Optimized Extraction of Sargahydroquinoic Acid, Major Bioactive Substance, from Sargassum yezoense Using Response Surface Methodology
Abstract
1. Introduction
2. Results
2.1. RSM Analysis of SHQA Extraction
2.2. Effects of Extraction Temperature, Time, and EtOH Concentration
2.3. Antioxidant Properties of Extracts from Sargassum yezoense
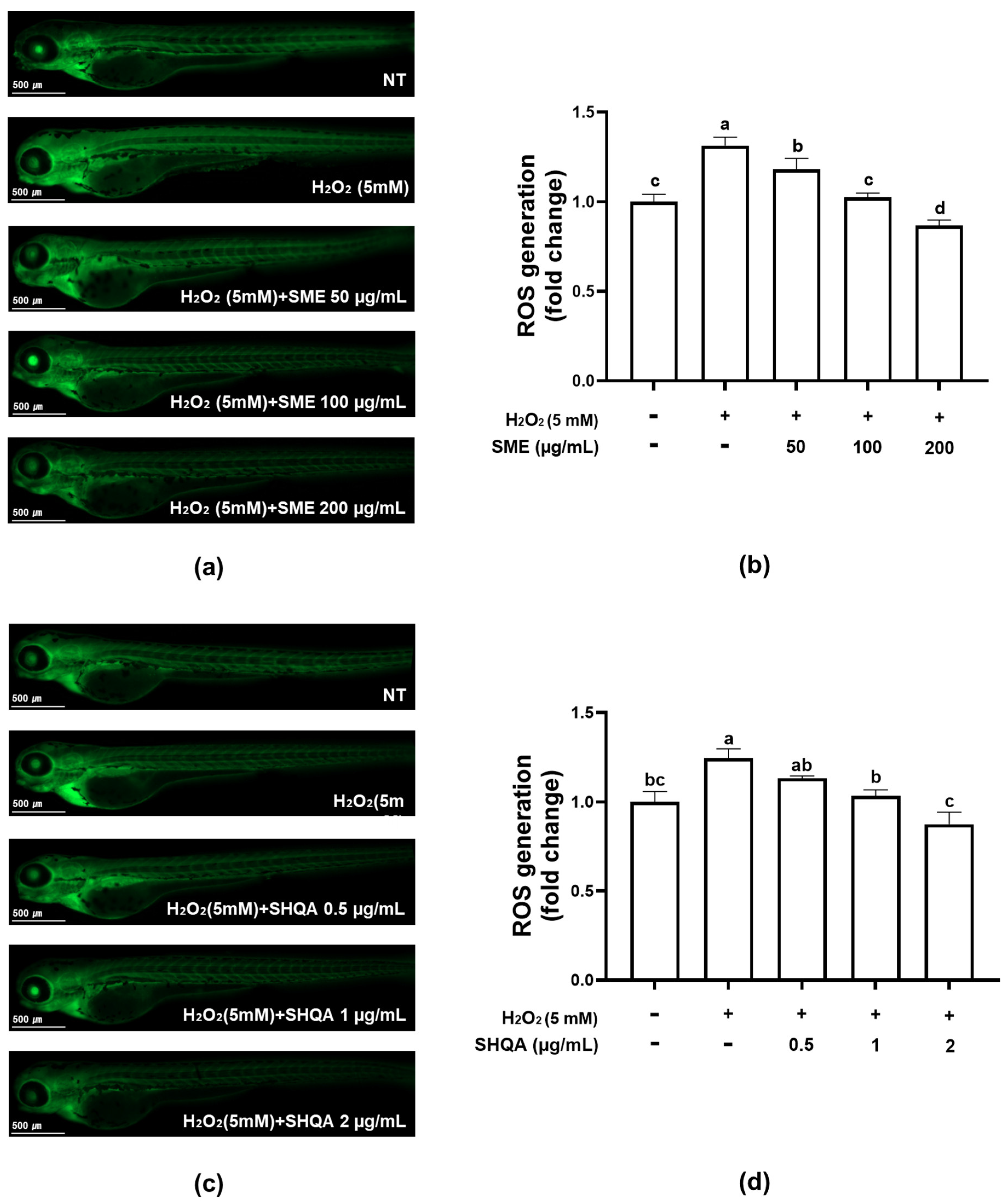
2.4. Protective Effect of SME and SHQA Against H2O2-Induced Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation and Extraction
4.3. Optimization of Extraction Conditions
4.3.1. Quantification of SHQA by High-Performance Liquid Chromatography (HPLC)
4.3.2. Experimental Design
4.4. Total Phenolic Content (TPC)
4.5. Total Antioxidant Capacity (TAC) by ABTS, DPPH, and FRAP Assays
4.5.1. ABTS Assay
4.5.2. DPPH Assay
4.5.3. Ferric Reducing Power (FRAP) Assay
4.6. Purification Method for SHQA
4.7. Estimation of Intracellular ROS Generation in Zebrafish Embryos
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yong, W.T.L.; Thien, V.Y.; Misson, M.; Chin, G.J.W.L.; Hussin, S.N.I.S.; Chong, H.L.H.; Yusof, N.A.; Ma, N.L.; Rodrigues, K.F. Seaweed: A bioindustrial game-changer for the green revolution. Biomass Bioenergy 2024, 183, 107122. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Goncalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum Seaweed as a Source of Anti-Inflammatory Substances and the Potential Insight of the Tropical Species: A Review. Mar. Drugs 2019, 17, 590. [Google Scholar] [CrossRef]
- Cao, L.; Lee, B.; Lee, B.-H.; Lee, S.; Kim, H.-R. Sargahydroquinoic acid from Sargassum macrocarpum attenuates TNF-α and UV-induced skin aging in human dermal fibroblasts. Algal Res. 2024, 78, 103410. [Google Scholar] [CrossRef]
- Lim, S.; Kwon, M.; Joung, E.-J.; Shin, T.; Oh, C.-W.; Choi, J.S.; Kim, H.-R. Meroterpenoid-rich fraction of the ethanolic extract from Sargassum serratifolium suppressed oxidative stress induced by tert-butyl hydroperoxide in HepG2 cells. Mar. Drugs 2018, 16, 374. [Google Scholar] [CrossRef]
- Joung, E.-J.; Cao, L.; Lee, B.; Gwon, W.-G.; Park, S.-H.; Kim, H.-R. Sargahydroquinoic acid, a cyclooxygenase-2 inhibitor, attenuates inflammatory responses by regulating NF-κB Inactivation and Nrf2 activation in lipopolysaccharide-stimulated cells. Inflammation 2021, 44, 2120–2131. [Google Scholar] [CrossRef]
- Blikra, M.J.; Altintzoglou, T.; Løvdal, T.; Rognså, G.; Skipnes, D.; Skåra, T.; Sivertsvik, M.; Fernández, E.N. Seaweed products for the future: Using current tools to develop a sustainable food industry. Trends Food Sci. Technol. 2021, 118, 765–776. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A critical comparison of the advanced extraction techniques applied to obtain health-promoting compounds from seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Putra, N.R.; Yustisia, Y.; Heryanto, R.B.; Asmaliyah, A.; Miswarti, M.; Rizkiyah, D.N.; Yunus, M.A.C.; Irianto, I.; Qomariyah, L.; Rohman, G.A.N. Advancements and challenges in green extraction techniques for Indonesian natural products: A review. S. Afr. J. Chem. Eng. 2023, 46, 88–98. [Google Scholar] [CrossRef]
- Box, G.E.; Wilson, K.B. On the experimental attainment of optimum conditions. In Breakthroughs in Statistics: Methodology and Distribution; Springer: Berlin/Heidelberg, Germany, 1992; pp. 270–310. [Google Scholar]
- Ferreira, S.C.; Bruns, R.; Ferreira, H.S.; Matos, G.D.; David, J.; Brandão, G.; da Silva, E.P.; Portugal, L.; Dos Reis, P.; Souza, A. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zeng, Y.; Nie, K.; Luo, D.; Wang, Z. Extraction optimization, characterization and bioactivities of a major polysaccharide from Sargassum thunbergii. PLoS ONE 2015, 10, e0144773. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Kishida, R.; Kasai, H. Cyclic bond formation of rhododendrol-quinone and dopamine-quinone: Effects of proton rearrangement. J. Phys. Soc. Jpn. 2018, 87, 084802. [Google Scholar] [CrossRef]
- Wang, F.; Qu, X.; Liu, D.; Ding, C.; Zhang, C.; Xian, Y. Upconversion nanoparticles-MoS2 nanoassembly as a fluorescent turn-on probe for bioimaging of reactive oxygen species in living cells and zebrafish. Sens. Actuators B Chem. 2018, 274, 180–187. [Google Scholar] [CrossRef]
- Kim, S.-N.; Lee, W.; Bae, G.-U.; Kim, Y.K. Anti-diabetic and hypolipidemic effects of Sargassum yezoense in db/db mice. Biochem. Biophys. Res. Commun. 2012, 424, 675–680. [Google Scholar] [CrossRef]
- Park, Y.; Cao, L.; Baek, S.; Jeong, S.; Yun, H.J.; Kim, M.-B.; Lee, S.G. The Role of Sargahydroquinoic Acid and Sargachromenol in the Anti-Inflammatory Effect of Sargassum yezoense. Mar. Drugs 2024, 22, 107. [Google Scholar] [CrossRef]
- Heo, S.-J.; Cha, S.-H.; Lee, K.-W.; Cho, S.-M.K.; Jeon, Y.-J. Antioxidant activities of chlorophyta and phaeophyta from Jeju Island. Algae 2005, 20, 251–260. [Google Scholar] [CrossRef]
- Barão, C.E.; Tanaka, M.R.R.; da Silva, C.; Madrona, G.S.; Rosset, M.; Pimentel, T.C. Extraction of natural food ingredients by modern techniques. In Extraction Processes in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 299–343. [Google Scholar]
- Barik, R.; Sugunan, S.; Shafri, M.A.B.M. Pressurized Liquid Extraction for the Isolation of Bioactive Compounds. In Bioactive Extraction and Application in Food and Nutraceutical Industries; Springer: Berlin/Heidelberg, Germany, 2024; pp. 275–298. [Google Scholar]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Yulianto, M.; Purwantisari, S.; Hartati, I.; Nisa, Q.; Nyamiati, R. Subcritical reactive extraction of shogaol and gingerol: Effect of time and temperature. Int. Food Res. J. 2022, 29, 857–863. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Soliman, A.A.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The potential of a new commercial seaweed extract in stimulating morpho-agronomic and bioactive properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Baek, S.; Cao, L.; Lee, H.; Lee, Y.; Lee, S. Effects of UV and Heating on the Stability of Fucoxanthin, Total Phlorotannin and Total Antioxidant Capacities in Saccharina japonica Ethanol Extract and Solvent Fractions. Appl. Sci. 2021, 11, 7831. [Google Scholar] [CrossRef]
- Lim, S.; Choi, A.-H.; Kwon, M.; Joung, E.-J.; Shin, T.; Lee, S.-G.; Kim, N.-G.; Kim, H.-R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef]
- Sasadara, M.; Wirawan, I. Effect of extraction solvent on total phenolic content, total flavonoid content, and antioxidant activity of Bulung Sangu (Gracilaria sp.) Seaweed. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Lombok, Indonesia, 12–14 October 2020; p. 012005. [Google Scholar]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Afrin, F.; Ahsan, T.; Mondal, M.; Rasul, M.; Afrin, M.; Silva, A.; Yuan, C.; Shah, A. Evaluation of antioxidant and antibacterial activities of some selected seaweeds from Saint Martin’s Island of Bangladesh. Food Chem. Adv. 2023, 3, 100393. [Google Scholar] [CrossRef]
- Husni, A.; Izmi, N.; Ayunani, F.Z.; Kartini, A.; Husnayain, N.; Isnansetyo, A. Characteristics and antioxidant activity of fucoidan from Sargassum hystrix: Effect of extraction method. Int. J. Food Sci. 2022, 2022, 3689724. [Google Scholar] [CrossRef]
- Park, J.-S.; Han, J.-M.; Surendhiran, D.; Chun, B.-S. Physicochemical and biofunctional properties of Sargassum thunbergii extracts obtained from subcritical water extraction and conventional solvent extraction. J. Supercrit. Fluids 2022, 182, 105535. [Google Scholar] [CrossRef]
- Zhao, T.; Dong, Q.; Zhou, H.; Yang, H. Drying kinetics, physicochemical properties, antioxidant activity and antidiabetic potential of Sargassum fusiforme processed under four drying techniques. LWT 2022, 163, 113578. [Google Scholar] [CrossRef]
- Seo, Y.; Park, K.E.; Kim, Y.A.; Lee, H.-J.; Yoo, J.-S.; Ahn, J.-W.; Lee, B.-J. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem. Pharm. Bull. 2006, 54, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Joung, E.-J.; Gwon, W.-G.; Shin, T.; Jung, B.-M.; Choi, J.; Kim, H.-R. Anti-inflammatory action of the ethanolic extract from Sargassum serratifolium on lipopolysaccharide-stimulated mouse peritoneal macrophages and identification of active components. J. Appl. Phycol. 2016, 29, 563–573. [Google Scholar] [CrossRef]
- van den Berg, R.; Haenen, G.R.; van den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]

| Run | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|
| Temperature | Time | Ethanol Concentration | SHQA Content (mg/g) | Error a (%) | ||
| Experimental Values | Predicted Values | |||||
| 1 | 50 °C | 16 h | 40% | 39.5 | 42.2 | 6.3 |
| 2 | 70 °C | 24 h | 60% | 52.2 | 54.2 | 3.8 |
| 3 | 70 °C | 16 h | 40% | 42.7 | 42.6 | −0.1 |
| 4 | 60 °C | 24 h | 40% | 44.4 | 42.3 | −4.8 |
| 5 | 60 °C | 24 h | 80% | 57.4 | 58.0 | 1.0 |
| 6 | 70 °C | 16 h | 80% | 61.5 | 58.8 | −4.5 |
| 7 | 60 °C | 16 h | 60% | 61.9 | 60.1 | −3.0 |
| 8 | 60 °C | 8 h | 80% | 64.1 | 66.1 | 3.1 |
| 9 | 60 °C | 16 h | 60% | 60.3 | 60.1 | −0.2 |
| 10 | 50 °C | 24 h | 60% | 59.0 | 58.4 | −1.1 |
| 11 | 50 °C | 16 h | 80% | 63.7 | 63.7 | 0.0 |
| 12 | 70 °C | 8 h | 60% | 60.5 | 61.1 | 1.0 |
| 13 | 60 °C | 16 h | 60% | 58.2 | 60.1 | 3.2 |
| 14 | 60 °C | 8 h | 40% | 44.7 | 44.1 | −1.4 |
| 15 | 50 °C | 8 h | 60% | 63.4 | 61.3 | −3.4 |
| Source * | DF a | Adj SS b | Adj MS c | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 985.07 | 109.45 | 13.86 | 0.005 |
| Linear | |||||
| A | 1 | 9.57 | 9.57 | 1.21 | 0.321 |
| B | 1 | 48.29 | 48.29 | 6.12 | 0.056 |
| C | 1 | 712.25 | 712.25 | 90.19 | 0.000 |
| Squares | |||||
| A*A | 1 | 4.38 | 4.38 | 0.55 | 0.490 |
| B*B | 1 | 0.33 | 0.33 | 0.04 | 0.847 |
| C*C | 1 | 192.51 | 192.51 | 24.38 | 0.004 |
| 2-way interactions | |||||
| A*B | 1 | 3.72 | 3.72 | 0.47 | 0.523 |
| A*C | 1 | 7.11 | 7.11 | 0.90 | 0.386 |
| B*C | 1 | 10.24 | 10.24 | 1.30 | 0.307 |
| Residual | 5 | 39.49 | 7.90 | 3.16 | |
| Lack of fit | 3 | 32.61 | 10.87 | 0.249 | |
| Pure error | 2 | 6.87 | 3.44 | ||
| Cor total | 14 | 1024.56 |
| Optimum Condition | Temperature | Time | EtOH Concentration | |||
| 52.8 °C | 8.3 h | 74.1% | ||||
| Response (SHQA content, mg/g) | Predicted value | Experimental value | 95% CI a | 95% PI b | ||
| 66.62 | 67.8 ± 0.60 | (60.80, 72.44) | (46.93, 86.30) | |||
| Sample | TPC (mg PGE/g) | ABTS (mg VCE/g) | DPPH (mg VCE/g) | FRAP (mM FeSO4/g) |
|---|---|---|---|---|
| SME | 25.00 ± 1.01 | 26.45 ± 0.66 | 28.74 ± 2.30 | 0.29 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.; Bae, J.-E.; Miao, Y.; Kim, G.; Ryu, B.; Lee, B.-H.; Lee, S. Optimized Extraction of Sargahydroquinoic Acid, Major Bioactive Substance, from Sargassum yezoense Using Response Surface Methodology. Mar. Drugs 2024, 22, 543. https://doi.org/10.3390/md22120543
Baek S, Bae J-E, Miao Y, Kim G, Ryu B, Lee B-H, Lee S. Optimized Extraction of Sargahydroquinoic Acid, Major Bioactive Substance, from Sargassum yezoense Using Response Surface Methodology. Marine Drugs. 2024; 22(12):543. https://doi.org/10.3390/md22120543
Chicago/Turabian StyleBaek, Suhyeon, Ji-Eun Bae, Yu Miao, Gahyeon Kim, Bomi Ryu, Byung-Hoo Lee, and Sanggil Lee. 2024. "Optimized Extraction of Sargahydroquinoic Acid, Major Bioactive Substance, from Sargassum yezoense Using Response Surface Methodology" Marine Drugs 22, no. 12: 543. https://doi.org/10.3390/md22120543
APA StyleBaek, S., Bae, J.-E., Miao, Y., Kim, G., Ryu, B., Lee, B.-H., & Lee, S. (2024). Optimized Extraction of Sargahydroquinoic Acid, Major Bioactive Substance, from Sargassum yezoense Using Response Surface Methodology. Marine Drugs, 22(12), 543. https://doi.org/10.3390/md22120543








