Thraustochytrium sp. and Aurantiochytrium sp.: Sustainable Alternatives for Squalene Production
Abstract
:1. Introduction
2. Results
2.1. Influence of Cultivation System on Biomass Productivity
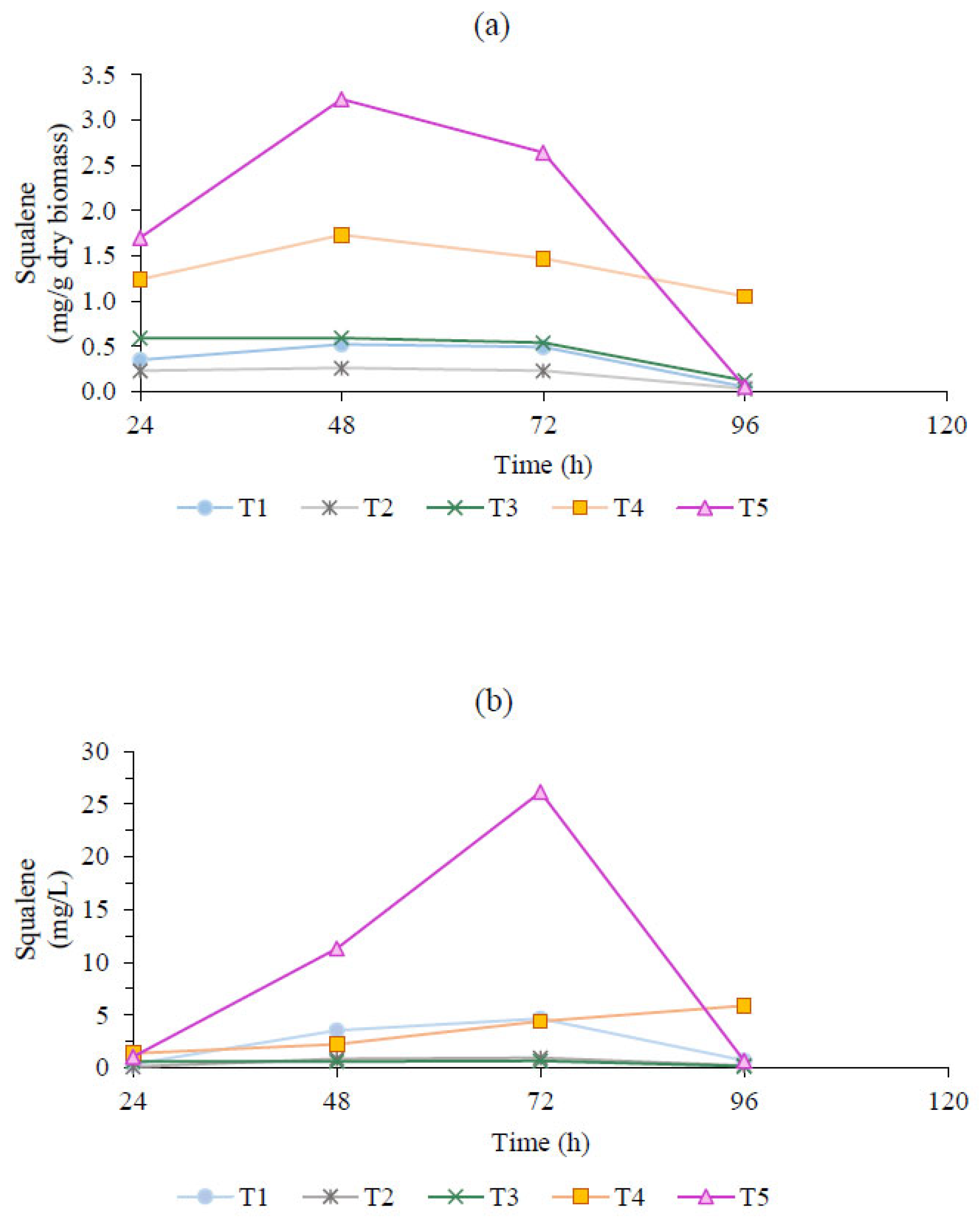
2.2. Influence of Cultivation System on Squalene Productivity
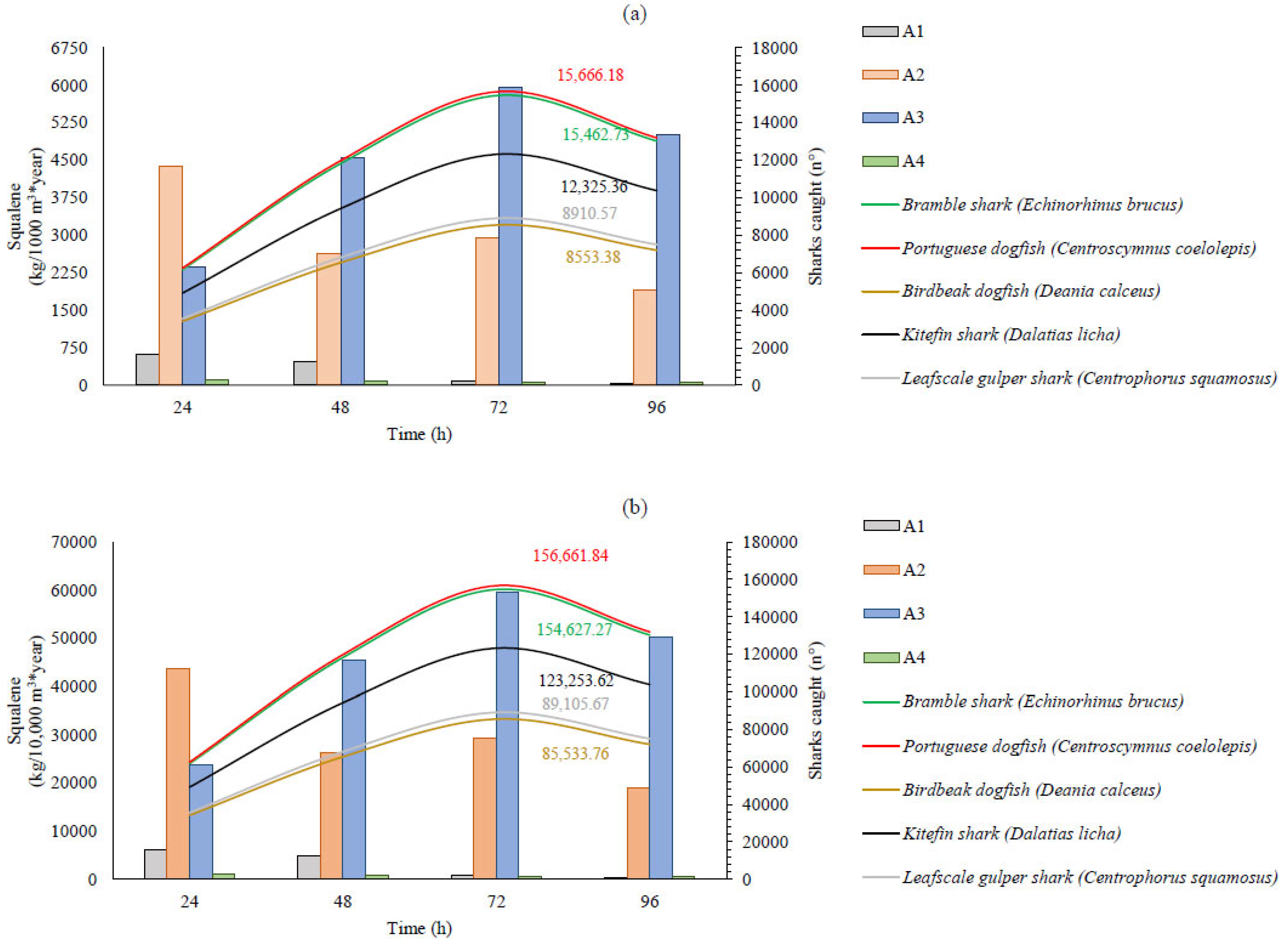
2.3. Squalene Sensitivity Analysis
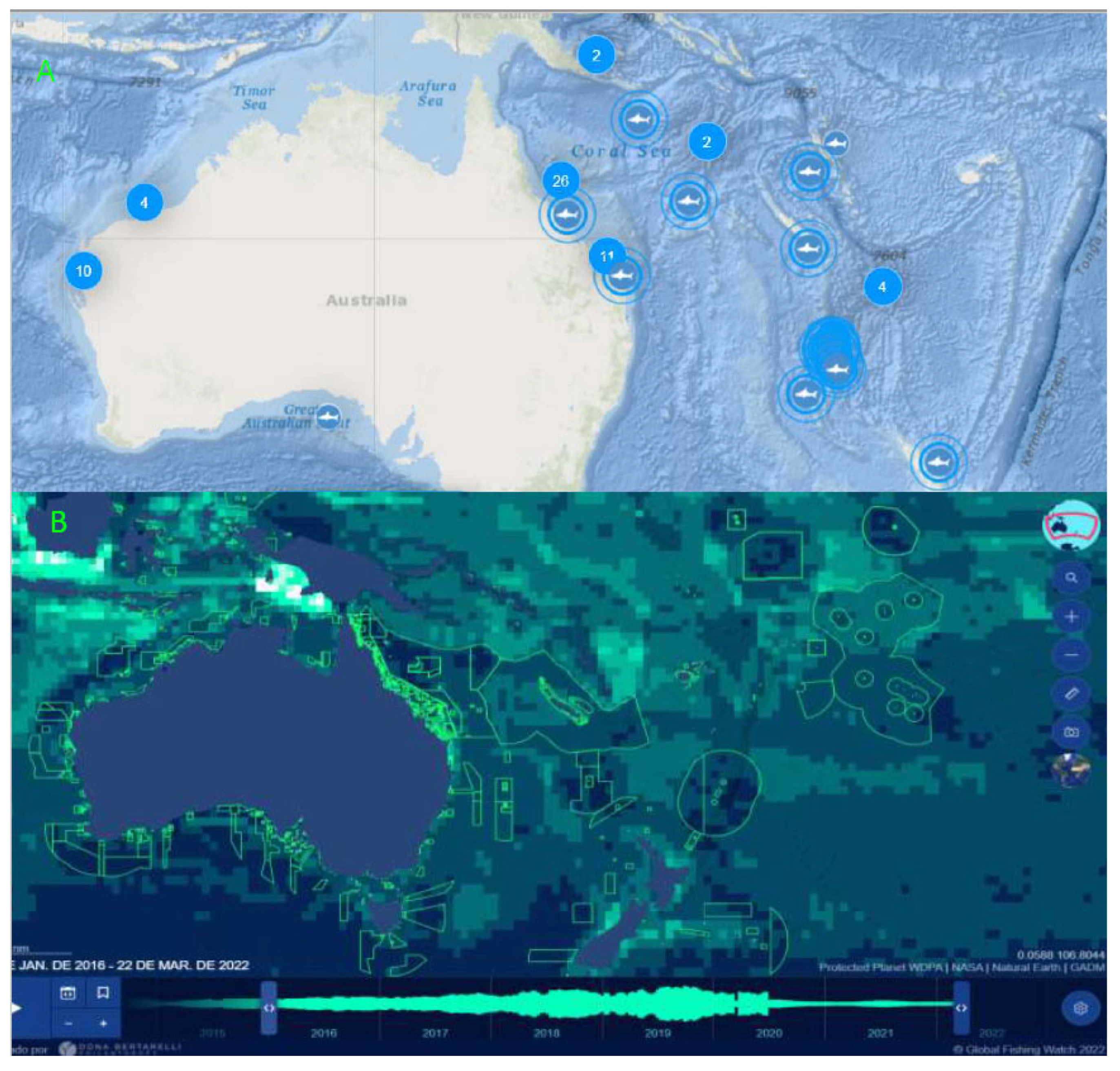
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Cultivation System
4.2. Culture Conditions
4.3. Productivity Parameters
4.4. Squalene Content Determination
4.5. Squalene Production Estimate
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, G.; Paulo, M.; Padilha, M.; Coutinho, J.; Cardoso, C.; Bandarra, N.; Batista, I. Simultaneous evaluation of the production of squalene and fatty acids by Aurantiochytrium. J. Mar. Biol. Aquac. 2018, 4, 14–20. [Google Scholar] [CrossRef]
- Rani, A.; Meghana, R.; Kush, A. Squalene production in the cell suspension cultures of Indian sandalwood (Santalum album L.) in shake flasks and air lift bioreactor. Plant Cell Tissue Organ Cult. 2018, 135, 155–167. [Google Scholar] [CrossRef]
- Hernández-Pérez, M.; Gallego, R.; Alayón, P.; Hernández, A.B. Squalene content in livers from deep-sea sharks caught in Canary Island waters. Mar. Freshw. Res. 1997, 48, 573–576. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Koide, M.; Yoshida, M.; Segawa, H.; Leproux, P.; Couderc, V.; Watanabe, M.; Kano, H. Identification of intracellular squalene in living algae, Aurantiochytrium mangrovei with hyper-spectral coherent anti-Stokes Raman microscopy using a sub-nanosecond supercontinuum laser source. J. Raman. Spectrosc. 2017, 48, 8–15. [Google Scholar] [CrossRef]
- Fagundes, M.; Vendruscolo, R.; Maroneze, M.; Barin, J.; de Menezes, C.; Zepka, L.; Jacob-Lopes, E.; Wagner, R. Towards a Sustainable Route for the Production of Squalene Using Cyanobacteria. Waste Biomass Valori. 2019, 10, 1295–1302. [Google Scholar] [CrossRef]
- Tsoi, K.; Chan, S.; Lee, Y.; Ip, B.; Cheang, C.C. Shark Conservation: An Educational Approach Based on Children’s Knowledge and Perceptions toward Sharks. PLoS ONE 2016, 11, e0163406. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pandarus, V.; Béland, F.; Pagliaro, M. Catalytic Hydrogenation of Squalene to Squalane. Org. Process. Res. Dev. 2014, 18, 1110–1115. [Google Scholar] [CrossRef]
- Xu, W.; Ma, X.; Wang, Y. Production of squalene by microbes: An update. World J. Microbiol. Biotechnol. 2016, 32, 195. [Google Scholar] [CrossRef]
- Paredes, P.; Larama, G.; Flores, L.; Leyton, A.; Ili, C.; Asenjo, J.; Chisti, Y.; Shene, C. Temperature Differentially Affects Gene Expression in Antarctic Thraustochytrid Oblongichytrium sp. RT2316-13. Mar. Drugs 2020, 18, 563. [Google Scholar] [CrossRef]
- Wei, L.; Kwak, S.; Liu, J.; Lane, S.; Hua, Q.; Kweon, D.; Jin, Y.S. Improved squalene production through increasing lipid contents in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2018, 115, 1793–1800. [Google Scholar] [CrossRef]
- Caamaño, E.; Loperena, L.; Hinzpeter, I.; Pradel, P.; Gordillo, F.; Corsini, G.; Tello, M.; Lavín, P.; González, A.R. Isolation and molecular characterization of Thraustochytrium strain isolated from Antarctic Peninsula and its biotechnological potential in the production of fatty acids. Brazilian J. Microbiol. 2017, 48, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Hrůzová, K.; Rova, U.; Christakopoulos, P.; Matsakas, L. Sustainable biorefinery concept for biofuel production through holistic volarization of food waste. Bioresour. Technol. 2019, 294, 122247. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Fan, K.; Lu, F.; Li, Q.; Aki, T.; Chen, F.; Jiang, Y. Optimization of nitrogen source for enhanced production of squalene from thraustochytrid Aurantiochytrium sp. New Biotechnol. 2010, 27, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Shene, C.; Leyton, A.; Rubilar, M.; Pinelo, M.; Acevedo, F.; Morales, E. Production of lipids and docosahexasaenoic acid (DHA) by a native Thraustochytrium strain. Eur. J. Lipid. Sci. Technol. 2013, 115, 890–900. [Google Scholar] [CrossRef]
- Furlan, V.; Maus, V.; Batista, I.; Bandarra, N.M. Production of docosahexaenoic acid by Aurantiochytrium sp. ATCC PRA-276. Brazilian J. Microbiol. 2017, 48, 359–365. [Google Scholar] [CrossRef]
- Furlan, V.J.M.; Paulo, M.d.C.; Batista, I.; Bandarra, N.M.; Santo, M.L.E.; Prentice, C. Effect of the concentration of glucose in the docosahexaenoic acid (DHA) production by Thraustochytrium sp., ATCC 26185. Adv. J. Food. Sci. Technol. 2012, 4, 257–264. [Google Scholar]
- Clarke, M.; Farrell, E.D.; Roche, W.; Murray, T.E.; Foster, S.; Marnell, F. Ireland Red List No. 11: Cartilaginous Fish [Sharks, Skates, Rays and Chimaeras]; National Parks and Wildlife Service: Sydney, Australia; Department of Arts, Heritage, Regional, Rural and Gaeltacht Affairs: Dublin, Ireland, 2016. [Google Scholar]
- Burja, A.; Radianingtyas, H.; Windust, A.; Barrow, C.J. Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: Screening of strains and optimization of omega-3 production. Appl. Microbiol. Biotechnol. 2006, 72, 1161–1169. [Google Scholar] [CrossRef]
- Morabito, C.; Bournaud, C.; Maës, C.; Schuler, M.; Aiese Cigliano, R.; Dellero, Y.; Maréchal, E.; Amato, A.; Rébeillé, F. The lipid metabolism in thraustochytrids. Prog. Lipid. Res. 2019, 76, 101007. [Google Scholar] [CrossRef]
- Kyne, P.; Heupel, M.; White, W.; Simpfendorfer, C.A. The Action Plan for Australian Sharks and Rays 2021; National Environmental Research Program Marine Biodiversity Hub: Hobart, Australia, 2021. [Google Scholar]
- Ketchum, J.; Hoyos-Padilla, M.; Aldana-Moreno, A.; Ayres, K.; Galván-Magaña, F.; Hearn, A.; Lara-Lizardi, F.; Muntaner-López, G.; Grau, M.; Trejo-Ramírez, A.; et al. Shark movement patterns in the Mexican Pacific: A conservation and management perspective. Adv. Mar. Biol. 2020, 85, 1–37. [Google Scholar] [CrossRef]
- Deprez, P.; Volkman, J.; Davenport, S. Squalene content and neutral lipis composition of Livers from Deep-sea sharks caught in Tasmanian waters. Mar. Freshw. Res. 1990, 41, 375. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase, Version 02/2022, World Wide Web Electronic Publication, 2022. Available online: www.fishbase.org.
- Kjerstad, H.; Fossen, I.; Willemsen, M.H. Utilization of Deep-sea Sharks at Hatton Bank in the North Atlantic. J. Northwest Atl. Fish. Sci. 2003, 31, 333–338. [Google Scholar] [CrossRef]
- Evans, D.; Claiborne, J.B. The Physiology of Fishes, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Rosales-Garcia, T.; Jimenez-Martinez, C.; Davila-Ortiz, G. Squalene Extraction: Biological Sources and Extraction Methods. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1662–1670. [Google Scholar] [CrossRef]
- Jeffreys, E. Communicating shark extinction. In Communicating Endangered Species; Routledge: London, UK, 2021; pp. 203–217. [Google Scholar] [CrossRef]
- Freedman, E.; Hiles, S.; Sachsman, D.B. Communicating Endangered Species, 1st ed.; Routledge: London, UK, 2021. [Google Scholar] [CrossRef]
- Dulvy, N.; Pacoureau, N.; Rigby, C.; Pollom, R.; Jabado, R.; Ebert, D.; Finucci, B.; Pollock, C.; Cheok, J.; Derrick, D.; et al. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021, 31, 4773–4787.e8. [Google Scholar] [CrossRef] [PubMed]
- Pethybridge, H.; Cossa, D.; Butler, E.C.V. Mercury in 16 demersal sharks from southeast Australia: Biotic and abiotic sources of variation and consumer health implications. Mar. Environ. Res. 2010, 69, 18–26. [Google Scholar] [CrossRef]
- Furlan, V.; Paulo, M.; Maus, V.; Ferreira, J.; Batista, I.; Bandarra, N.M. Production of docosahexaenoic acid (DHA) from Thraustochytrium sp. ATCC 26185 using differents nitrogen concentrations. Bol. Cent. Pesqui. Process. Aliment. 2016, 34, 1–11. [Google Scholar] [CrossRef]
- Nazir, Y.; Shuib, S.; Kalil, M.; Song, Y.; Hamid, A.A. Optimization of Culture Conditions for Enhanced Growth, Lipid and Docosahexaenoic Acid (DHA) Production of Aurantiochytrium SW1 by Response Surface Methodology. Sci. Rep. 2018, 8, 8909. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, D.; Barrow, C.; Puri, M. Exploring potential use of Australian thraustochytrids for the bioconversion of glycerol to omega-3 and carotenoids production. Biochem. Eng. J. 2013, 78, 11–17. [Google Scholar] [CrossRef]
- Furlan, V.; Batista, I.; Bandarra, N.; Mendes, R.; Cardoso, C. Conditions for the Production of Carotenoids by Thraustochytrium sp. ATCC 26185 and Aurantiochytrium sp. ATCC PRA-276. J. Aquat. Food. Prod. Technol. 2019, 28, 465–477. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, K.; Tsz-Yeung Wong, R.; Chen, F. Fatty Acid Composition and Squalene Content of the Marine Microalga Schizochytrium mangrovei. J. Agric. Food. Chem. 2004, 52, 1196–1200. [Google Scholar] [CrossRef]
- Patel, A.; Pruthi, V.; Pruthi, P.A. Synchronized nutrient stress conditions trigger the diversion of CDP-DG pathway of phospholipids synthesis towards de novo TAG synthesis in oleaginous yeast escalating biodiesel production. Energy 2017, 139, 962–974. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnol. Biofuels 2019, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Aki, T.; Chen, F.; Jiang, Y. Enhanced production of squalene in the thraustochytrid Aurantiochytrium mangrovei by medium optimization and treatment with terbinafine. World J. Microbiol. Biotechnol. 2010, 26, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Nichols, P.; McMeekin, T.A. Sterol and Squalene Content of a Docosahexaenoic-Acid-Producing Thraustochytrid: Influence of Culture Age, Temperature, and Dissolved Oxygen. Mar. Biotechnol. 2001, 3, 439–447. [Google Scholar] [CrossRef]
- Potijun, S.; Jaingam, S.; Sanevas, N.; Vajrodaya, S.; Sirikhachornkit, A. Green Microalgae Strain Improvement for the Production of Sterols and Squalene. Plants 2021, 10, 1673. [Google Scholar] [CrossRef]
- Nakazawa, A.; Kokubun, Y.; Matsuura, H.; Yonezawa, N.; Kose, R.; Yoshida, M.; Tanabe, Y.; Kusuda, E.; Van Thang, D.; Ueda, M.; et al. TLC screening of thraustochytrid strains for squalene production. J. Appl. Phycol. 2014, 26, 29–41. [Google Scholar] [CrossRef]
- Nakazawa, A.; Matsuura, H.; Kose, R.; Ito, K.; Ueda, M.; Honda, D.; Inouye, I.; Kaya, K.; Watanabe, M.M. Optimization of Biomass and Fatty Acid Production by Aurantiochytrium sp. Strain 4W-1b. Procedia. Environ. Sci. 2012, 15, 27–33. [Google Scholar] [CrossRef]
- Park, W.; Moon, M.; Shin, S.; Cho, J.; Suh, W.; Chang, Y.; Lee, B. Economical DHA (Docosahexaenoic acid) production from Aurantiochytrium sp. KRS101 using orange peel extract and low cost nitrogen sources. Algal. Res. 2018, 29, 71–79. [Google Scholar] [CrossRef]
- Squalene Market. Available online: https://www.globalinsightservices.com/reports/squalene-market (accessed on 16 February 2025).
- Russo, G.L.; Langellotti, A.L.; Sacchi, R.; Masi, P. Techno-economic assessment of DHA-rich Aurantiochytrium sp. production using food industry by-products and waste streams as alternative growth media. Bioresour. Technol. Rep. 2022, 18, 100997. [Google Scholar] [CrossRef]
- Min, K.; Lee, H.; Anbu, P.; Chaulagain, B.; Hur, B.K. The effects of culture condition on the growth property and docosahexaenoic acid production from Thraustochytrium aureum ATCC 34304. Korean. J. Chem. Eng. 2012, 29, 1211–1215. [Google Scholar] [CrossRef]
- Costello, C.; Ovando, D.; Hilborn, R.; Gaines, S.; Deschenes, O.; Lester, S. Status and Solutions for the World’s Unassessed Fisheries. Science 2012, 338, 517–520. [Google Scholar] [CrossRef]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rodríguez, T.; Dávila-Ortiz, G.; Vilchis-Gil, J. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 1829160. [Google Scholar] [CrossRef]



| Variables | Thraustochytrium sp. | Aurantiochytrium sp. |
|---|---|---|
| Yeast extract (g/L) | 1 | 1 |
| Peptone (g/L) | 1 | 15 |
| Glucose (g/L) * | 5 | 20 |
| Seawater (w/v) | 1.50% | 1.50% |
| Thraustochytrium sp. | Aurantiochytrium sp. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Components from Basal Medium (mg/L) | Components from Basal Medium (mg/L) | |||||||||
| KH2PO4 | 1540 | KH2PO4 | 300 | |||||||
| MgSO4·7H2O | 2620 | MgSO4·7H2O | 5000 | |||||||
| NaCl | 710 | NaCl | 10,000 | |||||||
| NaHCO3 | - | NaHCO3 | 100 | |||||||
| CaCl2·2H2O | - | CaCl2·2H2O | 300 | |||||||
| KCl | - | KCl | 280 | |||||||
| Dissolved components (mg/L) | Dissolved components (mg/L) | |||||||||
| MnCl2·4H2O | 3.00 | MnCl2·4H2O | 8.60 | |||||||
| ZnSO4·7H2O | 3.00 | ZnCl2 | 0.60 | |||||||
| CoCl2·6H2O | 0.04 | CoCl2·4H2O | 0.26 | |||||||
| Na2MoO4·2H2O | 0.04 | CuSO4·5H2O | 0.02 | |||||||
| CuSO4·5H2O | 2.00 | FeCl3·6H2O | 2.90 | |||||||
| NiSO4·6H2O | 2.00 | H3BO3 | 34.20 | |||||||
| FeSO4·7H2O | 10.00 | Na2EDTA | 30.00 | |||||||
| Thiamine | 9.50 | Thiamine | 9.50 | |||||||
| Calcium pantothenate | 3.20 | Calcium pantothenate | 3.20 | |||||||
| Treatment | T1 (C) | T2 | T3 | T4 | T5 | Treatment | A1 (C) | A2 | A3 | A4 |
| C/N | 5 | 10 | 15 | 13.9 | 1.6 | C/N | 4 | 27.2 | 54.5 | 40 |
| Glucose (g/L) | 30 | 60 | 30 | 30 | 0.1 * | Glucose (g/L) | 30 | 30 | 30 | 0.14 * |
| Total nitrogen (g/L) | 2.4 | 2.4 | 0.8 | 0.009 * | 2.4 | Total nitrogen (g/L) | 3 | 0.44 | 0.22 | 0.0014 * |
| (NH4)2SO4 (g/L) | 6.25 | 6.25 | 1.89 | 0.021 * | 6.3 | (NH4)2SO4 (g/L) | 1.36 | 0.20 | 0.1 | 0.00066 * |
| Yeast extract (g/L) | 8.8 | 8.8 | 3.23 | 0.036 * | 8.8 | Yeast extract (g/L) | 13.63 | 2 | 1 | 0.0066 * |
| Monosodium glutamate (g/L) | - | - | - | - | - | Monosodium glutamate (g/L) | 13.63 | 2 | 1 | 0.0066 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan, J.M.; Centenaro, G.S.; Bittencourt Fagundes, M.; Borges Filho, C.; Batista, I.; Bandarra, N. Thraustochytrium sp. and Aurantiochytrium sp.: Sustainable Alternatives for Squalene Production. Mar. Drugs 2025, 23, 132. https://doi.org/10.3390/md23030132
Furlan JM, Centenaro GS, Bittencourt Fagundes M, Borges Filho C, Batista I, Bandarra N. Thraustochytrium sp. and Aurantiochytrium sp.: Sustainable Alternatives for Squalene Production. Marine Drugs. 2025; 23(3):132. https://doi.org/10.3390/md23030132
Chicago/Turabian StyleFurlan, Júnior Mendes, Graciela Salete Centenaro, Mariane Bittencourt Fagundes, Carlos Borges Filho, Irineu Batista, and Narcisa Bandarra. 2025. "Thraustochytrium sp. and Aurantiochytrium sp.: Sustainable Alternatives for Squalene Production" Marine Drugs 23, no. 3: 132. https://doi.org/10.3390/md23030132
APA StyleFurlan, J. M., Centenaro, G. S., Bittencourt Fagundes, M., Borges Filho, C., Batista, I., & Bandarra, N. (2025). Thraustochytrium sp. and Aurantiochytrium sp.: Sustainable Alternatives for Squalene Production. Marine Drugs, 23(3), 132. https://doi.org/10.3390/md23030132








