialuhin, S.; Boyd, J.T.; Jeske, N.A.; Akopian, A.N. Sensitization of Small-diameter Sensory Neurons Is Controlled by TRPV1 and TRPA1 Association. FASEB J. 2020, 34, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Vassilevski, A.A.; Grishin, E.V. Cyanogen Bromide Cleavage of Proteins in Salt and Buffer Solutions. Anal. Biochem. 2010, 407, 144–146. [Google Scholar] [CrossRef]
- Gladkikh, I.; Monastyrnaya, M.; Leychenko, E.; Zelepuga, E.; Chausova, V.; Isaeva, M.; Anastyuk, S.; Andreev, Y.; Peigneur, S.; Tytgat, J.; et al. Atypical Reactive Center Kunitz-Type Inhibitor from the Sea Anemone Heteractis Crispa. Mar. Drugs 2012, 10, 1545–1565. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Shulepko, M.A.; Bychkov, M.L.; Kulbatskii, D.S.; Shlepova, O.V.; Vasilyeva, N.A.; Andreev-Andrievskiy, A.A.; Popova, A.S.; Lagereva, E.A.; Loktyushov, E.V.; et al. Water-soluble Variant of Human Lynx1 Positively Modulates Synaptic Plasticity and Ameliorates Cognitive Impairment Associated with α7-nAChR Dysfunction. J. Neurochem. 2020, 155, 45–61. [Google Scholar] [CrossRef]
- Hu, H.; Tian, J.; Zhu, Y.; Wang, C.; Xiao, R.; Herz, J.M.; Wood, J.D.; Zhu, M.X. Activation of TRPA1 Channels by Fenamate Nonsteroidal Anti-Inflammatory Drugs. Pflüg. Arch.—Eur. J. Physiol. 2010, 459, 579–592. [Google Scholar] [CrossRef]
- Raisinghani, M.; Zhong, L.; Jeffry, J.A.; Bishnoi, M.; Pabbidi, R.M.; Pimentel, F.; Cao, D.-S.; Steven Evans, M.; Premkumar, L.S. Activation Characteristics of Transient Receptor Potential Ankyrin 1 and Its Role in Nociception. Am. J. Physiol.-Cell Physiol. 2011, 301, C587–C600. [Google Scholar] [CrossRef]
- Kazimierczuk, K.; Orekhov, V.Y. Accelerated NMR Spectroscopy by Using Compressed Sensing. Angew. Chem. Int. Ed. 2011, 50, 5556–5559. [Google Scholar] [CrossRef]
- Cole, R. FAST-Modelfree: A Program for Rapid Automated Analysis of Solution NMR Spin-Relaxation Data. J. Biomol. NMR 2003, 26, 203–213. [Google Scholar] [CrossRef]
- Fiser, A.; Šali, A. Modeller: Generation and Refinement of Homology-Based Protein Structure Models. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 374, pp. 461–491. ISBN 978-0-12-182777-9. [Google Scholar]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; De Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Khan, H.M.; MacKerell, A.D.; Reuter, N. Cation-π Interactions between Methylated Ammonium Groups and Tryptophan in the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2019, 15, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bernetti, M.; Bussi, G. Pressure Control Using Stochastic Cell Rescaling. J. Chem. Phys. 2020, 153, 114107. [Google Scholar] [CrossRef]
- Ohue, M.; Shimoda, T.; Suzuki, S.; Matsuzaki, Y.; Ishida, T.; Akiyama, Y. MEGADOCK 4.0: An Ultra–High-Performance Protein–Protein Docking Software for Heterogeneous Supercomputers. Bioinformatics 2014, 30, 3281–3283. [Google Scholar] [CrossRef]
- Krylov, N.A.; Efremov, R.G. Libxtc: An Efficient Library for Reading XTC-Compressed MD Trajectory Data. BMC Res. Notes 2021, 14, 124. [Google Scholar] [CrossRef]
- Gowers, R.J.; Linke, M.; Barnoud, J.; Reddy, T.J.E.; Melo, M.N.; Seyler, S.L.; Domański, J.; Dotson, D.L.; Buchoux, S.; Kenney, I.M.; et al. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations. In Proceedings of the 15th Python in Science Conference, Austin, TX, USA, 11–17 July 2016; pp. 98–105. [Google Scholar] [CrossRef]




| TRPA1 | TRPA1/HCIQ2c1 complex | TRPA1 | TRPA1/HCIQ2c1 complex | |||||
| 1–4 | 2–1 | 5–1 | 1–4 | 2–1 | 5–1 | |||
| VSLD3 S1–S2 | PD2 S5–P1 | |||||||
| K741 | E27I 0.85 | E27I 0.89 | N896 | N4H 0.48 | R14H 0.51 | |||
| Q743 | S7H 0.31 | F897 | P33B 0.56 | R14P 0.39 | ||||
| S26H 0.21 | I35BH 0.92 | |||||||
| M746 | P33B 0.48 | P9B 0.66 | D899 | R16I 0.66 | R57I 0.35 | K11I 0.93 | ||
| I753 | P9B 0.57 | C15B 0.40 | I35H 0.52 | |||||
| C39B 0.91 | G37H 0.79 | |||||||
| I754 | T28H 0.67 | A900 | V12B 0.68 | |||||
| N755 | G29H 0.70 | G40H 0.62 | T903 | F24B 0.64 | V12B 0.75 | |||
| g755 2 | R53H 0.29 | G3H 0.58 | S26H 0.31 | I35B 0.76 | ||||
| E756 | I1I 0.22 | L906 | V12B 0.90 | V12B 0.74 | ||||
| T757 | F24B 0.27 | Y36B 0.95 | F34B 0.87 | |||||
| A58H 0.68 | I35B 0.72 | |||||||
| I758 | R21H 0.24 | D918 | R14I 0.23 | |||||
| Y36BH 0.93 | PD2 P2–P6 | |||||||
| S759 | E47H 0.48 | E933 | S18H 0.22 | |||||
| E762 | K11I 0.68 | A935 | I1B 0.77 | |||||
| E763 | V12H 0.43 | Y936 | I1B 0.79 | |||||
| R14I 0.52 | PD1 P2–P6 | |||||||
| R764 | R16H 0.48 | R922 | E27I 0.29 | |||||
| Y36P 0.85 | D923 | K30I 0.27 | ||||||
| G37H 0.92 | E927 | K11I 0.20 | K10I 0.70 | |||||
| I765 | P9B 0.28 | R14I 0.23 | ||||||
| V12B 0.23 | F930 | V12B 0.31 | F34B 0.58 | |||||
| N766 | E27H 0.20 | R14H 0.34 | I35B 0.75 | |||||
| VSLD3 S3–S4 | R931 | A58I 0.27 | ||||||
| A831 | I1B 0.45 | E933 | K30I 0.41 | |||||
| Y832 | I1B 0.80 | Lipids | ||||||
| Total number of interactions | 20 | 23 | 27 | POPC | K30I 0.43 | R16I 2.25 3 | ||
| T28B 0.41 | G17H 0.81 | |||||||
| Total lifetime | 10.4 | 11.3 | 18.9 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvetkina, A.N.; Oreshkov, S.D.; Mironov, P.A.; Zaigraev, M.M.; Klimovich, A.A.; Deriavko, Y.V.; Menshov, A.S.; Kulbatskii, D.S.; Logashina, Y.A.; Andreev, Y.A.; et al. Sea Anemone Kunitz Peptide HCIQ2c1: Structure, Modulation of TRPA1 Channel, and Suppression of Nociceptive Reaction In Vivo. Mar. Drugs 2024, 22, 542. https://doi.org/10.3390/md22120542
Kvetkina AN, Oreshkov SD, Mironov PA, Zaigraev MM, Klimovich AA, Deriavko YV, Menshov AS, Kulbatskii DS, Logashina YA, Andreev YA, et al. Sea Anemone Kunitz Peptide HCIQ2c1: Structure, Modulation of TRPA1 Channel, and Suppression of Nociceptive Reaction In Vivo. Marine Drugs. 2024; 22(12):542. https://doi.org/10.3390/md22120542
Chicago/Turabian StyleKvetkina, Aleksandra N., Sergey D. Oreshkov, Pavel A. Mironov, Maxim M. Zaigraev, Anna A. Klimovich, Yulia V. Deriavko, Aleksandr S. Menshov, Dmitrii S. Kulbatskii, Yulia A. Logashina, Yaroslav A. Andreev, and et al. 2024. "Sea Anemone Kunitz Peptide HCIQ2c1: Structure, Modulation of TRPA1 Channel, and Suppression of Nociceptive Reaction In Vivo" Marine Drugs 22, no. 12: 542. https://doi.org/10.3390/md22120542
APA StyleKvetkina, A. N., Oreshkov, S. D., Mironov, P. A., Zaigraev, M. M., Klimovich, A. A., Deriavko, Y. V., Menshov, A. S., Kulbatskii, D. S., Logashina, Y. A., Andreev, Y. A., Chugunov, A. O., Kirpichnikov, M. P., Lyukmanova, E. N., Leychenko, E. V., & Shenkarev, Z. O. (2024). Sea Anemone Kunitz Peptide HCIQ2c1: Structure, Modulation of TRPA1 Channel, and Suppression of Nociceptive Reaction In Vivo. Marine Drugs, 22(12), 542. https://doi.org/10.3390/md22120542











