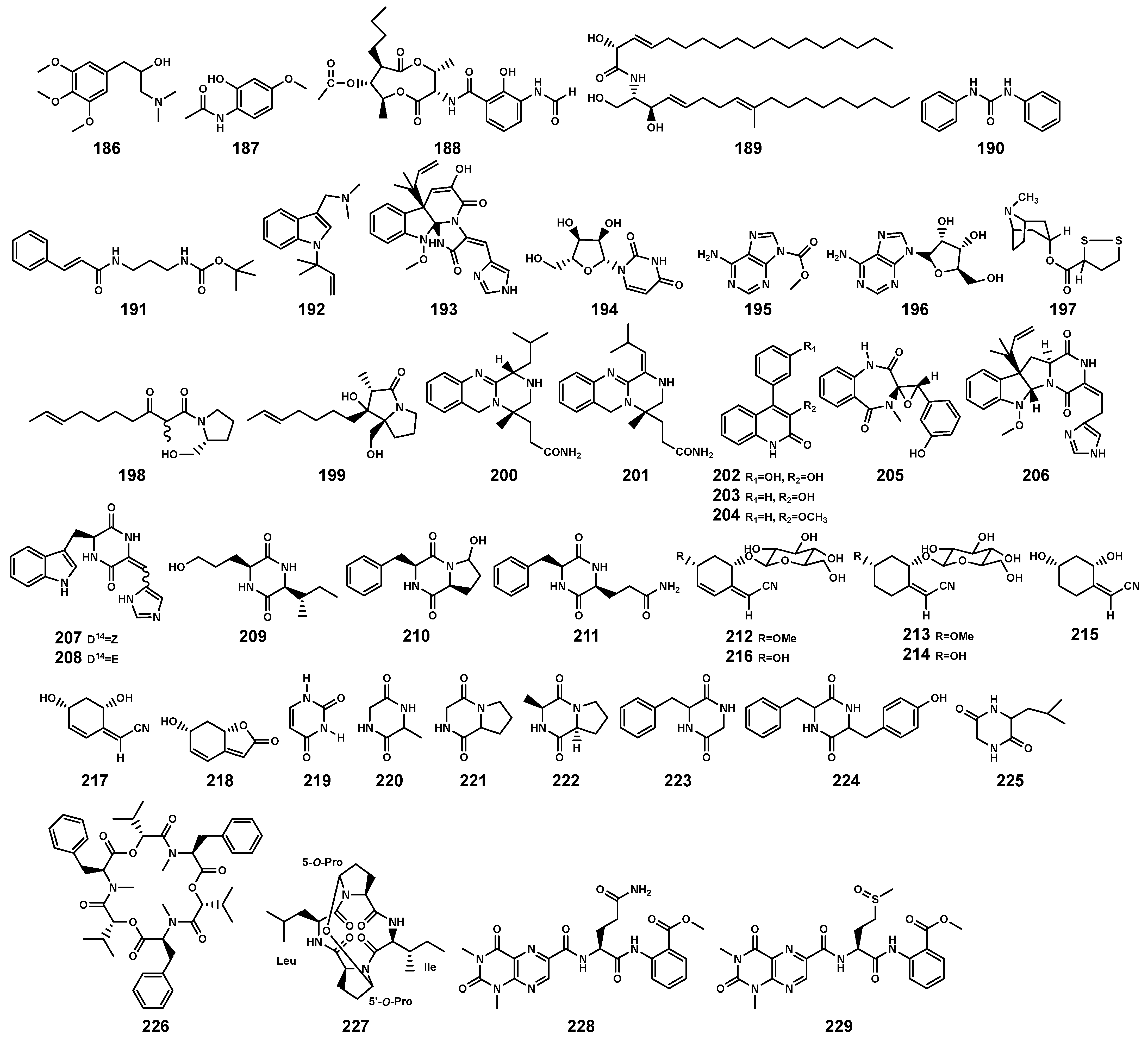Abstract
The genus Bruguiera, a member of the Rhizophoraceae family, is predominantly found in coastal areas as a mangrove plant, boasting a rich and diverse community of endophytes. This review systematically compiled approximately 496 compounds derived from both the Bruguiera genus and its associated endophytes, including 152 terpenoids, 17 steroids, 16 sulfides, 44 alkaloids and peptides, 66 quinones, 68 polyketides, 19 flavonoids, 38 phenylpropanoids, 54 aromatic compounds, and 22 other compounds. Among these, 201 compounds exhibited a spectrum of activities, including cytotoxicity, antimicrobial, antioxidant, anti-inflammatory, antiviral, antidiabetic, insecticidal and mosquito repellent, and enzyme inhibitory properties, etc. These findings provided promising lead compounds for drug discovery. Certain similar or identical compounds were found to be simultaneously present in both Bruguiera plants and their endophytes, and the phenomenon of their interaction relationship was discussed.
1. Introduction
Mangroves thriving in tropical and subtropical coastal regions, estuaries, and mudflats represent unique plant communities. Globally, these mangrove plants host a diverse distribution of 84 species belonging to 24 genera and 16 families [1]. The Rhizophoraceae family, in particular, encompasses approximately four genera, with Bruguiera being one of them [2]. Currently, the Bruguiera genus comprises six species, including Bruguiera cylindrica, B. exaristata, B. gymnorrhiza, B. hainessi, B. parviflora, and B. sexangula, which are mainly distributed along the coasts of Asia, the islands of the eastern Pacific Ocean, and the coasts of Oceania, and one variety B. sexangula var. rhynchopetala, found in the Hainan and Guangdong provinces of China [2,3,4]. It has been reported that B. gymnorrhiza, B. cylindrica, B. sexangula, and B. parviflora, have undergone pharmacological validation for their role as traditional medicinal plants [5]. Among these, B. gymnorrhiza stands out as the most extensively used for traditional purposes. In diverse regions such as the Sundarbans, the Pichavaram and Pichavaram forests of India, Guangxi province of China, South Andaman Island, Selangor of Malaysia, Indonesia, and the Comoros of Mauritius, inhabitants leverage different components of B. gymnorrhiza to address various health conditions [5]. For example, the bark and leaf were employed for treating diarrhea, malaria, fevers, burns, diabetes, liver disorders, and intestinal worms [6,7]. The root was found to have applications in the treatment of diabetes and hyperlipidemias [8]. The fruit is utilized for managing conditions like shingles, eye diseases, and diarrhea [9]. Additionally, the bark of B. cylindrica demonstrates efficacy in treating hemorrhages and ulcers [10], while the bark of B. parviflora has been utilized in diabetes treatment [5].
In 1966, Loder and Russell first reported the isolation of a new tropane alkaloid brugine from the stem bark of B. sexangula [11]. In 2008, Wu et al. conducted the first comprehensive review of natural products from true mangrove flora worldwide, encompassing their sources, chemistries, and bioactivities [3]. In 2014, Zheng et al. compiled 38 chemical constituents of the Bruguiera genus up to 2010 [12]. Subsequently, in 2018, Xie et al. conducted a literature search spanning from 1972 to 2017, providing an overview of the chemical compositions and biological activities of B. gymnorrhiza [13]; however, certain inaccuracies were identified. In 2021, Chen et al. summarized 1387 secondary metabolites from fungi associated with mangroves, covering the period from 1989 to 2020, detailing their sources, chemistries, and biological activities [14]. Our research team has undertaken partial studies on the isolation and activity screening of secondary metabolites from mangrove plants and marine microorganisms [15,16,17,18,19]. Currently, we are engaged in relevant research on the isolation and activity screening of secondary metabolites from Bruguiera plants and their endophytes.
The ongoing isolation endeavors for plants belonging to this genus are predominantly centered around four specific species: B. gymnorrhiza, B. cylindrica, B. sexangula, and B. sexangula var. rhynchopetala. A total of 167 secondary metabolites have been unearthed, with a unique class of sulfur-containing compounds discovered particularly in B. gymnorrhiza. Starting from 2018, the isolation of novel compounds from plants within the Bruguiera genus has encountered escalating challenges, and the enthusiasm for research in this area has gradually diminished. However, there is a current research focus on the secondary metabolites produced by endophytes within the Bruguiera genus.
The unique habitat of mangroves has nurtured a rich resource of fungi, bacteria, actinomycetes, and other microorganisms, particularly mangrove associated fungi, which constitute the second largest group of marine fungi [14,20]. These fungi play a crucial role in regulating the mangrove ecosystem, and produce unique structural and diverse bioactive secondary metabolites [14]. Since 2007, researchers have demonstrated considerable interest in the exploration of structurally novel and bioactive compounds derived from endophytes of mangrove genus Bruguiera. These endophytes, comprising fungi, bacteria, and actinomycetes, are predominantly sourced from various plant components, including branch, leaf, stem, bark, root, hypocotyl, and inner tissue. To better exploit the medicinal potential of microbial resources, researchers have extensively cultured and isolated endophytic fungi within the Bruguiera genus, conducting analyses on both their secondary metabolites and biological activities. Through a compilation of the relevant literature, it was revealed that these endophytes comprise 34 fungi strains, belonging to 19 genera (strains), namely Aspergillus (4), Cladosporium (1), Clonostachys (1), Daldinia (2), Epicoccum (1), Fusarium (1), Gloesporium (1), Nigrospora (1), Nectria (1), Peniophora (1), Penicillium (10), Pestalotiopsis (2), Pseudolagarobcasidium (1), Phomopsis (1), Phyllosticta (1), Rhytidhysteron (1), Stemphylium (1), Trichoderma (1), and Xylaria (2), along with four actinomycetes, affiliated with the genus Streptomyces (4). The above 38 strains of endophytes were derived from B. gymnorrhiza (63.2%), B. sexangula var. rhynchopetala (21.0%), B. sexangula (13.2%), and B. parviflora (2.6%).
Currently, 337 secondary metabolites have been found from the endophytes of the Bruguiera genus. Some of these compounds exhibit rare structural features and demonstrate significant bioactivity. For example, in the study by Zhang et al. [21], four novel 12-membered macrocyclic lactone compounds (179–182) with sulfur substitution at C-2 were obtained from the endophytic fungus Cladosporium cladosporioides MA-299, isolated from the leaves of B. gymnorrhiza, and the compounds exhibited notable antimicrobial activity against aquatic bacteria (Edwardsiella tarda and Edwardsiella ictarda) and plant pathogens (Bipolaris sorokiniana, Colletotrichum glecosporioides, Fusarium oxysporum f. sp. cucumerinum, and Physalospora piricola Nose). Additionally, Xu et al. [22] got a range of structurally diverse ansamycin compounds, named divergolides A–D (329–332), from the endophytic actinomycete Streptomyces sp. in B. gymnorrhiza, offering a detailed analysis of the polyketide synthase domain, which not only validated the stereochemical integrity of divergolides, but also yielded valuable insights into the formation mechanisms of the diverse aromatic chromophores. Evidently, endophytes of Bruguiera genus plants emerge as a promising reservoir for acquiring structurally novel compounds.
Research indicates that there is no significant distinction in marine fungal natural products obtained from different ecological niches [23]. In-depth exploration of understudied microbial phyla, particularly those unique to specific environments, is more likely to unveil structurally novel biologically active compounds [24]. Clearly, there is still a substantial knowledge gap in the current exploratory research on endophytic bacteria and actinomycetes within the Bruguiera genus, necessitating further in-depth investigation. Simultaneously, a significant amount of current research employs similar isolation and cultivation techniques targeting microorganisms of the same genus, leading to the production of structurally similar or the known compounds during the processes of microbial culture and secondary metabolite isolation [25]. Therefore, to extract a more diverse array of unique natural products from endophytes within the Bruguiera genus, we should adopt a targeted approach. This involves the targeted isolation of strains, guided by early genomic sequencing to inform strain selection, and innovations in culture media and cultivation techniques [25].
This review encompasses the relevant literature describing the chemical composition and biological activities of Bruguiera genus plants and their endophytes until 30 December 2023. It summarizes approximately 496 compounds, of which 201 exhibit biological activity. Of particular note, during the process of the literature organization, it was observed that certain compounds—such as cytochalasin D, zygosporin D, 2,6-dimethoxy-1,4-benzoquinone, scopoletin, and so on—either coexist in both plants and their endophytic fungi or play a crucial regulatory role in the interaction between plants and endophytic fungi. The noteworthy discovery will be further explored and discussed below.
2. Chemical Composition of Bruguiera Genus Plants and Their Endophytes
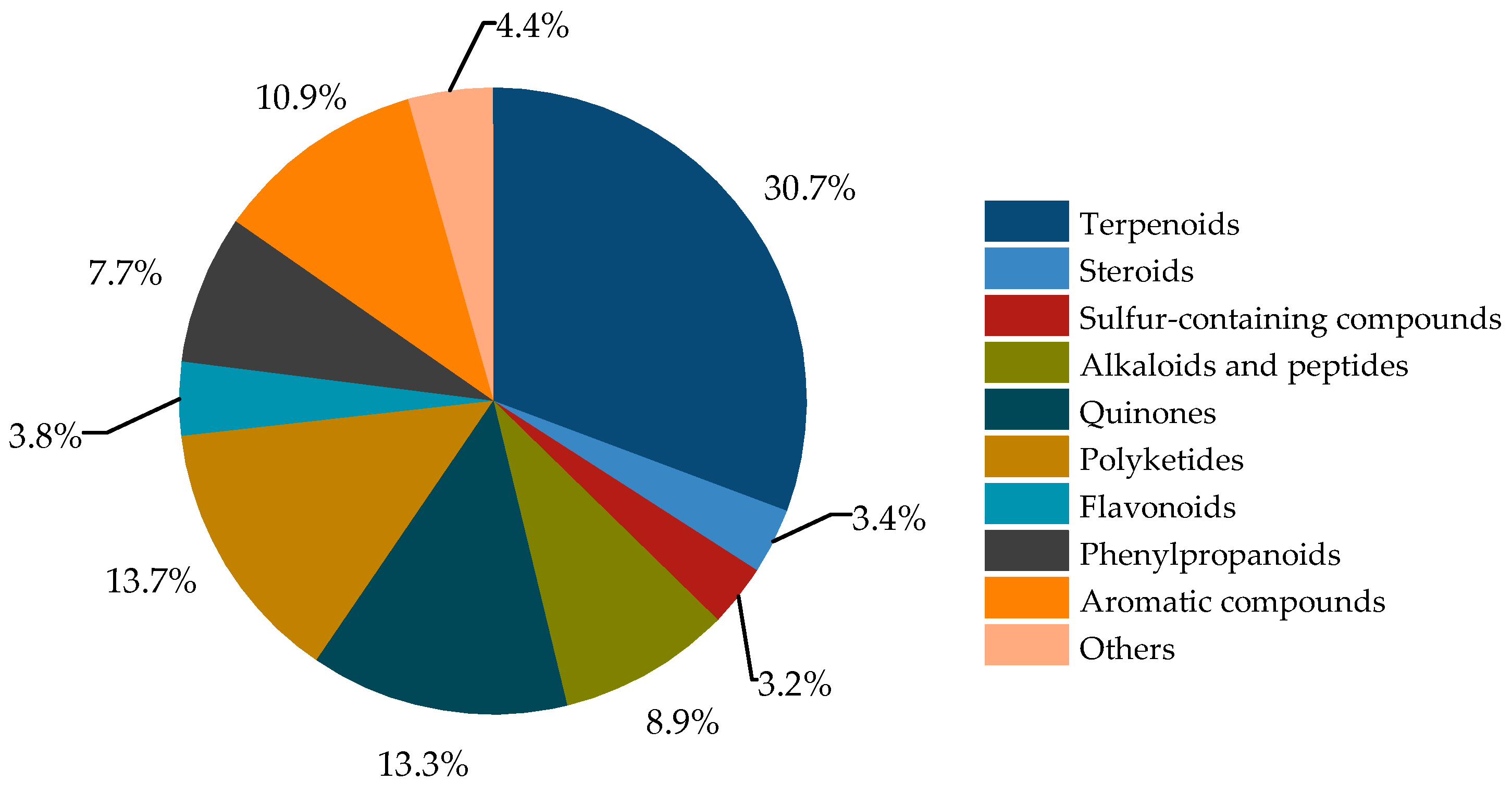
The secondary metabolites of Bruguiera genus plants and their endophytes demonstrate a diverse array (Figure 1), comprising terpenoids (30.7%), steroids (3.4%), sulfur-containing compounds (3.2%), alkaloids and peptides (8.9%), quinones (13.3%), polyketides (13.7%), flavonoids (3.8%), phenylpropanoids (7.7%), aromatic compounds (10.9%), and other compounds (4.4%).
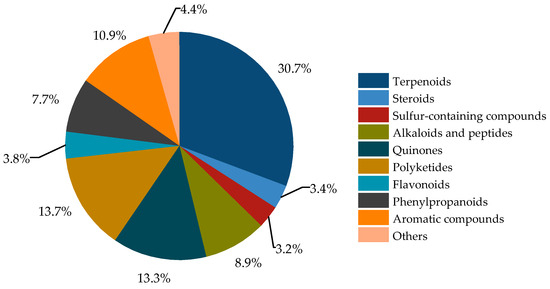
Figure 1.
Classification of secondary metabolites from plants of Bruguiera genus and their endophytes.
2.1. Terpenoids
Terpenoids constitute the largest category of chemical constituents in both Bruguiera genus plants and their endophytes, totaling 152 compounds. This diverse group includes 2 monoterpenes, 56 sesquiterpenes, 28 diterpenes, 4 sesterterpenes, 40 triterpenes, and 22 meroterpenes. Monoterpenes, sesterterpenes, and meroterpenes are exclusively found in endophytic fungi. Diterpenes are isolated only from Bruguiera plants, which suggests the potential absence or inhibition of the biosynthetic pathway for these compounds in their endophytes.
2.1.1. Monoterpenes
In the context of Bruguiera genus plants and their endophytes, the occurrence of monoterpenoid compound is infrequent. Only Xu et al. [26] isolated two antibacterially active monoterpenoid derivatives (Figure 2), (3R,4R,6R,7S)-7-hydroxyl-3,7-dimethyl-oxabicyclo[3.3.1]nonan-2-one (1) and (3R,4R)-3-(7-methylcyclohexenyl)-propanoic acid (2), from the fermentation product of Pestalotiopsis foedan, an endophytic fungus found in the branch of B. sexangula. While a variety of monoterpenes, such as trans-β-ocimene and linalool, are present in the essential oils of Bruguiera plants like the flower of B. gymnorrhiza [27], researchers have seldom conducted isolation studies on this particular fraction of crude extracts.
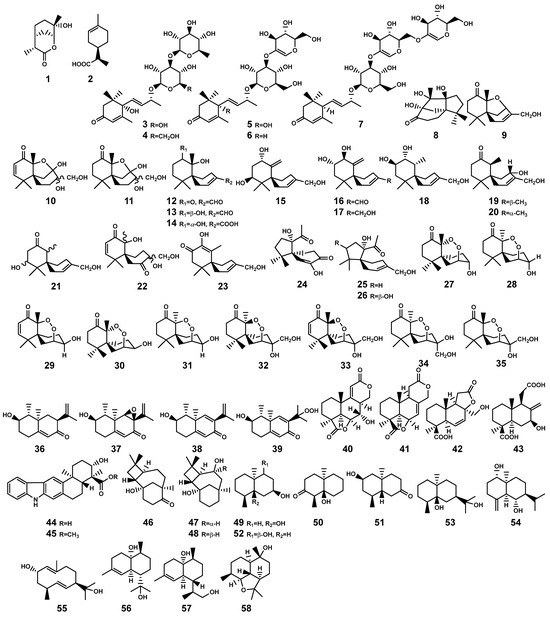
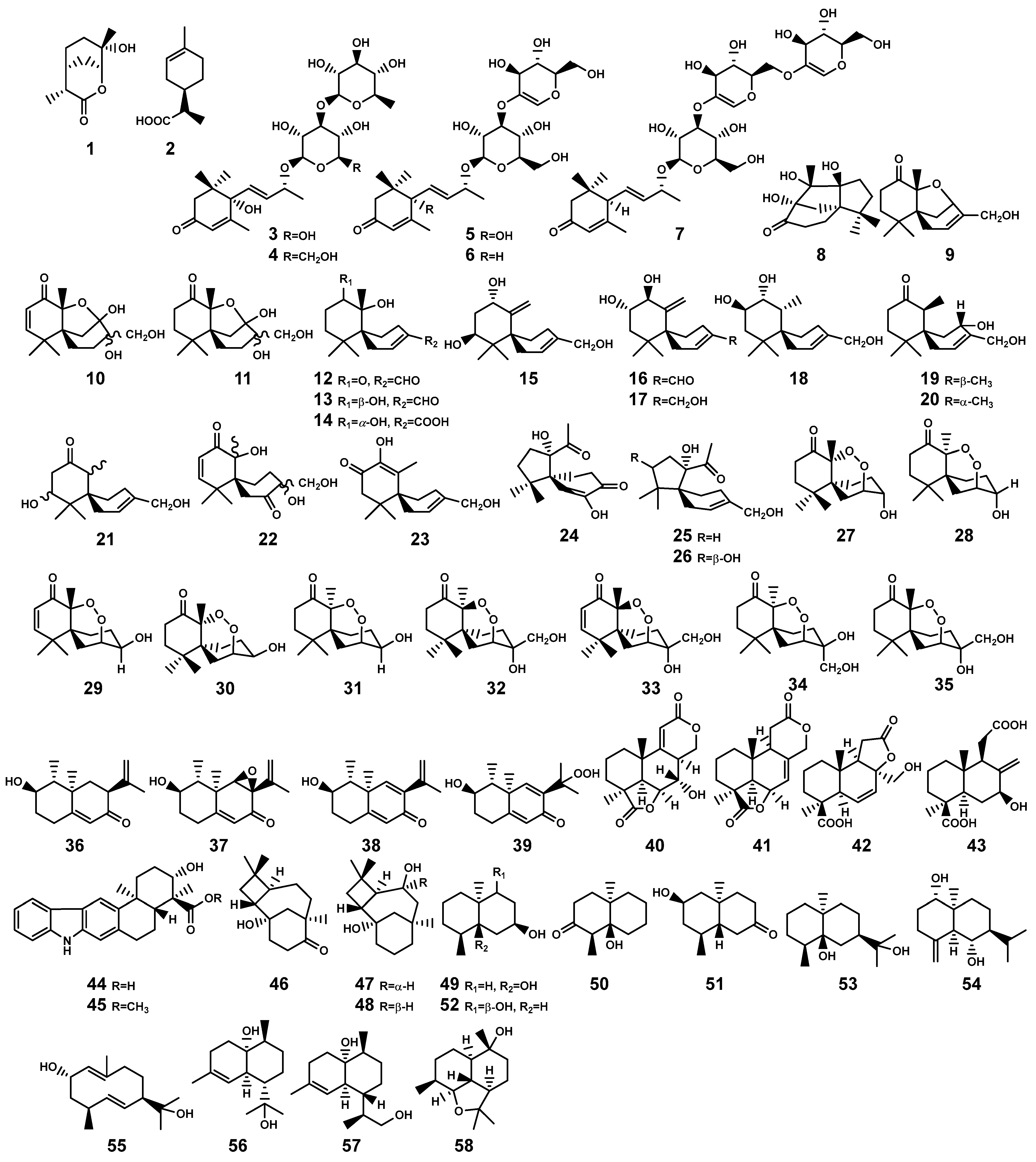
Figure 2.
Monoterpenes (1–2) and sesquiterpenes (3–58) isolated from Bruguiera genus plants and their endophytes.
2.1.2. Sesquiterpenes
In the Bruguiera genus and their endophytes, the biosynthetic pathway for sesquiterpenoids is exclusively present in both B. gymnorrhiza plant and its endophytes, having the production of 56 sesquiterpenoid compounds (3–58, Table 1 and Figure 2). In a study by Wibowo et al. [28,29], a total of 28 (nor)sesquiterpens (8–35) were isolated from the endophytic fungus Pseudolagarobasidium acaciicola in the roots of B. gymnorrhiza. Compound 8 is a novel tricyclic norsesquiterpene with a unique acaciicolane skeleton featuring a 6/5/5 ring system. Compounds 24–26 are identified as new spirobicyclic (nor)sesquiterpenes, characterized by a spiroacaciicolane skeleton with a 5/6 fused spirobicyclic ring system [28,29]. (Nor)sesquiterpenoid compounds (27–29, 35) are found as new nor-chamigrane endoperoxides [28,29]. Additionally, compounds 9–11 and 12–23 possess a novel 6/5/6 tricyclic ring system and a 6/6 spirobicyclic structure, respectively [29].
Ding and colleagues [30,31,32,33] identified five types of sesquiterpenes from two endophytic actinomycetes, Streptomyces sp. GT2002/1503 and Streptomyces sp. JMRC:ST027706, isolated from the stems of B. gymnorrhiza. These compounds encompass indolosesquiterpenes (44–45), plant-derived caryolanes (46–48), geosmins (49–52), plant-like eudesmanes (53–55), and plant-like cadinanes (56–58). Significantly, among these, compound 53 demonstrated noteworthy broad antimicrobial activity and also emerged as a principal constituent in the essential oil derived from the aromatic grass Cymbopogon distans, which may play a pivotal role as an active ingredient in the medicinal plant C. distans [32]. Nevertheless, there exists a plausible suspicion that this compound may also be produced by Streptomyces sp. JMRC:ST027706 in a manner to the plant’s defense response when the host B. gymnorrhiza plant is subjected to pathogenic threats. This implies a potential collaborative mechanism where the endophytic actinomycete aids the host plant in bolstering its resistance against diseases. Moreover, the discovery of oxygenated geosmins is interesting, as it has brought to light the potential presence of enzymes within the endophytic actinomycetes capable of modifying geosmin oxidizing the decalin core structure found in geosmin and analogous terpenoids [32]. This revelation not only expands our understanding but also introduces a promising prospect for employing biotechnological strategies to harness these enzymes for geosmin degradation.

Table 1.
Sesquiterpenes isolated from Bruguiera genus plants and their endophytes.
Table 1.
Sesquiterpenes isolated from Bruguiera genus plants and their endophytes.
| No. | Compound | Source | Reference |
|---|---|---|---|
| 3 | (S)-4-hydroxy-3,5,5-trimethyl-4-((R,E)-3-(((2R,3R,4S,5S,6S)-3,5,6-trihydroxy-4-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)cyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 4 | (S)-4-((R,E)-3-(((2R,3R,4S,5R,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-4-hydroxy-3,5,5-trimethylcyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 5 | (S)-4-((R,E)-3-(((2R,3R,4S,5R,6R)-4-(((2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-3,4-dihydro-2H-pyran-5-yl)oxy)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-4-hydroxy-3,5,5-trimethylcyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 6 | (R)-4-((R,E)-3-(((2R,3R,4S,5R,6R)-4-(((2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-3,4-dihydro-2H-pyran-5-yl)oxy)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-3,5,5-trimethylcyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 7 | (R)-4-((R,E)-3-(((2R,3R,4S,5R,6R)-4-(((2R,3S,4S)-2-((((2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-3,4-dihydro-2H-pyran-5-yl)oxy)methyl)-3,4-dihydroxy-3,4-dihydro-2H-pyran-5-yl)oxy)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-3,5,5-trimethylcyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 8 | Acaciicolin A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 9 | Acaciicolide A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 10 | Acaciicolide B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 11 | Acaciicolide C | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 12 | Acaciicolinol A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 13 | Acaciicolinol B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 14 | Acaciicolinol C | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 15 | Acaciicolinol D | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 16 | Acaciicolinol E | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 17 | Acaciicolinol F | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 18 | Acaciicolinol G | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 19 | Acaciicolinol H | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 20 | Acaciicolinol I | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 21 | Acaciicolinol J | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 22 | Acaciicolinol K | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 23 | Acaciicolinol L | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 24 | Spiroacaciicolide A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 25 | Spiroacaciicolide B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 26 | Spiroacaciicolide C | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 27 | 3-epi-Steperoxide A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 28 | 3-epi-merulin A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 29 | (3S,4R,6aS,10aR)-4-hydroxy-7,7,10a-trimethyl-5,6,7,10a-tetrahydro-3H-3,6a-methanobenzo[c] [1,2] dioxocin-10(4H)-one | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 30 | Steperoxide A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 31 | Merulin A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 32 | Merulin B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 33 | Merulin C | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 34 | Merulin D | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 35 | 7-epi-merulin B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 36 | Petasol | B. gymnorrhiza | [35] |
| 37 | Sporogen AO1 | B. gymnorrhiza | [35] |
| 38 | 6-dehydropetasol | B. gymnorrhiza | [35] |
| 39 | 3α-hydroxy-11-peroxyl-eremophila-6,9-dien-8-one | B. gymnorrhiza | [35] |
| 40 | Botryosphaerin F | Aspergillus terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [36] |
| 41 | 13,14,15,16-tetranorlabd-7-ene-19,6b:12,17-diolide | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [36] |
| 42 | Botryosphaerin B | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [36] |
| 43 | LLZ1271β | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [36] |
| 44 | Xiamycin | Streptomyces sp. GT2002/1503 (the stem of B. gymnorrhiza, endophytic actinomycete) | [30] |
| 45 | Methyl ester of xiamycin | Streptomyces sp. GT2002/1503 (the stem of B. gymnorrhiza, endophytic actinomycete) | [30] |
| 46 | Bacaryolane A | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [31] |
| 47 | Bacaryolane B | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [31] |
| 48 | Bacaryolane C | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [31] |
| 49 | 7R-hydroxygeosmin | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 50 | 3-oxogeosmin | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 51 | 2R-hydroxy-7-oxogeosmin | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 52 | 5-deoxy-7β,9β-dihydroxygeosmin | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 53 | (4S,5S,7R,10S)-4β,10α-eudesmane-5β,11-diol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 54 | (1S,5S,6S,7S,10S)-10α-eudesm-4(15)-ene-1α,6α-diol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 55 | 1(10)E,5E-germacradiene-2,11-diol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 56 | (+)-11-hydroxy-epicubenol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [33] |
| 57 | (+)-12-hydroxy-epicubenol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [33] |
| 58 | 5,11-epoxy-10-cadinanol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [33] |
2.1.3. Diterpenoids
Diterpenoids are a class of compounds derived from the precursor (geranylgeranyl diphosphate, GGPP) [37]. The diversity of diterpenoids in plants playing important roles in plant development, stress resistance, and interactions with environmental microorganisms, depends on the various skeletons biosynthesized by terpene synthases and the substrate promiscuity of cytochrome P450 monooxygenases (P450s), along with other post-modification enzymes [38]. In the genus Bruguiera, researchers have isolated five different skeletal types of diterpenoid (Table 2 and Figure 3), namely ent-pimarane (79–82), isopimarane (83–84), ent-beyerane (85–86), ent-kaurane (62–78), and ent-gibberellane (59–61), with the key intermediate pimarane in their biosynthesis.

Table 2.
Diterpenoids isolated from Bruguiera genus plants and their endophytes.
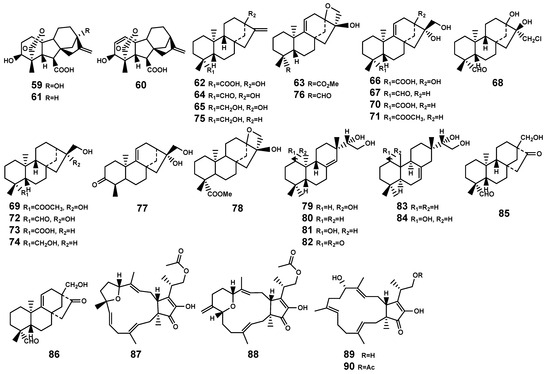
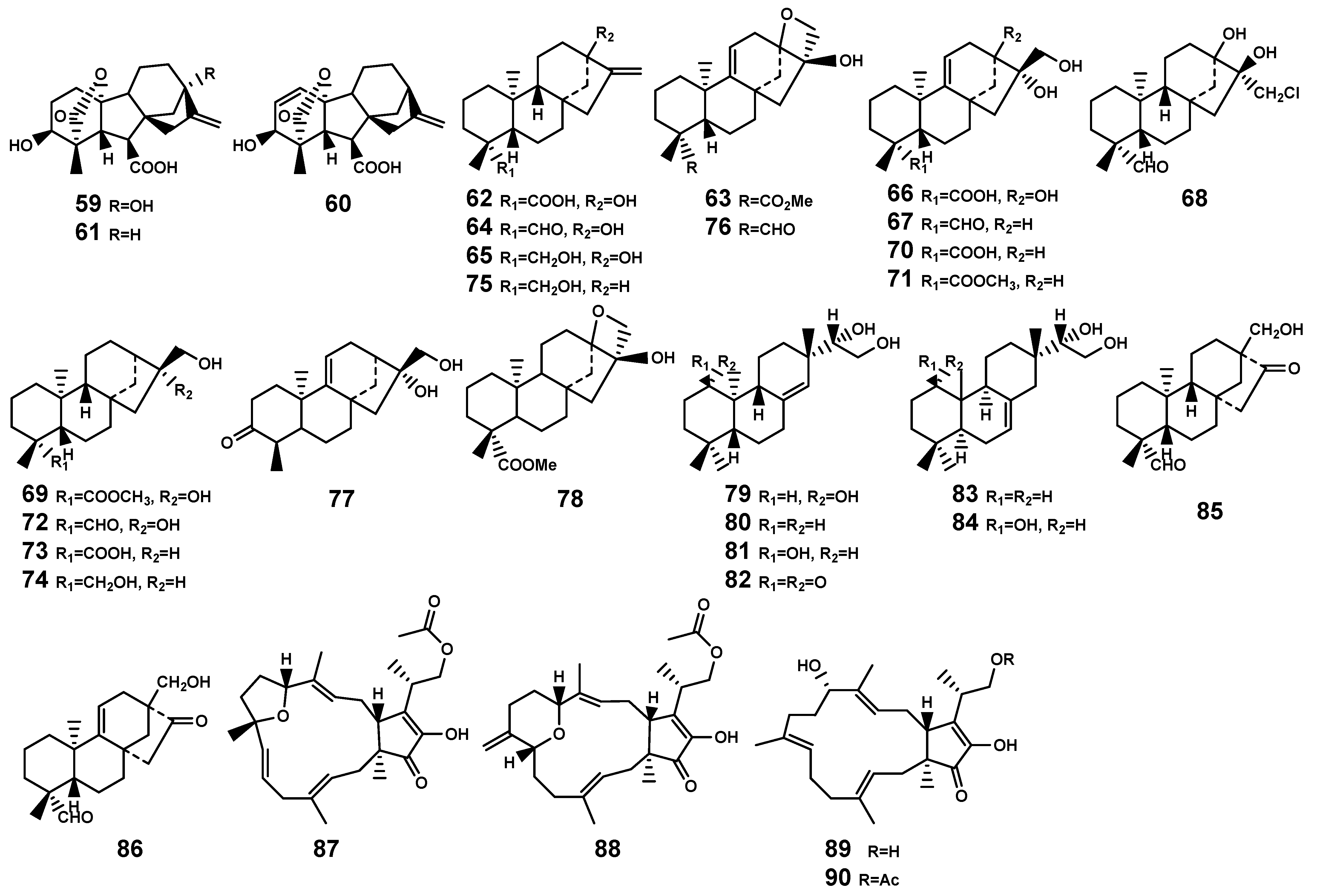
Figure 3.
Diterpenes (59–86) and sesterterpenes (87–90) isolated from Bruguiera genus plants and their endophytes.
2.1.4. Sesterpenoids
Most sesterpenoids are sourced from marine organisms, with approximately 15% of these compounds having been isolated from plants [44]. Currently, no such compounds have been identified in Bruguiera plants, emphasizing the need for further exploration in this area. Only Liu et al. [45] reported the discovery of two tricyclic sesterterpenes, fusaprolifin A and fusaprolifin B (87–88) with brine-shrimp lethality activity, and other two sesterterpenes, terpestacin and fusaproliferin (89–90), which isolated from the endophytic fungus Fusarium proliferatum MA-84 associated with B. sexangula plant (Figure 3).
2.1.5. Triterpenoids
In the Bruguiera plants and their endophytic fungi, triterpenoids (91–130, Table 3 and Figure 4) constitute a primary class of compounds. Distinguished by variations in carbon ring structures, there are tetracyclic triterpenoids, such as dammarane-type (91–93) and lanostane-type (94–98). Additionally, pentacyclic triterpenoids are present, including oleanane-type (99–104), ursane-type (105–107), lupane-type (108–117), and other types (118–130).

Table 3.
Triterpenoids isolated from Bruguiera genus plants and their endophytes.
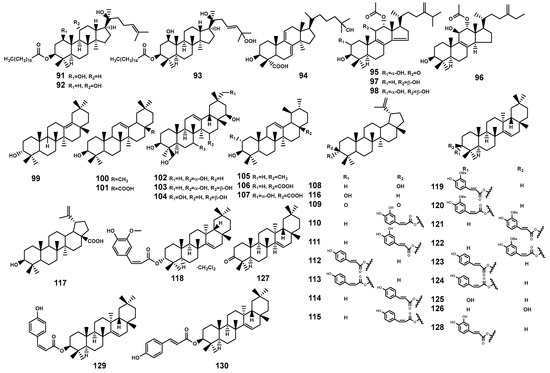
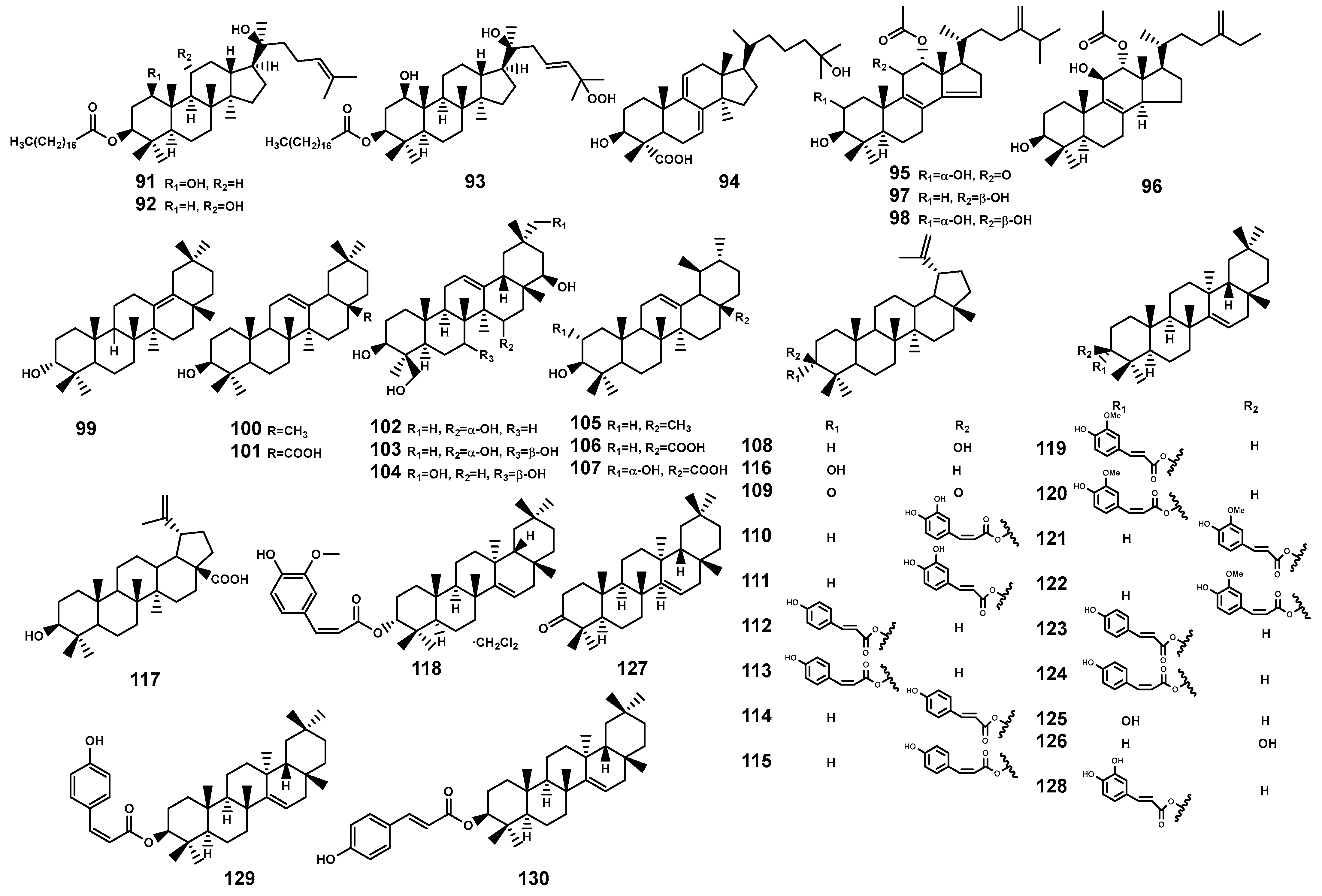
Figure 4.
Triterpenoids (91–130) isolated from Bruguiera genus plants and their endophytes.
2.1.6. Meroterpenoids
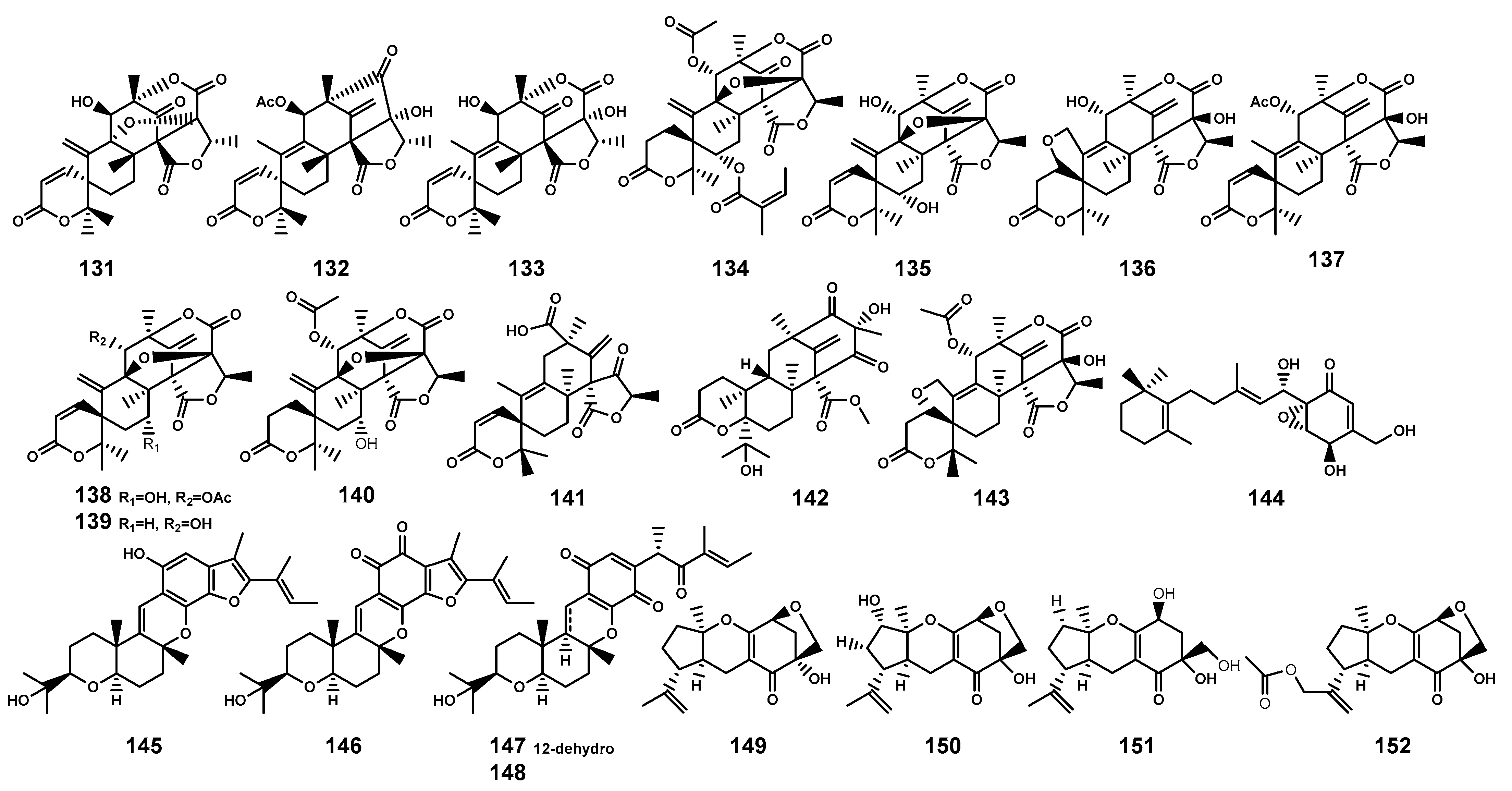
Meroterpenoids as secondary metabolites arising from hybrid terpenoid biosynthetic pathways, are characterized by the combination of terpenoid and non-terpenoid segments [59]. Based on their non-terpenoid starting moieties, these compounds were categorized into four classes: polyketide–terpenoids, indole–terpenoids, shikimate–terpenoids, and miscellaneous meroterpenoids [59]. This review summarized 22 meroterpenoids (Table 4 and Figure 5) isolated from the endophytic fungi found in B. gymnorrhiza, B. sexangula, and B. sexangula var. rhynchopetala. These meroterpenoids have been classified as shikimate-meroterpenoids of the tricycloalternarene type (149–152) and polyketide-meroterpenoids (131–148), which contain tetraketide moieties derived from 3,5-dimethylorsellinic acid (131–143) and 6-methylsalicylic acid (144), and hexaketide moiety (145–148), respectively.

Table 4.
Meroterpenoids isolated from Bruguiera genus plants and their endophytes.
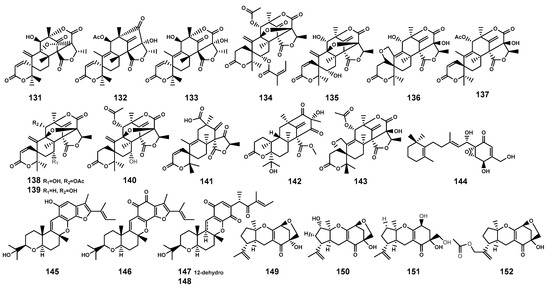
Figure 5.
Meroterpenoids (131–152) isolated from Bruguiera genus plants and their endophytes.
2.2. Steroids
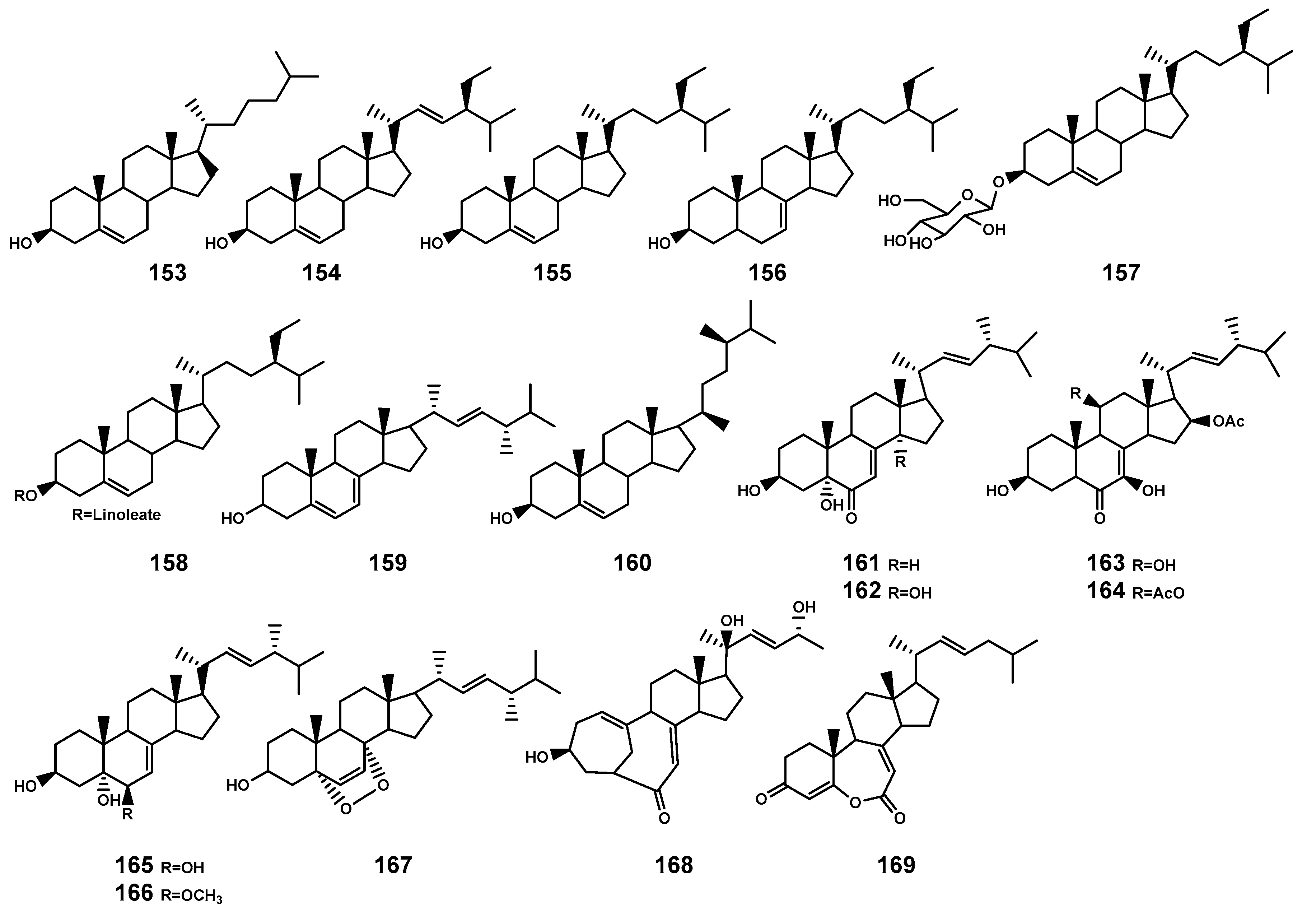
In plants and fungi, squalene serves as a precursor that undergoes oxidation catalyzed by squalene epoxidase to generate ox1idosqualene [65]. The further cyclization of oxidized squalene, catalyzed by oxidosqualene cyclases, leads to the biosynthesis of sterols representing a significant downstream structural derivative in the biosynthesis of triterpenoids [65]. Currently, 17 steroids (Table 5 and Figure 6) have been identified in the Bruguiera plants and their endophytic fungi. These compounds encompass three types: cholestrol (153), stigmastanol (154–158), and ergosterol (159–169), specifically noting the significance of two ergostanes (168–169) with a rearranged tetracyclic skeleton.

Table 5.
Steroids isolated from Bruguiera genus plants and their endophytes.
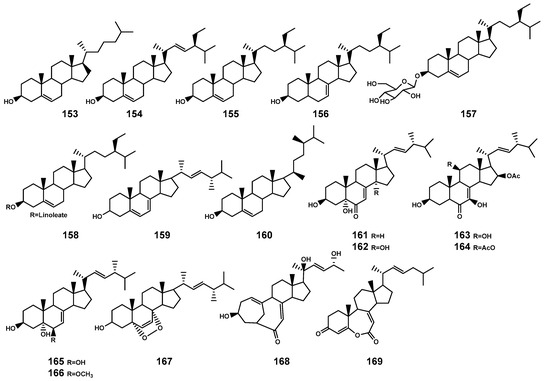
Figure 6.
Steroids (153–169) isolated from Bruguiera genus plants and their endophytes.
2.3. Sulfides
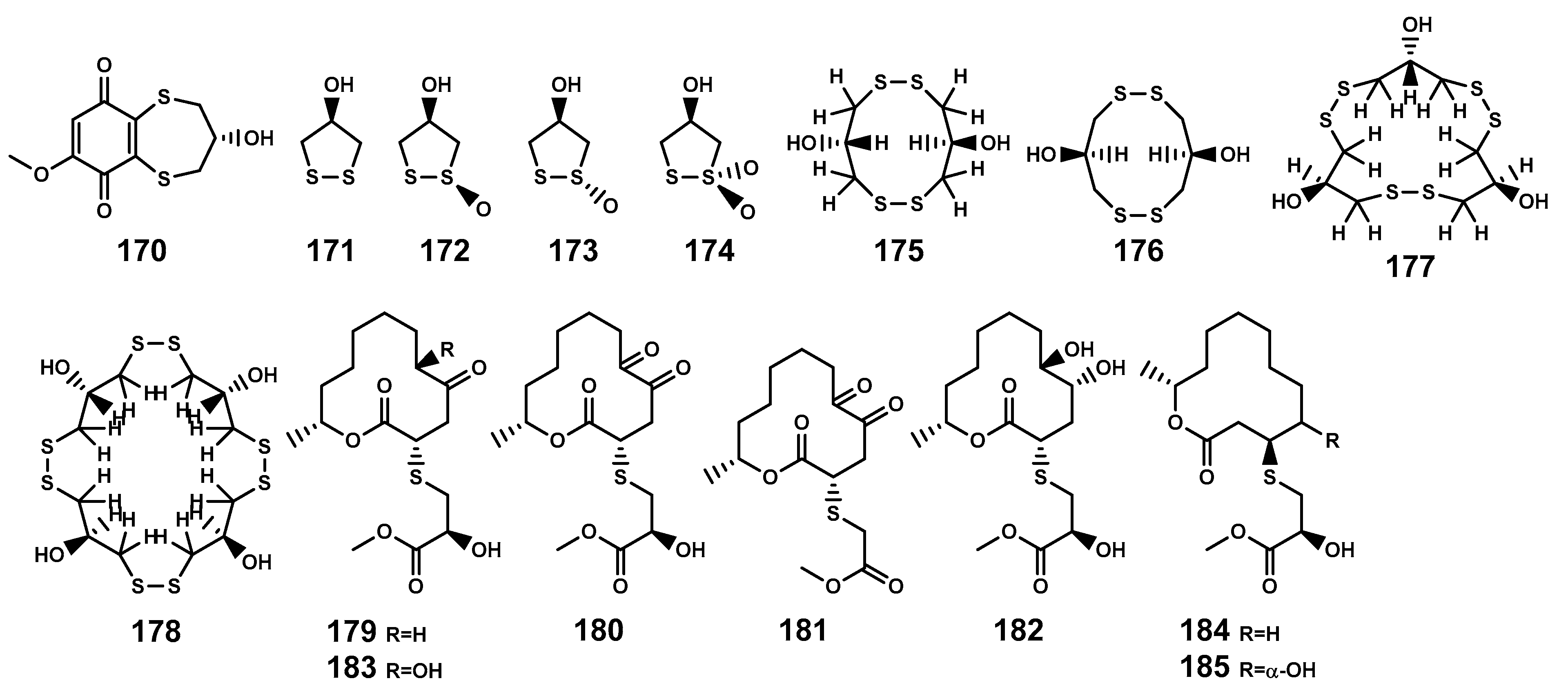
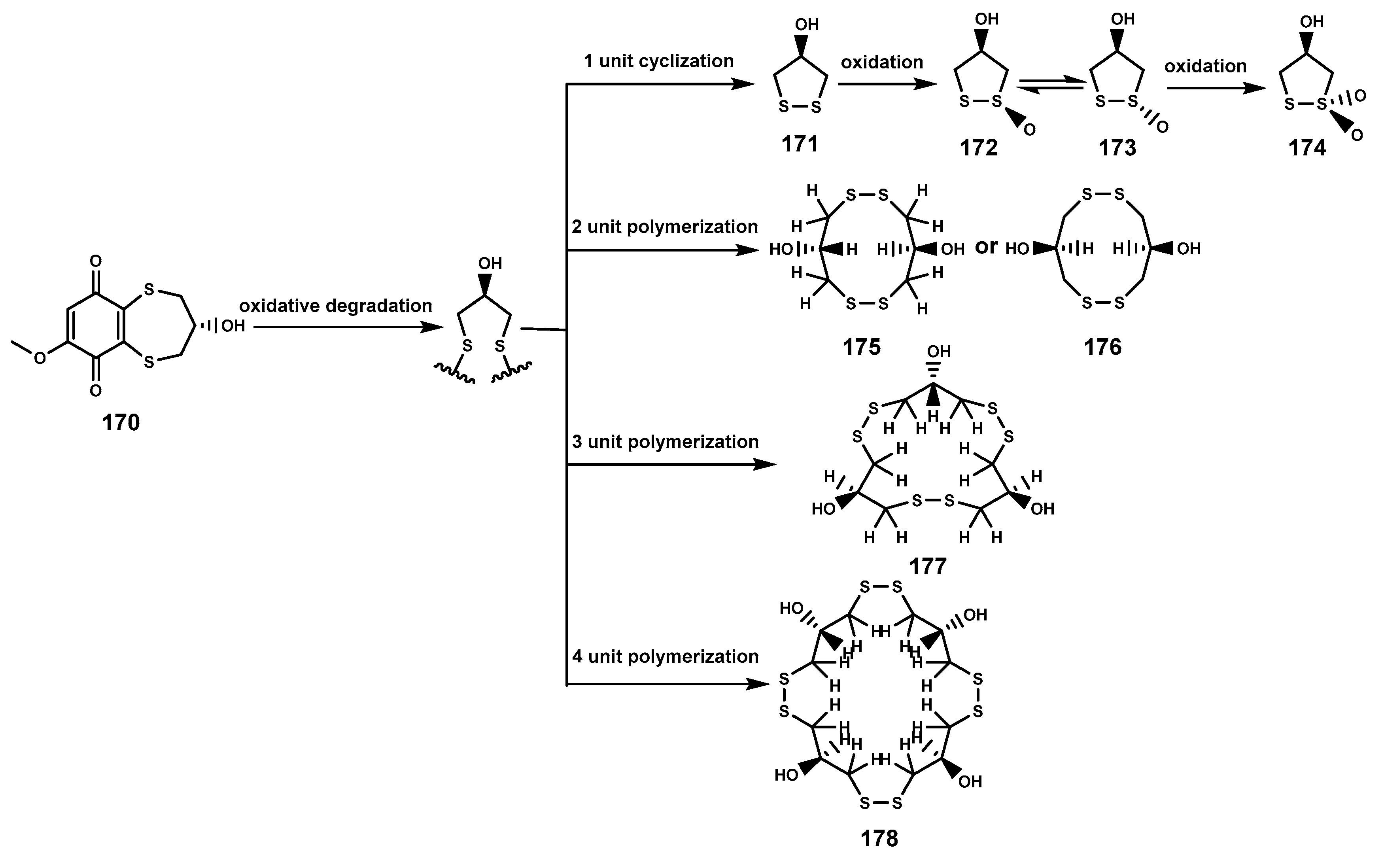
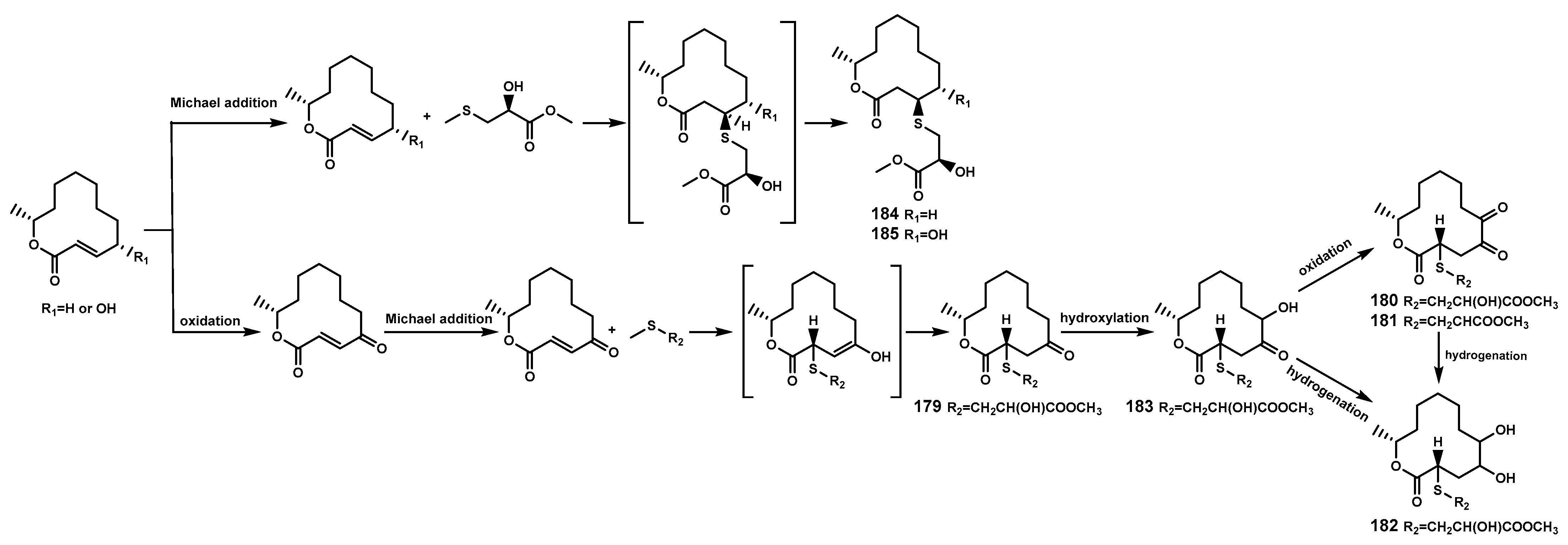
Sulfur-containing natural products are predominantly isolated from plants of the Alliaceae family or bacteria, with relatively fewer sulfur compounds being separated from fungi [71]. Now, this paper summarizes a total of 16 sulfur-containing compounds (Table 6 and Figure 7) from the Bruguiera genus of Rhizophoraceae family and their endophytes. Within the plants B. gymnorrhiza, B. sexangula var. rhynchopetala, and B. cylindrica, a class of (poly)disulfide compounds (170–178) has been found. These compounds belong to a characteristic type of compounds found in mangrove ecosystem of the Bruguiera genus, and exhibit a proposed unique biosynthetic pathway (Figure 8) [41,72]. Previous research indicates that sulfides play a crucial role in plant defense against various pathogens and pests [73]. Dahibhate et al. [74] discovered that a mixture of brugierol and isobrugierol exhibited inhibitory activity against Pseudomonas aeruginosa by reducing the formation of virulence biofilms controlled by the quorum sensing system, lowering the level of virulence factors, thereby attenuating the pathogenicity of the bacterium against Bruguiera plants. Simultaneously, Zhang et al. [21,75] cultured and isolated the endophytic fungus C. cladosporioides MA-299 from the leaves of B. gymnorrhiza, obtaining seven sulfur-containing 12-membered macrocyclic lactones (179–185). The potential biosynthetic pathway of these compounds is illustrated in Figure 9, with the precursor cladocladosin A featuring the bicyclo 5/9 ring system. These compounds (179–185) demonstrate activity against aquatic pathogens and plant-pathogenic fungi [21,75], thereby assisting B. gymnorrhiza leaves in resisting microbial pathogen invasion.

Table 6.
Sulfides isolated from Bruguiera genus plants and their endophytes.
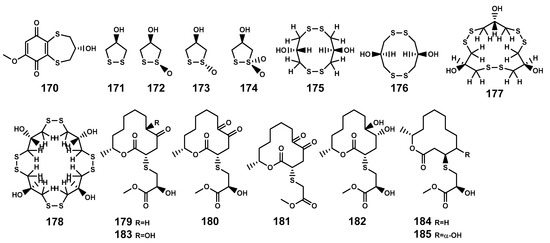
Figure 7.
Sulfides (170–185) isolated from Bruguiera genus plants and their endophytes.
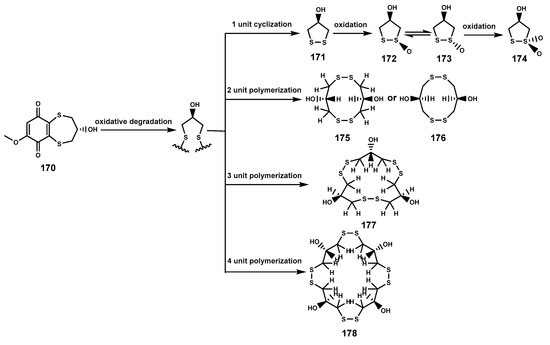
Figure 8.
The proposed biosynthetic pathway of disulfide compounds (170–178).
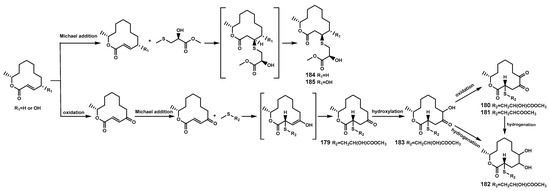
Figure 9.
The proposed biosynthetic pathway of sulfur-containing 12-membered macrocyclic lactones (179–185).
2.4. Alkaloids and Peptides
Alkaloids constitute a class of naturally occurring organic compounds with at least one nitrogen atom, primarily derived from amino acids. They typically exhibit complex cyclic structures, with some playing crucial roles in plant defense mechanisms [81]. Currently, researchers have identified 33 alkaloids (Table 7 and Figure 10) with different types from B. gymnorrhiza plant and endophytic fungi in B. gymnorrhiza and B. sexangula var. rhynchopetala, including amine (186) and amide types (187–191), indole (192–193), pyrimidine (194) and purine types (195–196), tropane (197), pyrrolidine (198), pyrrolizidine (199), quinazoline (200–201), quinoline (202–204), benzodiazepine (205), diketopiperazine (206–211), and cyclohexylideneacetonitrile derivatives (212–218). Studies indicate a significant correlation between endophytes and alkaloid [82]. Among the alkaloid-producing endophytic fungi, 66% belong to the Penicillium genus, and 75% of the alkaloids isolated from endophytic fungi are derived from the Penicillium genus. Clearly, within the endophytic communities of Bruguiera, the Penicillium genus likely serves as the dominant producer of alkaloids. Additionally, Li et al. [68] isolated a 7-membered 2,5-dioxopiperazine alkaloid (+)-cyclopenol (205), from an endophytic fungus Penicillium sclerotiorum in the inner bark of B. gymnorrhiza. In the terrestrial plant Garcinia atroviridis, the endophytic fungus P. sclerotiorum produces azaphilone-type derivatives [83]. (-)-cyclopenol, which shares a biosynthetic pathway similar to 205, was also isolated from terrestrial soil Penicillium genus [68,84]. This suggests that the mechanism of (+)-cyclopenol production may be associated with the unique habitat of mangroves.

Table 7.
Alkaloids and Peptides isolated from Bruguiera genus plants and their endophytes.
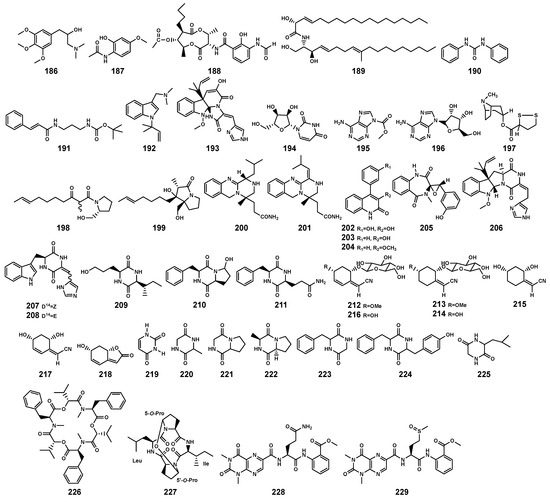
Figure 10.
Alkaloids (186–218) and peptides (219–229) isolated from Bruguiera genus plants and their endophytes.
2.5. Quinones
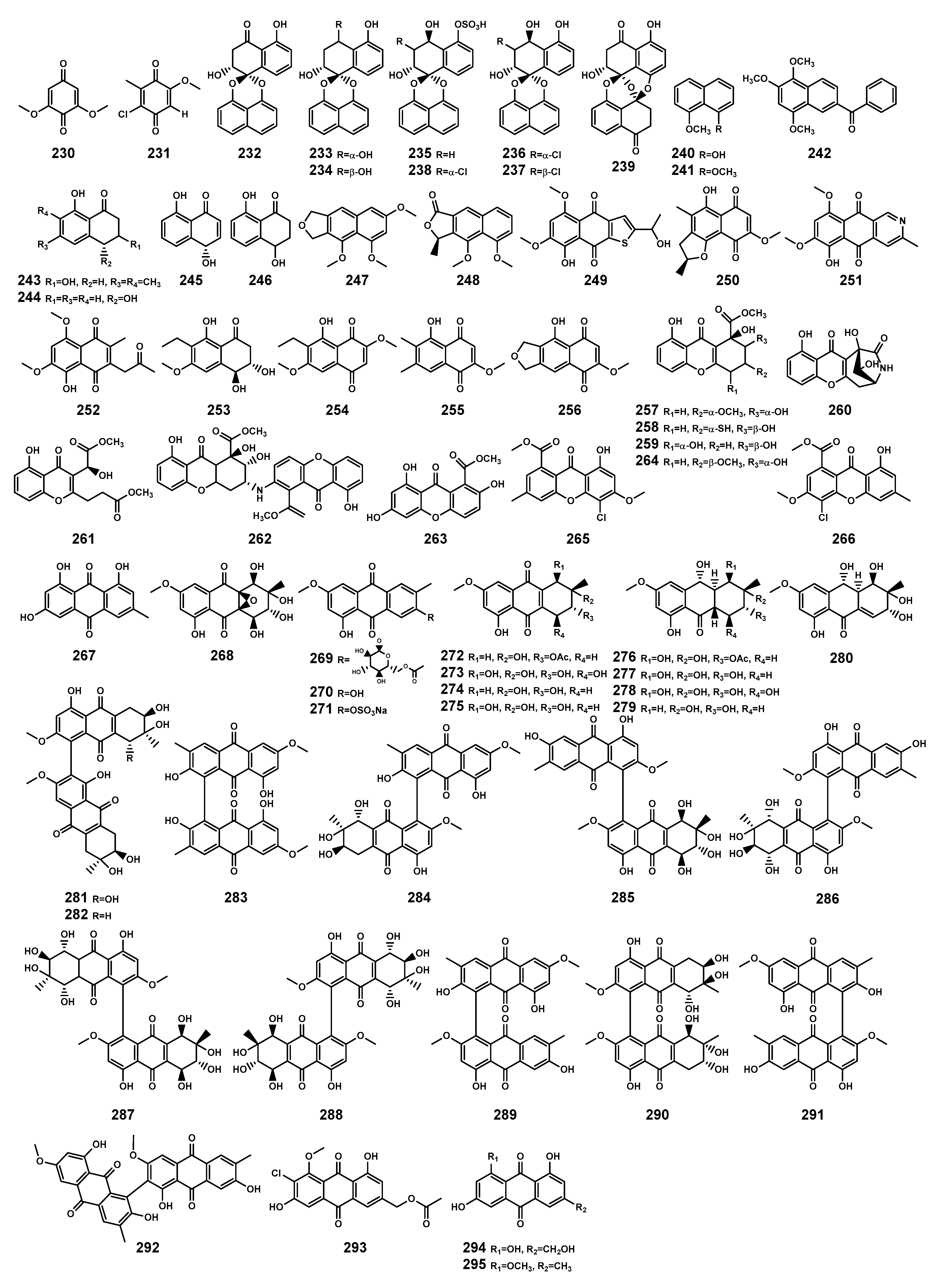
Plants are the primary producers of quinones [95], but in Bruguiera genus plants and their endophytes, 87.5% of quinones originate from endophytic fungi. The identified quinones (Table 8 and Figure 11) include benzoquinones (230–231), naphthalenes (232–242), naphthalenones (243–246), naphthofurans (247–248), naphthoquinones (249–256), xanthones (257–266), and anthraquinones (267–295). Among these, 41.2% of quinones sourced from endophytic fungi exhibit antibacterial activity. Studies have shown that fungi can commonly reduce 2,6-dimethoxy-1,4-benzoquinone (230) to hydroquinones, and some fungi showed effects of quinone-dependent polymer polystyrene sulfonate degradation, contributing to driving fungi to utilize the redox cycling of quinones for biodegradation in the ecological environment [96]. In plant immunity, quinone-related molecules could play a role as pathogen- or danger-associated molecular patterns [95]. The series of evidence underscores the ecological significance of quinone substances.

Table 8.
Quinones isolated from Bruguiera genus plants and their endophytes.
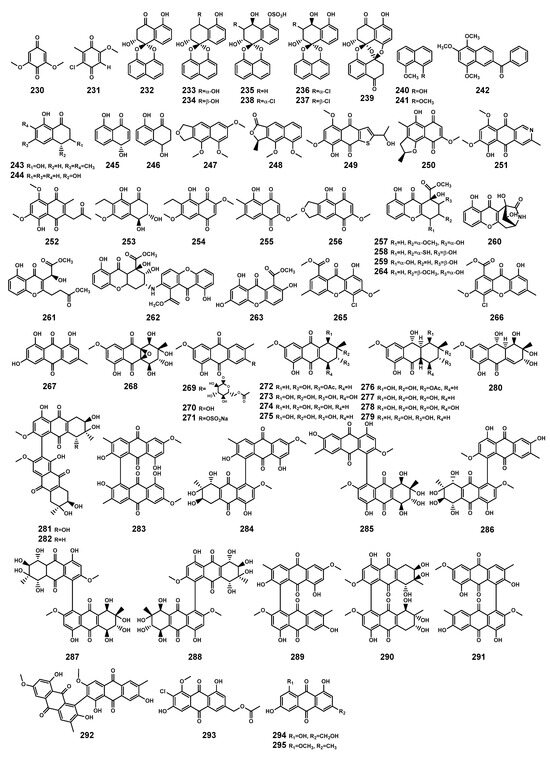
Figure 11.
Quinones (230–295) isolated from Bruguiera genus plants and their endophytes.
2.6. Polyketides
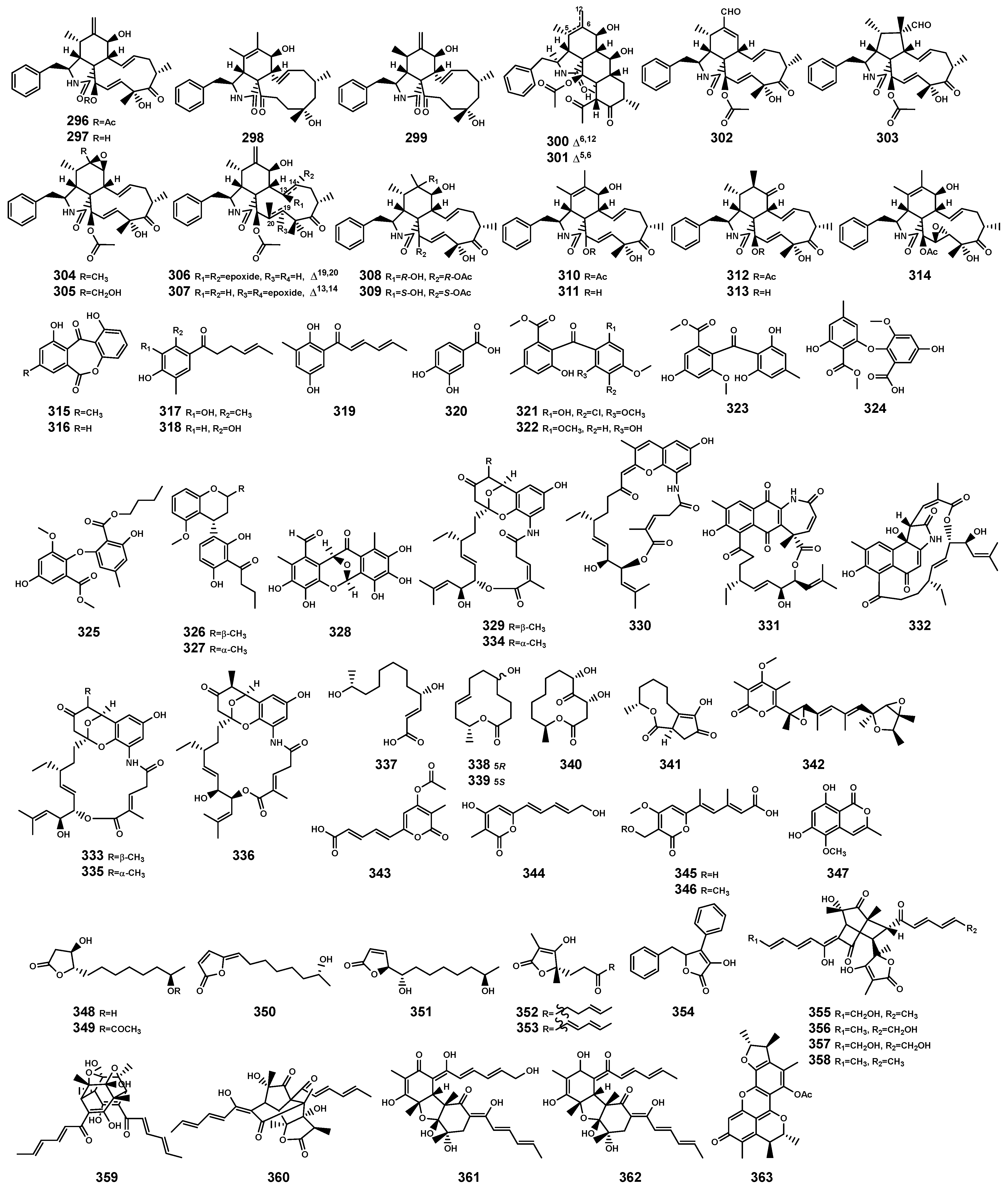
In the genus Bruguiera and its endophytes, polyketides primarily exhibit antibacterial and cytotoxic activities, predominantly sourced from endophytes of the Bruguiera genus. These polyketides (Table 9 and Figure 12) encompass various types, including cytochalasins (296–314), phenols (315–328), ansamycins (329–336), 12-membered macrolides (337–341), α-pyrone (342–346), chromanone (347), furanones (348–354), sorbicillinoids (355–362), and benzofuranones (363). Supratman et al. [108] isolated (-)-dihydrovertinolide (352) from endophytic fungus C. rosea B5-2 obtained from the branch of B. gymnorrhiza. The compound 352 exhibited no antibacterial activity but demonstrated phytotoxicity to lettuce seedlings (Lactuca sativa L.) [108]. This effect may enhance the competitive abilities of B. gymnorrhiza with other plants in terms of nutrients and space [109], contributing to the reinforcement of B. gymnorrhiza plant defense mechanisms. It could serve as a lead compound for the development of potential bioherbicides.

Table 9.
Polyketides isolated from Bruguiera genus plants and their endophytes.
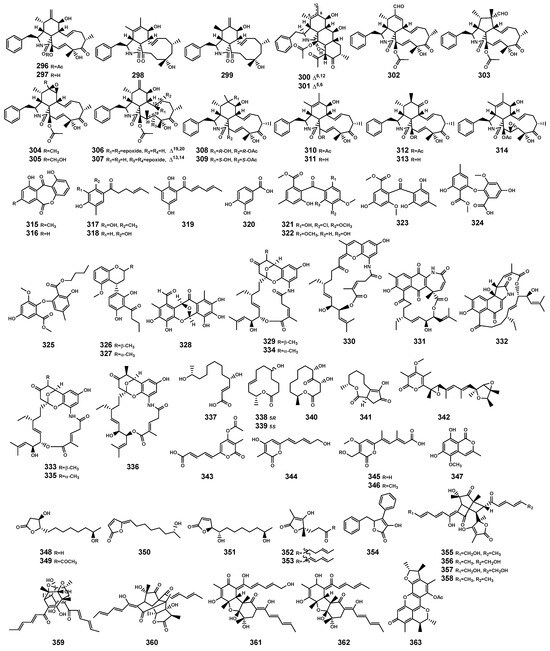
Figure 12.
Polyketides (296–363) isolated from Bruguiera genus plants and their endophytes.
2.7. Flavonoids
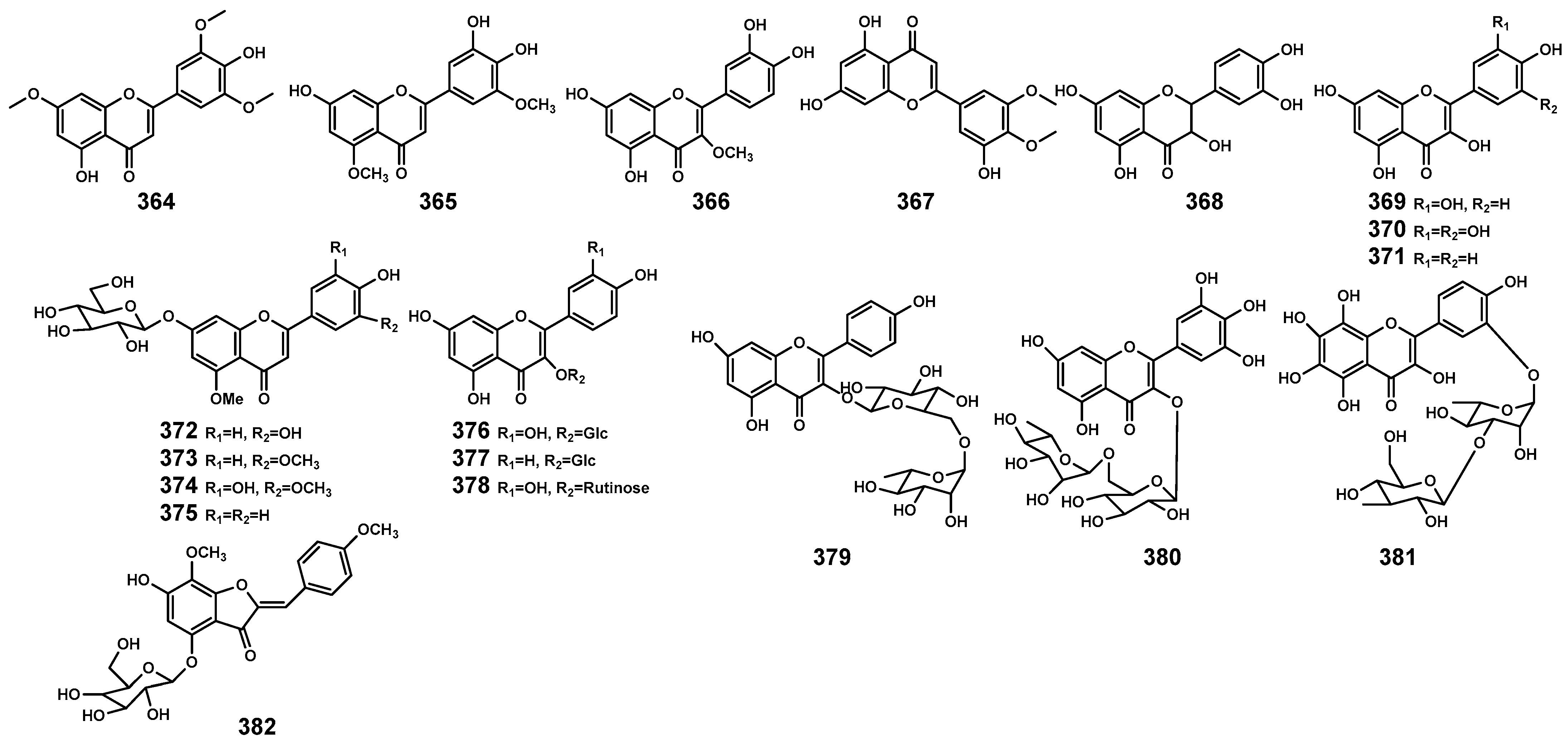
Flavonoids are commonly present in the organs of plants, participating in the plant’s response to environmental stressors [116]. Currently, in the genus Bruguiera and its endophytes, flavonoid (Table 10 and Figure 13) primarily originates from B. gymnorrhiza plants, including flavones (364–367), flavone glycosides (372–375), and flavonol glycosides (376–381). In the B. parviflora plant, dihydroflavonol (368) and flavonol (369–371) have been identified. Additionally, WU et al. [117] isolated a novel aurone glycoside compound (Z)-7,4’-dimethoxy-6-hydroxy-aurone-4-O-β-glucopyranoside (382) from the endophytic fungus Penicillium citrinum associated with B. gymnorrhiza, exhibiting significant neuroprotective activity.

Table 10.
Flavonoids isolated from Bruguiera genus plants and their endophytes.
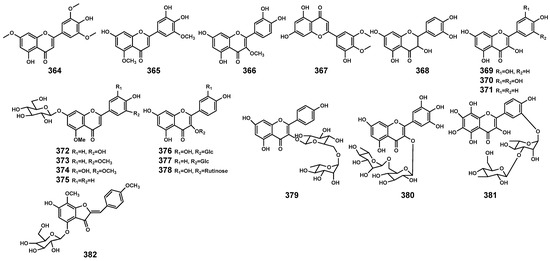
Figure 13.
Flavonoids (364–382) isolated from Bruguiera genus plants and their endophytes.
2.8. Phenylpropanoids
The 38 phenylpropanoids (Table 11 and Figure 14) include phenylpropanoic acid (383), coumarins (384–385) and isocoumarins (386–409), and lignans (410–420). Among these, isocoumarins and lignans are the main compounds, originating from endophytic fungi associated with the Bruguiera genus and Bruguiera plants, respectively. Previous research has indicated that plant-derived coumarins have the potential to resist infections in both plants and animals [122], particularly scopoletin (384). When different plant species are exposed to multiple pathogens such as bacteria, fungi, oomycetes, and viruses, it can lead to the accumulation of 384, thereby enhancing their resistance to diseases [123]. So, compound 384 in B. gymnorrhiza plants may serve as a crucial participant in the plant’s chemical defense strategy against various pathogen invasions, contributing to the plant’s ability to resist diseases.

Table 11.
Phenylpropanoids isolated from Bruguiera genus plants and their endophytes.
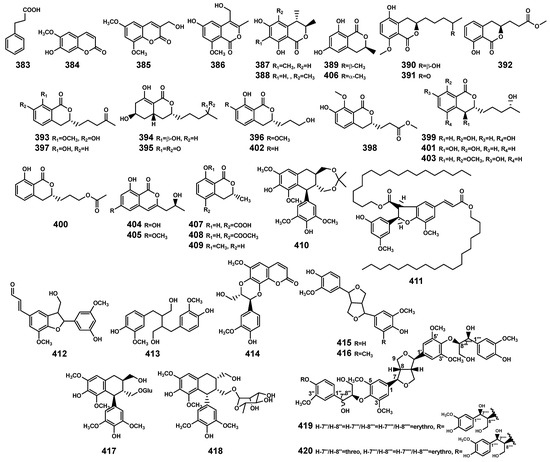
Figure 14.
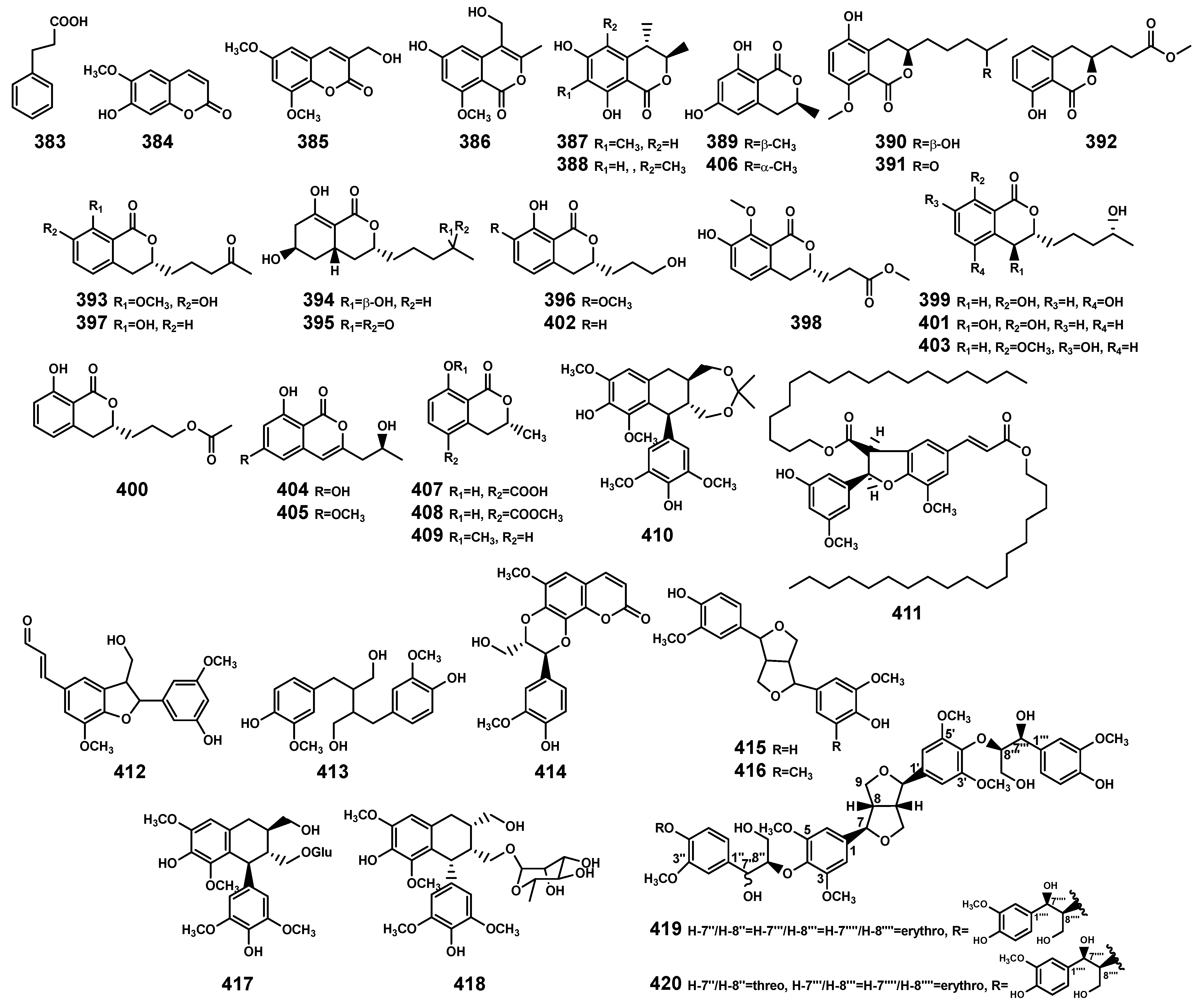
Phenylpropanoids (383–420) isolated from Bruguiera genus plants and their endophytes.
2.9. Aromatic Compounds
The aromatic compounds (Table 12 and Figure 15) are prevalent in both plants and endophytic fungi secondary metabolites. The majority of the aromatic compounds summarized in this study are phenolic-related aromatic compounds. Previous reports have highlighted the crucial role of phenolic compounds in plants’ defense against various biotic and abiotic stresses [129]. In mangrove plants, some compounds have been identified as substrates of fungal metabolism or (and) signals of plant origin, such as compounds 431, 435, and 436. Additionally, there are other types of aromatic compounds present, including biphenyl derivatives (451–453), (iso) benzopyrans (454–458), chromones (459–465), (iso) benzofurans (467–468), and (iso) benzofuranones (471–474), among others.

Table 12.
Aromatic compounds isolated from Bruguiera genus plants and their endophytes.
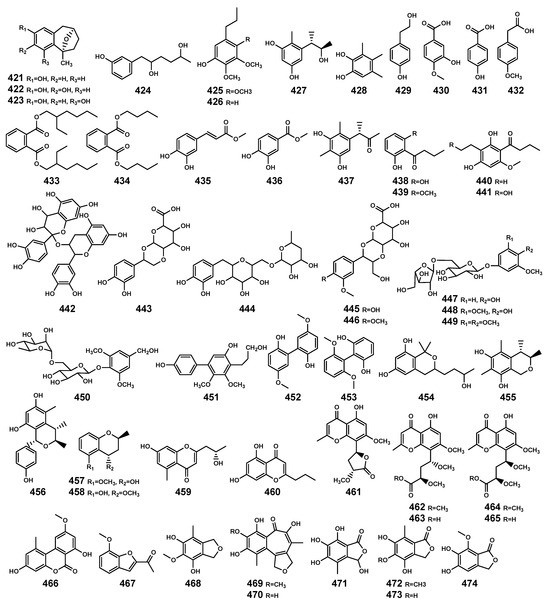
Figure 15.
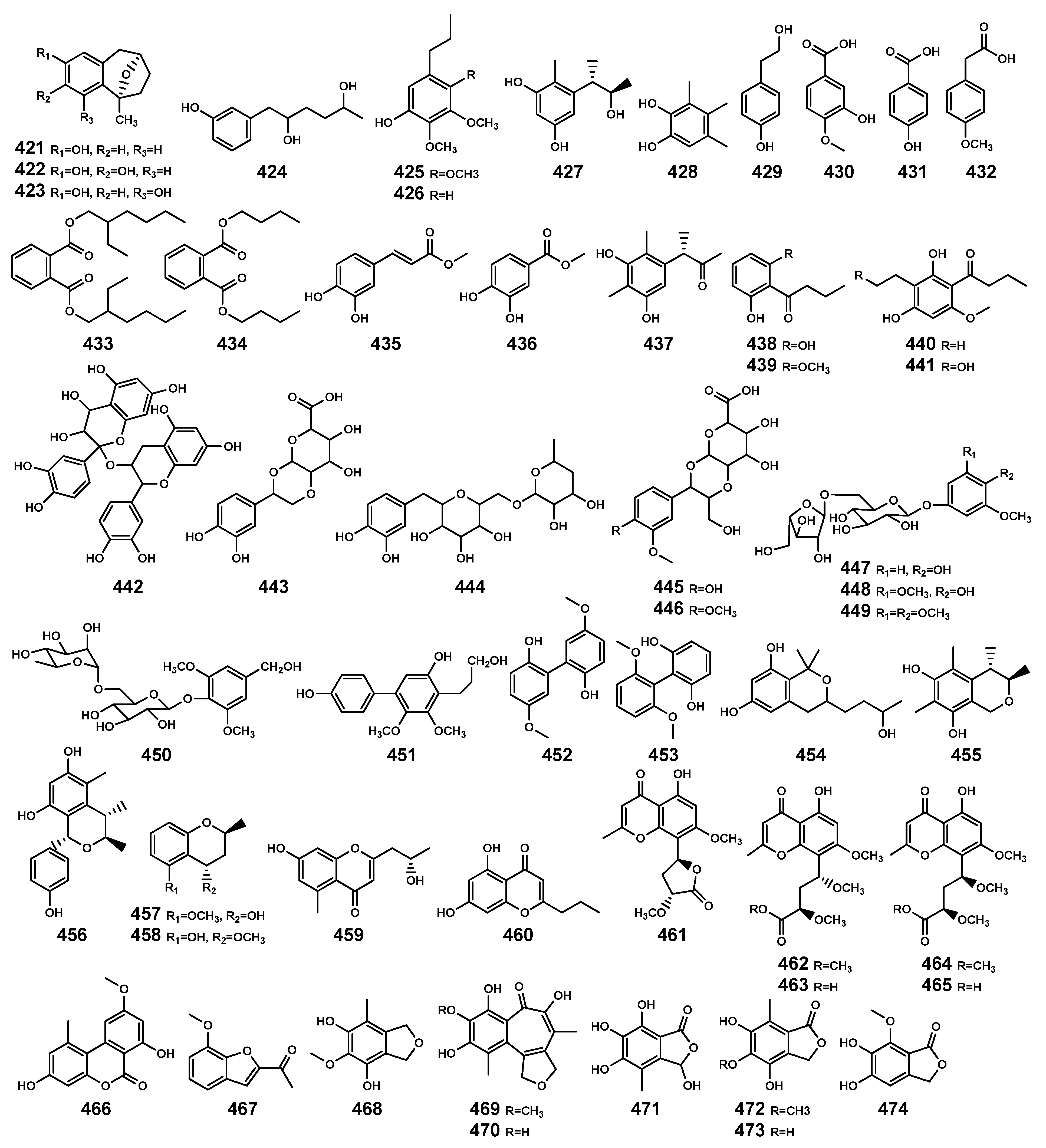
Aromatic compounds (421–474) isolated from Bruguiera genus plants and their endophytes.
2.10. Other Compounds
Apart from the previously mentioned compounds, fatty acids (475–486), 2H-pyran-2-ones (487–490), alcohols (491–493), and additional ones (494–496) were also identified (Table 13 and Figure 16). Specifically, the secondary metabolite 495, originating from the endophytic fungus Aspergillus terreus, was discovered for the first time in the ripe fruits of Garcinia cowa [134,135]. It belonged to polyprenylated benzoylphloroglucinols with a unique tetracyclo[7.3.3.33,11.03,7]tetradecane-2,12,14-trione skeleton, and exhibited significant anti-inflammatory and alpha-glucosidase inhibition activities [135].

Table 13.
Other compounds isolated from Bruguiera genus plants and their endophytes.
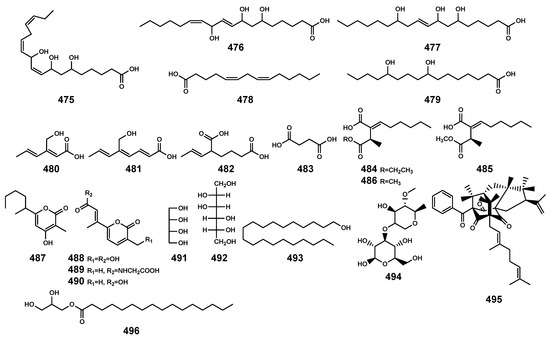
Figure 16.
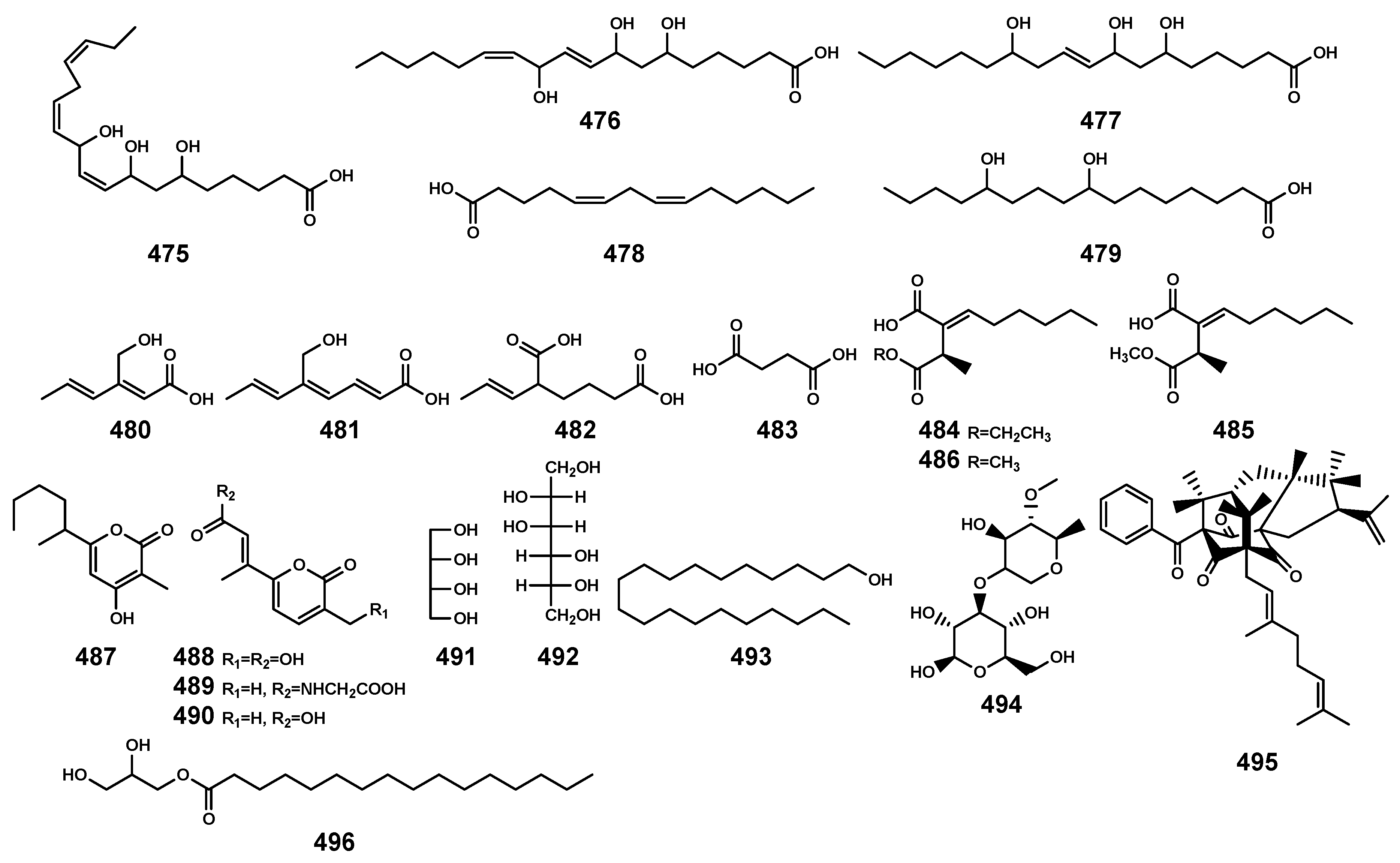
Other compounds (475–496) isolated from Bruguiera genus plants and their endophytes.
3. Bioactivities
3.1. Cytotoxic Activity
Cytotoxic activity stands out as a pharmacological attribute of secondary metabolites derived from Bruguiera genus plants and their associated endophytes. The n-butanol extract from B. gymnorrhiza showed antitumor activity against A-549 and HL-60 [76]. Methanol extract of B. gymnorrhiza displayed selective cytotoxicity against breast ductal carcinoma cells (MDA-MB-435S) with IC50 1.38 mg/mL [137]. Leaves extract of B. sexangula demonstrated inhibitory effect on the proliferation of gastric cancer cells in both in vitro and in vivo studies [138]. Qayoom et al. [139] utilized network pharmacology methods and unveiled that brugine (197) possesses significant anti-breast cancer activity, exerting its effects through various pathways such as the calcium signaling pathway, cAMP signaling pathway, PI3K-Akt pathway, and others. Additionally, anticancer compounds have been identified in B. gymnorrhiza plants and the endophytic fungi of both B. gymnorrhiza and B. sexangula var. rhynchopetala. The lethality assay using brine shrimp (Artemia salina L.) reveals significant cytotoxicity in B. gymnorrhiza extracts and certain compounds [9,140]. Table 14 provides a summary of the cytotoxic activity of monomeric compounds.

Table 14.
Cytotoxic activity of the compounds isolated from Bruguiera genus and its endophytes.
3.2. Antimicrobial Activity
Previous studies have indicated that crude extracts of B. gymnorrhiza exhibit substantial antibacterial activity against a range of bacteria, including the fungal pathogen Candida albicans and bacterial pathogens, such as Micrococcus sp., Staphylococcus aureus (MTCC 3160), Klebsiella pneumoniae (MTCC 4030), Escherichia coli (MTCC 42), E. coli (NX, AMP, OF resistant strain) [141]. Bibi Sadeer et al. [142] found that ethyl acetate extract of B. gymnorrhiza twig, when used in combination with streptomycin and ciprofloxacin, respectively, potentiates their inhibitory effects against MRSA and P. aeruginosa. Ethyl acetate extracts obtained from mature leaves, immature leaves, and the bark of B. sexangula reveal antibacterial properties against S. aureus [143]. Currently, numerous compounds isolated from B. gymnorrhiza and endophytes of plants (B. gymnorrhiza, B. sexangula, B. sexangula var. rhynchopetala, and B. parviflora) have shown considerable antibacterial activity, as presented in Table 15.

Table 15.
Antimicrobial activity of the compounds isolated from Bruguiera genus and its endophytes.
3.3. Antioxidant Activity
Studies have revealed significant antioxidant activity in extracts derived from B. gymnorrhiza, B. sexangula, and B. cylindrica [10,144,145]. The antioxidant and polyphenol-rich leaves of B. gymnorrhiza can exert hepatoprotective effects by ameliorating liver tissue injury [7]. Condensed tannins from B. gymnorrhiza possessed notable anti-tyrosinase and antioxidant capabilities, effectively inhibiting browning reactions in fresh-cut lotus roots [146]. Ethanol extract of B. sexangula leaves had antioxidant and melanin inhibition activities without skin irritation [145]. In the in vitro antioxidant activity studies, compounds 469, 470, 471, 472, 473, 468, and 328 showed DPPH radical scavenging activity with IC50 values of 57.6, 26.5, 29.3, 85.2, 16.5, 53.1, and 14.7 µM, and ABTS radical scavenging activity with IC50 values of 46.4, 29.2, 23.7, 43.1, 23.3, 24.0, and 18.8 µM, respectively [113]. Yao et al. [121] conducted the cellular antioxidant assay and identified that compound 381 exhibited antioxidant activity with an EC50 value of 11.79 μM. Compound 382 exhibited effective neuroprotective activity against 1-methyl-4-phenylpyridinium-induced oxidative damage in PC12 cells, and its mechanism involved inhibiting apoptosis in PC12 cells through the mitochondrial pathway [117]. It also attenuated oxidative stress and inflammatory responses induced by lipoteichoic acid in embryonic rat heart cells (H9c2) [147].
3.4. Anti-Inflammatory Activity
Studies have indicated the anti-inflammatory activity in extracts from plants within the Bruguiera genus, such as B. gymnorrhiza, B. sexangula, and B. parviflora, as well as endophytic fungi associated with B. gymnorrhiza. Chen et al. [148] conducted research revealing that aqueous extract of B. gymnorrhiza leaves can alleviate dextran sulfate sodium (DSS)-induced ulcerative colitis by inhibiting of NF-κB activation and modulating the gut microbiota. Subsequently, Lin et al. [149] observed that aqueous extracts from B. gymnorrhiza fruit also exhibited a protective effect against DSS-induced ulcerative colitis, and the mechanism may be associated with the attenuation of inflammation, activation of the Keap1/Nrf2 signaling pathway, and modulation of the gut microbiota. Furthermore, Zhang et al. [150] reported that methanol extracts of B. gymnorrhiza fruits mitigate inflammation in gastric injury by activating the NF-κB pathway, thus conferring gastroprotective effects. Moreover, consumption of B. gymnorrhiza fruits has been shown to ameliorate systemic inflammations in obesity, increase circulating satiety hormones, reduce lipid profiles, elevate short-chain fatty acids levels, and promote weight loss [151]. Eldeen et al. [152] discovered that metabolites obtained from B. cylindrica possessed inhibitory effects on pro-inflammatory enzymes, including 5-lipoxygenase, cyclooxygenase, and acetylcholinesterase, and on the growth of an induced rheumatoid arthritis synovial fibroblasts. According to reports, compound 367 may serve as the potential primary anti-inflammatory substance in B. gymnorrhiza leaves through various possible mechanisms, such as regulation of oxidative stress, suppression of arachidonic acid metabolism, and downregulation of pro-inflammatory cytokines by inhibiting NF-κB [120]. Ukwatta et al. [101,135] employed a cell-based assay for THP-1 cytokine-release assay to quantify the anti-inflammatory activity of compounds 242 and 495, showcasing IC50 values of 6.2 μM and 12.1 µg/mL, respectively. Furthermore, compounds 368, 369, 370, 378, and 371 from B. parviflora leaves exhibited significant inhibitory effects on the inflammatory response of the RAW 264.7 cells induced by lipopolysaccharide with a range of NO production between 11.77 and 13.92 μM, at the concentration of 100 μg/mL [52].
3.5. Antiviral Activity
Hou et al. [153]detected that endophytic fungal strains (GXIMD07366, GXIMD07616, GXIMD07384, GXIMD07550, GXIMD07445X) from B. gymnorrhiza have anti-hepatitis B virus (HBV) activity. At a concentration of 125 µg/mL, their extracts significantly reduced the HBV-DNA levels in the supernatant of HepG2.2.15 cells, and the inhibition rates were 51%, 47%, 63%, 52%, and 47%, respectively. Compounds 212–218 derived from the hypocotyls of B. gymnorrhiza displayed moderate anti-HBV activity [91]. Furthermore, the compound 186 exhibited anti-HBV activity, with IC50 values of 4.37 mmol/L for HbsAg and 4.89 mmol/L for HBeAg, and therapeutic indices of 2.68 and 2.40 [85]. In addition to anti-HBV activity, compound 44 showed selective anti-HIV activity [30]. It is worth noting that the compound 356 demonstrated moderate antiviral activity against the pathogen SARS-CoV-2, the causative agent of COVID-19, with an EC50 value of 29.0 µM [112]. Aqueous extracts from the roots and fruits of B. gymnorrhiza inhibited Zika virus (ZIKV) infection at non-cytotoxic concentrations [154]. Moreover, B. cylindrica-synthesized AgNP, at a concentration of 30 μg/mL, significantly suppressed the production of dengue viral E protein and downregulated the expression of dengue viral E gene in Vero cells [155].
3.6. Antidiabetic Activity
The decoctions of both roots and leaves of B. gymnorrhiza have demonstrated varying degrees of antidiabetic activity, ranging from low to moderate efficacy [156]. B. gymnorrhiza leaf extracts displayed inhibitory effects on α-glucosidase, with an IC50 value of 2.670 mg/mL [144]. Extracts derived from B. cylindrica leaves contained antidiabetic components, and the ethanol extract of which was considered potential sources of novel bioactive compounds for treating type 2 diabetes [157,158]. Notably, several compounds, including 108, 117, 107, 368, 369, 370, 378, 371, and 467 exhibited significant α-glucosidase inhibitory activity, with IC50 values ranging from 1.1 to 98.0 μg/mL (better than the positive control acarbose) [52,103]. Compounds 242, 256, and 495 also show α-glucosidase inhibitory activity, with IC50 values of 6.9 μM, 5.7 μg/mL, and 7.8 μM, respectively [101,103,135]. Additionally, through in vitro bioactivity assays, it was found that compounds 177 and 174 exhibited significant inhibitory activity against the target molecule associated with type II diabetes, human protein tyrosine phosphatase 1B (PTP1B), with IC50 values of 14.9 and 17.6 μM, respectively [72,80,159].
3.7. Insecticidal and Mosquito Repellent Activity
Currently, in the Bruguiera genus and its endophytes, researchers have primarily investigated the anti-plasmodial, anti-Caenorhabditis elegans, and mosquito-repellent activities. Bai et al. [61] identified insecticidal activity in meroterpenoids and isocoumarin compounds obtained from the fungus Penicillium sp. TGM112, isolated from B. sexangula var. rhynchopetala. Compounds 133, 134, 135, 137, 140, 141, 143, 394, 395, 398, 399, and 401 showed insecticidal activity against newly hatched larvae of Helicoverpa armigera Hubner, with IC50 values ranging from 50 to 200 μg/mL [61,62]. Compounds 133, 134, 135, 136, 137, 138, and 139 displayed anti-C. elegans activity, with EC50 values ranging from 9.4 to 38.2 μg/mL [61]. These types of compounds are expected to be potential candidates for effective and low-toxicity novel biopesticides [62]. The leaf and hypocotyl extracts of B. cylindrica exhibited in vitro antiplasmodial activities, with IC50 values of 173.75 and 74.81 μg/mL, respectively [160,161]. And compound 110 showed anti-malarial activity, with an EC50 value of 8.6 mg/mL [54]. Compound 364 significantly reduced the lifespan of C. elegans [118]. Compound 479 displays good nematocidal activity [136]. Compound 495 had anti-filarial activity, with MIC, IC50, and LC50 values of 0.358, 0.708, and 3.89 mg/mL, respectively [135]. Murugan et al. [155] discovered that B. cylindrica-synthesized AgNP had mosquito larvicidal properties and effectively reduced the populations of Aedes aegypti larvae and pupae when applied at low doses.
3.8. Enzyme Inhibitory Activity
Homhual et al. [46,79] discovered that in stably transfected HepG2 cells, compounds 91–93 and 172–174 from the flowers of B. gymnorrhiza activated antioxidant response elements (ARE) luciferase activity, with respective EC50 values were 7.8, 9.4, 15.7, 3.7, 1.8, and 56.7 µM. Compounds 91, 172, and 173 were found to inhibit phorbol ester-induced NF-κB luciferase activity, with IC50 values of 1.4, 85.0, and 14.5 μM, respectively [46,79]. Compounds 91 and 172 also exhibited inhibitory effects on cyclooxygenase-2 (COX-2) activity, with IC50 values of 0.37 and 6.1 μM, respectively [46,79]. In vitro bioactivity tests revealed that compounds 126, 129, 130, 163, 226, 250, and 251 significantly inhibited α-acetylcholinesterase (AChE) with IC50 values of 84.26, 5.28, 12.00, 1.89, 3.09, 2.01, and 6.71 µM, respectively [58,69]. Compound 348 demonstrated potent activity against acetylcholinesterase, with an IC50 value of 40.26 µM [114].
3.9. Other Activities
In addition to the aforementioned biological activities, it has also been reported to possess analgesic, anti-diarrheal, anti-hemolytic activities, and various other pharmacological effects.
The extracts from B. gymnorrhiza stem, leaf, and hypocotyl have demonstrated significant analgesic and anti-diarrheal effects [144,162]. When administered at doses of 250 and 500 mg/kg body weight, the leaf and hypocotyl extracts exhibited a remarkable inhibitory effect on castor oil-induced diarrhea mice [144]. Moreover, B. cylindrica extracts have shown the ability to inhibit H2O2-induced hemolysis of bovine erythrocytes [10]. The methanol extract of B. gymnorrhiza also exhibited anti-hemolytic activity with an IC50 value of 311.28 μg/mL [9]. Extracts from B. gymnorrhiza, prepared using different solvents (n-hexane, ethyl acetate, n-butanol, and an aqueous phase), and from leaves of different ages (senescent, mature, and young leaves), exhibited significant inhibitory effects on algae growth [34,163].
4. Interactions between Bruguiera Genus and Its Endophytes
Plants constitute a complex ecological community, engaging in symbiotic relationships with endophytic fungi, wherein they coexist and evolve together over an extended period [164]. Recent study has reported that the endophytic Streptomyces parvulus VCCM 22513 from B. gymnorrhiza exhibited significant adaptive responses to abiotic environmental stressors, including antioxidation, salt tolerance, and degradation of aromatic compounds [165]. Moreover, through the review of the literature, it has been observed that both the Bruguiera genus and its associated endophytes produce similar or closely related secondary metabolites, including cholesterol (153), cytochalasin D (296), zygosporin D (297), and dibutylphthalate (434). This phenomenon may be attributed to the fact that the plants flourish in high-salinity and waterlogged environments. In response to the complex environmental stresses, endophytes associated with Bruguiera genus have evolved similar signaling pathways to their host counterparts, enabling information exchange [164,166,167]. This allows the endophytes to elicit defense responses akin to those of the Bruguiera genus plants, consequently, synthesize the shared metabolites.
In the genus Bruguiera and its endophytic fungi, cytochalasins (296–314) were isolated from various sources, including B. gymnorrhiza (296–297), endophytic fungus Xylaria arbuscula GZS74 from B. gymnorrhiza (296–297, 300–314), endophytic fungus Daldinia eschscholtzii HJ001 from B. sexangula var. rhynchopetala (298–299), and endophytic fungus Xylaria cubensis PSU-MA34 from B. parviflora (296). Cytochalasins are typically derived from the Xylaria genus of endophytic fungi [168]. Structurally, they consist of a highly substituted isoindolone ring, featuring a benzyl group at C-3 and fused to an 11- to 14-membered macrocyclic ring [169]. According to their structure–activity relationship, one of the significant toxic structural features of cytochalasins is the presence of a complete perihydroisoindolyl-1-one motif fused to either a [11] or a [13] carbocyclic or a [14] lactone macrocycle ring [169]. Additionally, the C7-OH group may also exhibit toxicity depending on the crop species, sensitivity, and type of cytochalasins [169]. Compound 296 exhibits inhibitory activity against plant pathogens such as Botrytis cinerea (at a concentration of 25 μg/disc) [170], Cladosporium cladosporioides (at a concentration of 10 μg/mL) [171], C. sphaerospermum (at a concentration of 25 μg/mL) [171], and C. gloeosporioides (MIC value of 2.46 μmol/mL) [168]. Compound 297 effectively inhibits the shoot elongation of rice seedlings [172]. Compounds 307 and 314 both display nonspecific moderate phytotoxicity against monocotyledonous plant bentgrass and dicotyledonous plant lettuce [173]. Evidently, endophytic fungi of the Bruguiera genus aid in plant growth competition and defense against plant pathogens by producing cytochalasins with phytotoxic and antifungal activities, thereby providing protection for Bruguiera species’ growth.
Simultaneously, existing research confirms that dibutylphthalate (434), a secondary metabolite derived from the B. gymnorrhiza plant and its endophytic fungus Penicillium thomi, also serves as the primary active metabolite of endophyte Bacillus sp. KL5 from the plant Rumex dentatus, exhibits significant antagonistic activity against plant pathogens Fusarium oxysporum and Verticillium dahliae, making it a promising candidate for the prevention and control of postharvest diseases Fusarium root rot in potato [174]. Compound 434 may therefore also contribute to bolstering the B. gymnorrhiza plant’s resilience against plant pathogens.
In addition to the shared compounds mentioned above, specific secondary metabolites have been identified in Bruguiera genus plants and their endophytes that play crucial roles in promoting plant growth and development, as well as enhancing the plant’s resistance to both biotic and abiotic stresses, like 2,6-dimethoxy-1,4-benzoquinone (230), scopoletin (384), erythritol (491), and mannitol (492).
According to the research analysis by Laohavisit et al. [95], quinone molecules may serve as pathogen or danger-associated molecular patterns, such as 2,6-dimethoxy-1,4-benzoquinone (230). This quinone signal is perceived by leucine-rich-repeat receptor-like kinases, triggering the expression of defense-related genes and immune responses against bacterial pathogens [95]. Compound 230 produced by host plants can also act as an inducing factor for the development of root parasitic plant absorbers, potentially playing a regulatory role during the parasitic process of these plants [175].
Scopoletin (384), as a crucial component in the plant’s natural immune response, plays a role in resisting the invasion of pathogenic microorganisms and promoting the proliferation of beneficial microbes [123]. In healthy plant leaves, due to the instability and toxicity of scopoletin, it is converted into the glycosylated form, scopolin, by glucosyltransferases, then transferred intracellularly and stored in vacuoles, with their accumulation typically maintained at low levels [123]. Upon activation of the leaf defense system by pathogens (fungi, bacteria, and viruses, etc.) or elicitors (flg22, MYB15, MPK3, etc.), scopolin is released from the vacuoles in infected tissues [123,176]. It is then converted to scopoletin by β-glucosidases, leading to an increase in scopoletin production [176]. This, in turn, exhibits antimicrobial activity and clears H2O2 in infected tissues to prevent cell death [176]. The level of disease resistance is correlated with the extent and timing of scopoletin accumulation [177]. The toxic effects of scopoletin may be attributed to the presence of methoxy (-OCH3) and hydroxy (-OH) groups on the benzene ring [123]. Upon glycosylation, scopoletin is confined by the cell wall, limiting its toxicity [178]. Depending on the environmental conditions, scopolin and scopoletin in tissues of B. gymnorrhiza plant can mutually convert within cells, thereby regulating the level of scopoletin to aid in resisting pathogen invasion.
The compound erythritol (491) with antibacterial activity, is one of the main chemical components of the endophytic fungus Lasiodiplodia pseudotheobromae APR5 [179], and it is also produced by the endophytic fungus P. citrinum ZD6 within the stems of the Chinese medicinal plant B. gymnorrhiza [92]. The endophytic fungus L. pseudotheobromae APR5, originating from the host plant Andrographis paniculata, effectively inhibits the growth of plant pathogenic fungi [179]. Moreover, it produces the plant hormone IAA (indole-3-acetic acid) and iron carrier, promoting the growth and development of the host plant and enhancing its resistance to adverse environmental conditions [179]. Furthermore, the genus Penicillium has been confirmed to enhance the resistance of host plants to both biotic and abiotic stresses [180]. As a major member of endophytic fungi within the Bruguiera genus, Penicillium (26.3%) undoubtedly plays a crucial role in conferring resistance to pathogenic fungal invasion in Bruguiera genus plants.
Mannitol (492) is present in B. gymnorrhiza plant and its endophytic fungus P. citrinum ZD6 [92], serving as both an osmoprotectant and an antioxidant against oxidative stress [181]. However, research suggests that fungal pathogens also secrete mannitol, playing a role in fungal pathogenicity [181]. Mannitol can protect fungal pathogens against plant defense mechanisms based on reactive oxygen [182]. Given the complexity of the existing interactions, the specific mechanisms underlying these processes have not been elucidated as of now.
In summary, utilizing secondary metabolites as a starting point for research, exploring the interactive relationships between the Bruguiera genus plants and their endophytic fungi presents an intriguing direction. To delve deeper into the mechanisms underlying the interaction between Bruguiera plants and their endophytic fungi, modern “omics” tools, including genome sequencing, comparative genomics, microarrays, next-generation sequencing, metagenomics, metatranscriptomics, and others, can be integrated with various systems biology techniques to explore the role of endophytic fungi in the ecology of Bruguiera plants [183].
5. Conclusions
The Bruguiera genus encompasses a diverse range of plant species housing a wide array of endophytic fungal communities. These organisms are prolific producers of secondary metabolites, which manifest extensive pharmacological effects. Many compounds derived from these sources have demonstrated notable biological activities. Despite the discovery of numerous pharmacological effects, a more comprehensive investigation into the underlying mechanisms is still imperative. The presence of certain compounds in both plants and their endophytes suggests the potential for further exploration into the interaction mechanisms between Bruguiera plants and their endophytic fungi. Future research endeavors can be directed towards developing highly active lead compounds with therapeutic potential from the Bruguiera genus and its endophytes.
Author Contributions
Conceptualization, X.C., X.L. and X.J.; writing—original draft preparation, X.C. and L.Z.; writing—review and editing, X.C. and X.L.; visualization, B.L., L.X. and M.Z.; resources, X.L., X.J. and Y.M., X.L. and X.C. contributed equally to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31670349, and the Young Scientists Fund of the National Natural Science Foundation of China, grant number 81802678.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to express our gratitude to Jun Wu from Guangdong Medical University for his guidance and assistance in writing this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, B.S.; Liang, S.C.; Zhang, W.Y.; Zan, Q.J. Mangrove flora of the world. Acta Bot. Sin. 2003, 45, 644–653. [Google Scholar]
- Lin, P. Distribution of mangrove species. Sci. Silvae Sin. 1987, 23, 481–490. [Google Scholar]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zhang, X.; Wang, J.S.; Shen, X.X.; Tang, L.L.; Li, R.L. Utilization Status and Development Countermeasures of Mangrove Medicinal Resources in the Marine-Terrestrial Interlaced Zone. Acta Sci. Nat. Univ. Pekin. 2023, 59, 704–718. [Google Scholar]
- Bibi, S.N.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Kannan, R.R.R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves—A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Sani, W.; Hossain, A.B.M.S.; Taha, R.M.; Monneruzzaman, K.M. Total phenolic contents, antioxidant and antimicrobial activities of Bruguiera gymnorrhiza. J. Med. Plants Res. 2011, 5, 4112–4118. [Google Scholar]
- Sur, T.K.; Hazra, A.; Hazra, A.K.; Bhattacharyya, D. Antioxidant and hepatoprotective properties of Indian Sunderban mangrove Bruguiera gymnorrhiza L. leave. J. Basic Clin. Pharm. 2016, 7, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Karimulla, S.; Kumar, B.P. Anti diabetic and Anti hyperlipidemic activity of bark of Bruguiera gymnorrhiza on streptozotocin induced diabetic rats. Asian J. Pharm. Sci. Technol. 2011, 1, 4–7. [Google Scholar]
- Karim, M.A.; Islam, M.A.; Islam, M.M.; Rahman, M.S.; Sultana, S.; Biswas, S.; Hosen, M.J.; Mazumder, K.; Rahman, M.M.; Hasan, M.N. Evaluation of antioxidant, anti-hemolytic, cytotoxic effects and anti-bacterial activity of selected mangrove plants (Bruguiera gymnorrhiza and Heritiera littoralis) in Bangladesh. Clin. Phytosci. 2020, 6, 8. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Sasikumar, J.M.; Shamna, R.; Pandiarajan, C.; Sofia, P.; Nagarajan, B. Antioxidant activities of bark extract from mangroves, Bruguiera cylindrica (L.) Blume and Ceriops decandra Perr. Indian J. Pharmacol. 2011, 43, 557–562. [Google Scholar]
- Loder, J.W.; Russell, G.B. Tropine 1,2-dithiolane-3-carboxylate, a new alkaloid from Bruguiera sexangula. Tetrahedron Lett. 1966, 51, 6327–6329. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Wang, D.N.; Ming, X.C.; Li, W.; Han, B.; Zhao, J.; Huang, G.L. Recent Advances of Chemical Constituents of Genus Bruguiera. Guangdong Chem. Ind. 2014, 41, 45–46,50. [Google Scholar]
- Xie, L.H.; Hou, X.T.; Deng, J.G.; Wei, L.Y.; Xia, Z.S.; Du, Z.C. Research Progress on Chemical Constituents and Pharmacological Effect of Bruguiera gymnorrhiza. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 225–234. [Google Scholar]
- Chen, S.H.; Cai, R.L.; Liu, Z.M.; Cui, H.; She, Z.G. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2021, 39, 560–595. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, S.; Luo, X.M.; Liu, Y.H. Preparative isolation and purification of two benzoxazinoid glucosides from Acanthus ilicifolius L. by high-speed counter-current chromatography. J. Chromatogr. A 2008, 1205, 177–181. [Google Scholar] [CrossRef]
- Gao, C.H.; Tian, X.P.; Qi, S.H.; Luo, X.M.; Wang, P.; Zhang, S. Antibacterial and antilarval compounds from marine gorgonian-associated bacterium Bacillus amyloliquefaciens SCSIO 00856. J. Antibiot. 2010, 63, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Luo, M.Q.; Xia, M.; Wu, Q.; Long, S.M.; Hu, Y.H.; Gao, G.C.; Yao, X.L.; He, M.; Su, H.X.; et al. Marine Compound Catunaregin Inhibits Angiogenesis through the Modulation of Phosphorylation of Akt and eNOS in vivo and in vitro. Mar. Drugs 2014, 12, 2790–2801. [Google Scholar] [CrossRef]
- Tang, H.; Ge, H.; Chen, Z.B.; Luo, X.M.; Su, F.J.; Liang, Y.B.; Li, Z.Y.; Wu, J.G.; Yang, Q.; Zeng, L.J.; et al. Micrometam C Protects against Oxidative Stress in Inflammation Models in Zebrafish and RAW264.7 Macrophages. Mar. Drugs 2015, 13, 5593–5605. [Google Scholar] [CrossRef]
- Liu, B.; Jin, X.B.; Chen, X.H.; Wang, X.; Zhang, W.B.; Luo, X.M. Two New Lactam Derivatives from Micromelum falcatum (Lour.) Tan. with Brine Shrimp Larvae Toxicity. Molecules 2023, 28, 7157. [Google Scholar] [CrossRef]
- Jia, S.L.; Chi, Z.; Liu, G.L.; Hu, Z.; Chi, Z.M. Fungi in mangrove ecosystems and their potential applications. Crit. Rev. Biotechnol. 2020, 40, 852–864. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Li, X.M.; Yang, S.Q.; Meng, L.H.; Wang, B.G. Thiocladospolides A−D, 12-Membered Macrolides from the Mangrove-Derived Endophytic Fungus Cladosporium cladosporioides MA-299 and Structure Revision of Pandangolide 3. J. Nat. Prod. 2019, 82, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Baunach, M.; Ding, L.; Peng, H.Y.; Franke, J.; Hertweck, C. Biosynthetic Code for Divergolide Assembly in a Bacterial Mangrove Endophyte. Chembiochem 2014, 15, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Nguyen, L.T.; Dhakal, R.; Murphy, B.T. The need to innovate sample collection and library generation in microbial drug discovery: A focus on academia. Nat. Prod. Rep. 2021, 38, 292–300. [Google Scholar] [CrossRef]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How different are marine microbial natural products compared to their terrestrial counterparts? Nat. Prod. Rep. 2022, 39, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, B.Y.; Yang, X.L. Antifungal Monoterpene Derivatives from the Plant Endophytic Fungus Pestalotiopsis foedan. Chem. Biodivers. 2016, 13, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Toyota, M.; Asakawa, Y.; Takaso, T.; Tobe, H. Floral scent chemistry of mangrove plants. J. Plant Res. 2002, 115, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Tricyclic and Spirobicyclic Norsesquiterpenes from the Endophytic Fungus Pseudolagarobasidium acaciicola. Eur. J. Org. Chem. 2014, 2014, 3976–3980. [Google Scholar] [CrossRef]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cytotoxic sesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Phytochemistry 2016, 122, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Münich, J.; Goerls, H.; Maier, A.; Fiebig, H.H.; Lin, W.H.; Hertweck, C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg. Med. Chem. Lett. 2010, 20, 6685–6687. [Google Scholar] [CrossRef]
- Ding, L.; Goerls, H.; Dornblut, K.; Lin, W.; Maier, A.; Fiebig, H.H.; Hertweck, C. Bacaryolanes A-C, Rare Bacterial Caryolanes from a Mangrove Endophyte. J. Nat. Prod. 2015, 78, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Hertweck, C. Oxygenated Geosmins and Plant-like Eudesmanes from a Bacterial Mangrove Endophyte. J. Nat. Prod. 2020, 83, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Görls, H.; Hertweck, C. Plant-like Cadinane Sesquiterpenes from an Actinobacterial Mangrove Endophyte. Magn. Reson. Chem. MRC 2021, 59, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiao, H.; Sun, D.; Duan, S.S. Investigation of the Inhibitory Effects of Mangrove Leaves and Analysis of Their Active Components on Phaeocystis globosa during Different Stages of Leaf Age. Int. J. Environ. Res. Public Health 2018, 15, 2434. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.S.; Liu, H.L.; Gong, J.X.; Guo, Y.W. Chemical constituents of mangrove plant Bruguiera gymnorrhiza. Chin. J. Mar. Drugs 2011, 30, 15–18. [Google Scholar]
- Deng, C.M.; Huang, C.H.; Wu, Q.L.; Pang, J.Y.; Lin, Y.C. A new sesquiterpene from the mangrove endophytic fungus Aspergillus terreus (No. GX7-3B). Nat. Prod. Res. 2013, 27, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.D.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Sun, Y.W.; Liu, H.L.; Wang, Y. Pathway elucidation and engineering of plant-derived diterpenoids. Curr. Opin. Biotechnol. 2021, 69, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.N.; Sircar, S.M. Gibberellins from mangrove plants. Phytochemistry 1974, 13, 1911–1913. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Rao, B.V.; Ward, R.S.; Hursthouse, M.B.; Hibbs, D.E. Diterpenes from the marine mangrove Bruguiera gymnorhiza. Phytochemistry 1999, 51, 83–90. [Google Scholar] [CrossRef]
- Bao, S.Y.; Deng, Z.W.; Fu, H.Z.; Proksch, P.; Lin, W.H. Diterpenes and Disulfides from the Marine Mangrove Plant Bruguiera sexangula var. rhynchopetala. Helv. Chim. Acta 2005, 88, 2757–2763. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.S.; Sattler, I.; Dahse, H.M.; Fu, H.Z.; Lin, W.H.; Grabley, S. New Diterpenoids from the Marine Mangrove Bruguiera gymnorrhiza. J. Nat. Prod. 2004, 67, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Huang, X.S.; Sattler, I.; Dahse, H.M.; Fu, H.Z.; Grabley, S.; Lin, W.H. Three new pimaren diterpenoids from marine mangrove plant, Bruguiera gymnorrhiza. Pharmazie 2005, 60, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Liu, Y.; Li, S.H. The untapped potential of plant sesterterpenoids: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.; Li, C.; Wang, B. Sesterterpenes and 2H-Pyran-2-ones (=α-Pyrones) from the Mangrove-Derived Endophytic Fungus Fusarium proliferatum MA-84. Helv. Chim. Acta 2013, 96, 437–444. [Google Scholar] [CrossRef]
- Homhual, S.; Bunyapraphatsara, N.; Kondratyuk, T.; Herunsalee, A.; Chaukul, W.; Pezzuto, J.M.; Fong, H.H.S.; Zhang, H.J. Bioactive Dammarane Triterpenes from the Mangrove Plant Bruguiera gymnorrhiza. J. Nat. Prod. 2006, 69, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Li, L.A.; Huang, C.G.; Wang, C.Y.; Guo, Y.W. Sexangulic acid, a new cytotoxic triterpenoid from the Chinese mangrove Bruguiera sexangula. Nat. Prod. Res. 2010, 24, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Huang, G.L.; Tang, X.Z.; Wang, D.N.; Gong, X.L.; Zhang, Q.; Song, X.P.; Chen, G.Y. Secondary Metabolites and Antibacterial Activities of a Bruguiera sexangula var. Rhynchopetala-Derived Fungus Penicillium sp. (J41221). Chin. J. Org. Chem. 2014, 34, 1172–1176. [Google Scholar] [CrossRef]
- Sarkar, A.; Ganguly, S.N. Gymnorhizol, a new triterpene alcohol from Brugiera gymnorhiza. Chem. Informationsdienst 1979, 10, 138. [Google Scholar]
- Ghosh, A.; Misra, S.; Dutta, A.K.; Choudhury, A. Pentacyclic triterpenoids and sterols from seven species of mangrove. Phytochemistry 1985, 24, 1725–1727. [Google Scholar] [CrossRef]
- Luo, D.Q.; Deng, H.Y.; Yang, X.L.; Shi, B.Z.; Zhang, J.Z. Oleanane-Type Triterpenoids from the Endophytic Fungus Pestalotiopsis clavispora Isolated from the Chinese Mangrove Plant Bruguiera sexangula. Helv. Chim. Acta 2011, 94, 1041–1047. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, K.P.T.; Nguyen, P.P.K.; Le, D.T.; Nguyen, T.L. Anti-Inflammatory and α-Glucosidase Inhibitory Activities of Chemical Constituents from Bruguiera parviflora Leaves. J. Chem. 2022, 2022, 3049994. [Google Scholar] [CrossRef]
- Li, F.; Li, X.M.; Wang, B.G. Chemical constituents of marine mangrove plant Bruguiera gymnorrhiza. Mar. Sci. 2010, 34, 24–27. [Google Scholar]
- Chumkaew, P.; Kato, S.; Chantrapromma, K. A New Triterpenoid Ester from the Fruits of Bruguiera parviflora. Chem. Pharm. Bull. 2005, 53, 95–96. [Google Scholar] [CrossRef]
- Karalai, C.; Laphookhieo, S. Triterpenoid Esters from Bruguiera cylindrica. Aust. J. Chem. 2005, 58, 556–559. [Google Scholar] [CrossRef]
- Chantrapromma, S.; Fun, H.-K.; Razak, I.A.; Laphookhieo, S.; Karalai, C. Absolute configuration of 3-feruloyltaraxerol dichloromethane solvate. Acta Cryst. E 2003, 59, o1864–o1866. [Google Scholar] [CrossRef]
- Laphookhieo, S.; Karalai, C.; Ponglimanont, C.; Chantrapromma, K. Pentacyclic Triterpenoid Esters from the Fruits of Bruguiera cylindrica. J. Nat. Prod. 2004, 67, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Kalappurakkal, V.N.; Bhattacharya, D.; Chakravarty, S.; Uppuluri, M.V. Isolation, Synthesis and AChE Inhibitory Potential of Some Novel Cinnamyl Esters of Taraxerol, the Major Metabolite of the Mangrove Bruguiera cylindrica. Chem. Biodivers. 2018, 15, e1800008. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Liu, L.; Chen, S. The chemistry and biology of fungal meroterpenoids (2009–2019). Org. Biomol. Chem. 2020, 19, 1644–1704. [Google Scholar] [CrossRef]
- Huang, G.L.; Zhou, X.M.; Bai, M.; Liu, Y.X.; Zhao, Y.L.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.J.; Chen, G.Y. Dihydroisocoumarins from the Mangrove-Derived Fungus Penicillium citrinum. Mar. Drugs 2016, 14, 177. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Huang, G.L.; Mei, R.Q.; Wang, B.; Luo, Y.P.; Zheng, C.; Niu, Z.G.; Chen, G.Y. Bioactive Meroterpenoids and Isocoumarins from the Mangrove Derived Fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zheng, C.J.; Chen, G.Y. Austins-Type Meroterpenoids from a Mangrove-Derived Penicillium sp. J. Nat. Prod. 2021, 84, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Sofian, F.F.; Suzuki, T.; Supratman, U.; Harneti, D.; Maharani, R.; Salam, S.; Abdullah, F.F.; Koseki, T.; Tanaka, K.; Kimura, K.I.; et al. Cochlioquinone derivatives produced by coculture of endophytes, Clonostachys rosea and Nectria pseudotrichia. Fitoterapia 2021, 155, 105056. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Xiong, B.X.; Xu, J. Chemical Investigation Of Secondary Metabolites Produced By Mangrove Endophytic Fungus Phyllosticta Capitalensis. Nat. Prod. Res. 2019, 35, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G.; Lathe, R. Terpenes, hormones and life: Isoprene rule revisited. J. Endocrinol. 2019, 242, R9–R22. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, Y.; Wang, H.Z.; Wang, S.J.; Zhang, R.Q. The metabolites of a mangrove endophytic fungus, Penicillium thomi. J. Asian Nat. Prod. Res. 2007, 9, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Song, X.M.; Zhou, X.M.; Li, X.B.; Zheng, C.J.; Huang, G.L.; Yu, Z.X.; Song, X.P.; Chen, G.Y. Secondary Metabolites of a Bruguiera sexangula var. Rhynchopetala Derived Fungus Phomopsis longicolla HL-2232. Chin. J. Org. Chem. 2015, 35, 2102–2107. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Jiang, C.S.; Li, G.; Guo, Y.W. (+)-Cyclopenol, a new naturally occurring 7-membered 2,5-dioxopiperazine alkaloid from the fungus Penicillium sclerotiorum endogenous with the Chinese mangrove Bruguiera gymnorrhiza. J. Asian Nat. Prod. Res. 2014, 16, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.M.; Liu, S.X.; Huang, C.H.; Pang, J.Y.; Lin, Y.C. Secondary Metabolites of a Mangrove Endophytic Fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616–2624. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Yang, X.H.; Liu, H.L.; Gu, Y.C.; Ye, B.P.; Guo, Y.W. A New Cyclic Peptide and a New Steroid from the Fungus Penicillium sp. GD6 Isolated from the Mangrove Bruguiera gymnorrhiza. Helv. Chim. Acta 2014, 97, 1564–1570. [Google Scholar] [CrossRef]
- Liu, Z.M.; Li, M.Q.; Wang, S.; Huang, H.B.; Zhang, W.M. Sulfur-Containing Metabolites from Marine and Terrestrial Fungal Sources: Origin, Structures, and Bioactivities. Mar. Drugs 2022, 20, 765. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Shen, X.; Jiang, H.L.; Guo, Y.W. Structural Studies on an Unusual Novel Macrocyclic Polydisulfide from the Chinese Mangrove Bruguiera gymnorrhiza. Chin. J. Org. Chem. 2008, 28, 246–251. [Google Scholar]
- Nwachukwu, I.D.; Slusarenko, A.J.; Gruhlke, M.C. Sulfur and Sulfur Compounds in Plant Defence. Nat. Prod. Commun. 2012, 7, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Dahibhate, N.L.; Shukla, S.K.; Kumar, K. A Cyclic Disulfide Diastereomer From Bioactive Fraction of Bruguiera gymnorhiza Shows Anti–Pseudomonas aeruginosa Activity. Front. Pharmacol. 2022, 13, 890790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Z.; Li, X.M.; Meng, L.H.; Wang, B.G. Cladocladosin A, an unusual macrolide with bicyclo 5/9 ring system, and two thiomacrolides from the marine mangrove-derived endophytic fungus, Cladosporium cladosporioides MA-299. Bioorg Chem. 2020, 101, 103950. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Guo, Y.W. Gymnorrhizol, an unusual macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Tetrahedron Lett. 2004, 45, 5533–5535. [Google Scholar] [CrossRef]
- Kato, A.; Takahashi, J. A new naturally occurring 1,2-dithiolane from Bruguiera cylindrica. Phytochem. Lett. 1976, 15, 220–221. [Google Scholar] [CrossRef]
- Kato, A.; Numata, M. Brugierol and Isobrugierol, trans- and cis-1,2-dithiolane-1-oxide, from Brugiera conjugata. Tetrahedron Lett. 1972, 13, 203–206. [Google Scholar] [CrossRef]
- Homhual, S.; Zhang, H.J.; Bunyapraphatsara, N.; Kondratyuk, T.P.; Santarsiero, B.D.; Mesecar, A.D.; Herunsalee, A.; Chaukul, W.; Pezzuto, J.M.; Fong, H.H. Bruguiesulfurol, A New Sulfur Compound from Bruguiera gymnorrhiza. Planta Med. 2006, 72, 255–260. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, Q.; Liu, H.L.; Zhang, Y.; Xin, G.R.; Shen, X.; Dong, M.L.; Guo, Y.W. Diastereoisomeric macrocyclic polydisulfides from the mangrove Bruguiera gymnorrhiza. Phytochemistry 2009, 70, 2096–2100. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamada, Y. Alkaloid Biogenesis: Molecular Aspects. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 257–285. [Google Scholar] [CrossRef]
- Lei, F.Y.; Liu, X.D.; Huang, H.N.; Fu, S.D.; Zou, K.; Zhang, S.F.; Zhou, L.; Zeng, J.G.; Liu, H.W.; Jiang, L.H.; et al. The Macleaya cordata Symbiont: Revealing the Effects of Plant Niches and Alkaloids on the Bacterial Community. Front. Microbiol. 2021, 12, 681210. [Google Scholar] [CrossRef] [PubMed]
- Arunpanichlert, J.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Tewtrakul, S.; Rungjindamai, N.; Sakayaroj, J. Azaphilone and Isocoumarin Derivatives from the Endophytic Fungus Penicillium sclerotiorum PSU-A13. Chem. Pharm. Bull. 2010, 58, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Bracken, A.; Pocker, A.; Raistrick, H. Studies in the biochemistry of microorganisms. 93. Cyclopenin, a nitrogen-containing metabolic product of Penicillium cyclopium Westling. Biochem. J. 1954, 57, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Qu, C.H.; Lu, J.; Cheng, G.; Gao, C.H. A new antiviral alkaloid from the hypocotyl of Bruguiera gymnorrhiza. Guihaia 2016, 36, 236–239. [Google Scholar]
- Yan, L.L.; Han, N.N.; Zhang, Y.Q.; Yu, L.Y.; Chen, J.; Wei, Y.Z.; Li, Q.P.; Tao, L.; Zheng, G.H.; Yang, S.E.; et al. Antimycin A18 produced by an endophytic Streptomyces albidoflavus isolated from a mangrove plant. J. Antibiot. 2010, 63, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Liao, H.X.; Mei, R.Q.; Huang, G.L.; Yang, L.J.; Zhou, X.M.; Shao, T.M.; Chen, G.Y.; Wang, C.Y. Two new benzophenones and one new natural amide alkaloid isolated from a mangrove-derived Fungus Penicillium citrinum. Nat. Prod. Res. 2018, 33, 1127–1134. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Kurtán, T.; Yang, X.H.; Mándi, A.; Geng, M.Y.; Ye, B.P.; Taglialatela-Scafati, O.; Guo, Y.W. Penibruguieramine A, a Novel Pyrrolizidine Alkaloid from the Endophytic Fungus Penicillium sp. GD6 Associated with Chinese Mangrove Bruguiera gymnorrhiza. Org. Lett. 2014, 16, 1390–1393. [Google Scholar] [CrossRef]
- Kato, A. Brugine from Bruguiera cylindrica. Phytochemistry 1975, 14, 1458. [Google Scholar] [CrossRef]
- Jiang, C.S.; Zhou, Z.F.; Yang, X.H.; Lan, L.F.; Gu, Y.C.; Ye, B.P.; Guo, Y.W. Antibacterial sorbicillin and diketopiperazines from the endogenous fungus Penicillium sp. GD6 associated Chinese mangrove Bruguiera gymnorrhiza. Chin. J. Nat. Med. 2018, 16, 358–365. [Google Scholar] [CrossRef]
- Yi, X.X.; Deng, J.G.; Gao, C.H.; Hou, X.T.; Li, F.; Wang, Z.P.; Hao, E.W.; Xie, Y.; Du, Z.C.; Huang, H.X.; et al. Four New Cyclohexylideneacetonitrile Derivatives from the Hypocotyl of Mangrove (Bruguiera gymnorrhiza). Molecules 2015, 20, 14565–14575. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Chang, M.; Zhang, Q.H.; He, R.F.; Ye, B.P. The endophytic fungus strain ZD6 isolated from the stem of Bruguiera gymnorrhiza and the antibacterial activity of its metabolites. Mycosystema 2010, 29, 739–745. [Google Scholar]
- Li, C.Y.; Chen, M.; Ding, W.J.; Lin, Y.C.; Zhou, S.N. Study on the Metabolites of Endophytic Fungus (Gloesporium sp.) Isolated from Bruguiera gymnoihiza Grown in the South China Sea. J. South. China Agric. Univ. 2008, 29, 122–124. [Google Scholar]
- Zheng, C.J.; Wu, L.Y.; Li, X.B.; Song, X.M.; Niu, Z.G.; Song, X.P.; Chen, G.Y.; Wang, C.Y. Structure and Absolute Configuration of Aspergilumamide A, a Novel Lumazine Peptide from the Mangrove-Derived Fungus Aspergillus Sp. Helv. Chim. Acta 2015, 98, 368–373. [Google Scholar] [CrossRef]
- Laohavisit, A.; Wakatake, T.; Ishihama, N.; Mulvey, H.; Takizawa, K.; Suzuki, T.; Shirasu, K. Quinone perception in plants via leucine-rich-repeat receptor-like kinases. Nature 2020, 587, 92–97. [Google Scholar] [CrossRef]
- Krueger, M.C.; Bergmann, M.; Schlosser, D. Widespread ability of fungi to drive quinone redox cycling for biodegradation. Fems Microbiol. Lett. 2016, 363, fnw105. [Google Scholar] [CrossRef]
- Klaiklay, S.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Buatong, J.; Bussaban, B. Metabolites from the Mangrove-derived Fungus Xylaria cubensis PSU-MA34. Arch. Pharm. Res. 2012, 35, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.S.; Kurtán, T.; Miao, Z.H.; Mándi, A.; Komáromi, I.; Liu, H.L.; Ding, J.; Guo, Y.W. Palmarumycins BG1-BG7 and Preussomerin BG1: Establishment of Their Absolute Configurations Using Theoretical Calculations of Electronic Circular Dichroism Spectra. J. Org. Chem. 2011, 76, 1821–1830. [Google Scholar] [CrossRef]
- Kongyen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Sakayaroj, J. A new hydronaphthalenone from the mangrove-derived Daldinia eschscholtzii PSU-STD57. Nat. Prod. Res. 2015, 29, 1995–1999. [Google Scholar] [CrossRef]
- Yang, L.J.; Liao, H.X.; Bai, M.; Huang, G.L.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.J.; Wang, C.Y. One new cytochalasin metabolite isolated from a mangrove-derived fungus Daldinia eschscholtzii HJ001. Nat. Prod. Res. 2018, 32, 208–213. [Google Scholar] [CrossRef]
- Ukwatta, K.M.; Lawrence, J.L.; Wijayarathna, C.D. The study of antimicrobial, anti-cancer, antiinflammatory and α-glucosidase inhibitory activities of Nigronapthaphenyl, isolated from an extract of Nigrospora sphaerica. Mycology 2019, 10, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Huang, G.L.; Xu, Y.; Song, X.M.; Yao, J.; Liu, H.; Wang, R.P.; Sun, X.P. A new benzopyrans derivatives from a mangrove-derived fungus Penicillium citrinum from the South China Sea. Nat. Prod. Res. 2015, 30, 821–825. [Google Scholar] [CrossRef]
- Liao, H.X.; Shao, T.M.; Mei, R.Q.; Huang, G.L.; Zhou, X.M.; Zheng, C.J.; Wang, C.Y. Bioactive Secondary Metabolites from the Culture of the Mangrove-Derived Fungus Daldinia eschscholtzii HJ004. Mar. Drugs 2019, 17, 710. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Jiao, F.W.; Li, W.; Zhang, X.; Yan, W.; Jiao, R.H. Cytotoxic Xanthone Derivatives from the Mangrove-Derived Endophytic Fungus Peniophora incarnata Z4. J. Nat. Prod. 2020, 83, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- He, K.Y.; Zhang, C.; Duan, Y.R.; Huang, G.L.; Yang, C.Y.; Lu, X.R.; Zheng, C.J.; Chen, G.Y. New chlorinated xanthone and anthraquinone produced by a mangrove-derived fungus Penicillium citrinum HL-5126. J. Antibiot. 2017, 70, 823–827. [Google Scholar] [CrossRef]
- Zhou, X.M.; Zheng, C.J.; Chen, G.Y.; Song, X.P.; Han, C.R.; Li, G.N.; Fu, Y.H.; Chen, W.H.; Niu, Z.G. Bioactive Anthraquinone Derivatives from the Mangrove-Derived Fungus Stemphylium sp. 33231. J. Nat. Prod. 2014, 77, 2021–2028. [Google Scholar] [CrossRef]
- Li, X.B.; Chen, G.Y.; Liu, R.J.; Zheng, C.J.; Song, X.M.; Han, C.R. A new biphenyl derivative from the mangrove endophytic fungus Phomopsis longicolla HL-2232. Nat. Prod. Res. 2017, 31, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Supratman, U.; Suzuki, T.; Nakamura, T.; Yokoyama, Y.; Harneti, D.; Maharani, R.; Salam, S.; Abdullah, F.F.; Koseki, T.; Shiono, Y. New metabolites produced by endophyte Clonostachys rosea B5-2. Nat. Prod. Res. 2019, 35, 1525–1531. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Garrido-Santos, M.Y. Phytotoxic compounds from endophytic fungi. Appl. Microbiol. Biotechnol. 2022, 106, 931–950. [Google Scholar] [CrossRef]
- Su, J.H.; Wang, M.Q.; Li, Y.Z.; Lin, Y.S.; Gu, J.Y.; Zhu, L.P.; Yang, W.Q.; Jiang, S.Q.; Zhao, Z.X.; Sun, Z.H. Rare cytochalasans isolated from the mangrove endophytic fungus Xylaria arbuscula. Fitoterapia 2022, 157, 105124. [Google Scholar] [CrossRef]
- Huang, H.B.; Li, Q.; Feng, X.J.; Chen, B.; Wang, J.; Liu, L.; She, Z.G.; Lin, Y.C. Structural elucidation and NMR assignments of four aromatic lactones from amangrove endophytic fungus (No. GX4-1B). Magn. Reson. Chem. MRC 2010, 48, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Chen, T.; Yu, J.C.; Jia, H.; Chen, C.; Long, Y.H. New Sorbicillinoids from the Mangrove Endophytic Fungus Trichoderma reesei SCNU-F0042. Mar. Drugs 2023, 21, 442. [Google Scholar] [CrossRef]
- Zou, G.; Tan, Q.; Chen, Y.; Yang, W.C.; Zang, Z.M.; Jiang, H.M.; Chen, S.Y.; Wang, B.; She, Z.G. Furobenzotropolones A, B and 3-Hydroxyepicoccone B with Antioxidative Activity from Mangrove Endophytic Fungus Epicoccum nigrum MLY-3. Mar. Drugs 2021, 19, 395. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Li, X.M.; Li, X.; Yang, S.Q.; Meng, L.H.; Wang, B.G. Polyketides from the Mangrove-Derived Endophytic Fungus Cladosporium cladosporioides. Mar. Drugs 2019, 17, 296. [Google Scholar] [CrossRef]
- Deng, P.F.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.J.; Chen, G.Y.; Chen, J.; Ma, W.H. A New Penicitrinone Derivative from the Endophytic Fungus Penicillium sp. from Bruguiera sexangula var. rhynchopetala. Chem. Nat. Compd. 2016, 52, 810–812. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.J.; Cheng, Y.; Gao, H.S.; Chen, X.H. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Qiao, F.; Xu, G.W.; Zhao, J.; Teng, J.F.; Li, C.; Deng, W.J. Neuroprotective metabolites from the endophytic fungus Penicillium citrinum of the mangrove Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 12, 148–152. [Google Scholar] [CrossRef]
- Hu, Y.O.; Ye, B.P.; Wang, D.Y. Study on the bioactivity of 3′,4′,5′-trihydroxy-7-hydroxy-5-methoxyflavone extracted from Bruguiera gymnorrhiza by C.elegans. Chin. J. Mar. Drugs 2007, 26, 20–26. [Google Scholar]
- Panyadee, A.; Sahakitpichan, P.; Ruchirawat, S.; Kanchanapoom, T. 5-Methyl ether flavone glucosides from the leaves of Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 11, 215–219. [Google Scholar] [CrossRef]
- Barik, R.; Sarkar, R.; Biswas, P.; Bera, R.; Sharma, S.; Nath, S.; Karmakar, S.; Sen, T. 5,7-dihydroxy-2-(3-hydroxy-4, 5-dimethoxy-phenyl)-chromen-4-one-a flavone from Bruguiera gymnorrhiza displaying anti-inflammatory properties. Indian. J. Pharmacol. 2016, 48, 304–311. [Google Scholar]
- Yao, J.E.; Shen, M.R.; Yi, X.X.; Yang, Y.; Gao, C.H. A New 8-Hydroxyquercetagetin Glycoside from the Hypocotyls of Mangrove Bruguiera gymnorrhiza. Chem. Nat. Comp. 2017, 53, 33–35. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Stringlis, I.A.; de Jonge, R.; Pieterse, C.M.J. The Age of Coumarins in Plant–Microbe Interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef]
- Yi, X.X.; Gao, C.H.; He, B.J.; Chen, B. Study on phenylpropanoids from hypocotyls of the mangrove plant Bruguiera gymnorrhiza. Guihaia 2013, 33, 191–194. [Google Scholar]
- Han, Z.; Mei, W.L.; Zhao, Y.X.; Deng, Y.Y.; Dai, H.F. A new cytotoxic isocoumarin from endophytic fungus Penicillium sp. 091402 of the mangrove plant Bruguiera sexangula. Chem. Nat. Compd. 2009, 45, 805–807. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.S.; Sattler, I.; Fu, H.Z.; Grabley, S.; Lin, W.H. Two new constituents from mangrove Bruguiera gymnorrhiza. J. Asian Nat. Prod. Res. 2007, 9, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.S.; Long, S.J. Brugnanin, a new syn-2,3-dihydrobenzofuran neolignan dioate from the mangrove Bruguiera gymnorrhiza. Chem. Nat. Compd. 2008, 44, 186–189. [Google Scholar] [CrossRef]
- Bao, S.; Ding, Y.; Deng, Z.; Proksch, P.; Lin, W. Rhyncosides A-F, phenolic constituents from the Chinese mangrove plant Bruguiera sexangula var. rhynchopetala. Chem. Pharm. Bull. 2007, 55, 1175–1180. [Google Scholar] [CrossRef]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal-plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.S.; Sattler, I.; Moellmann, U.; Fu, H.Z.; Lin, W.H.; Grabley, S. New aromatic compounds from the marine mangrove Bruguiera gymnorrhiza. Planta Med. 2005, 71, 160–164. [Google Scholar] [CrossRef]
- Gao, C.H.; Long, B.; Su, Z.W.; Yu, L.; Yan, D.M.; Wen, L.J.; He, B.J. Study on Aromatic Compounds from Hypocotyls of Mangrove Bruguiera gymnorrhiza. J. Guangxi Acad. Sci. 2015, 31, 28–31. [Google Scholar]
- Seshadri, T.R.; Trikha, R.K. Procyanidin of Bruguiera parviflora. Indian. J. Chem. 1971, 9, 302–304. [Google Scholar]
- Chokpaiboon, S.; Choodej, S.; Boonyuen, N.; Teerawatananond, T.; Pudhom, K. Highly oxygenated chromones from mangrove-derived endophytic fungus Rhytidhysteron rufulum. Phytochemistry 2016, 122, 172–177. [Google Scholar] [CrossRef]
- Sriyatep, T.; Maneerat, W.; Sripisut, T.; Cheenpracha, S.; Machan, T.; Phakhodee, W.; Laphookhieo, S. Cowabenzophenones A and B, two new tetracyclo[7.3.3.33,11.03,7]tetradecane-2,12,14-trione derivatives, from ripe fruits of Garcinia cowa. Fitoterapia 2014, 92, 285–289. [Google Scholar] [CrossRef]
- Ukwatta, K.M.; Lawrence, J.L.; Wijayarathne, C.D. Antimicrobial, anti-cancer, anti-filarial and antiinflammatory activities of Cowabenzophenone A extracted from the endophytic fungus Aspergillus terreus isolated from a mangrove plant Bruguiera gymnorrhyza. Mycology 2019, 11, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Habden, X.; Han, N.N.; Yu, S.Y.; Yan, L.L.; Luan, Y.C.; Liu, S.W.; Guo, L.J.; Zhong, K.; Yu, L.Y.; et al. Separation, purification and structural identification of an active nematicidal component from the fermentation broth of Endophytic streptomyces I07A-01824 from Mangrove ecosystem. Chin. Med. Biotechnol. 2012, 7, 5–8. [Google Scholar]
- Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic Effects of Bangladeshi Medicinal Plant Extracts. Evid.-Based Complement. Altern. Med. Ecam. 2011, 2011, 578092. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Q.; Tao, G.L.; Tian, S.H.; Yang, Z.X.; Yao, M.Z.; Huang, L.; Fu, J. Inhibitory effect of Bruguiera sexangula leaves extract on gastric cancer cells proliferation and its acute toxicity in mice. Chin. J. Mar. Drugs 2018, 37, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Qayoom, H.; Alkhanani, M.; Almilaibary, A.; Alsagaby, S.A.; Mir, M.A. A network pharmacology-based investigation of brugine reveals its multi-target molecular mechanism against Breast Cancer. Med. Oncol. 2023, 40, 202. [Google Scholar] [CrossRef]
- Hosen, M.Z.; Biswas, A.; Islam, M.R.; Hossain, S.J. Anti-Bacterial, Anti-Diarrheal, and Cytotoxic Activities of Edible Fruits in the Sundarbans Mangrove Forest of Bangladesh. Prev. Nutr. Food Sci. 2021, 26, 192–199. [Google Scholar] [CrossRef]
- Acharya, S.; Patra, D.K.; Pradhan, C.; Mohapatra, P.K. Anti-bacterial, anti-fungal and anti-oxidative properties of different extracts of Bruguiera gymnorrhiza L. (Mangrove). Eur. J. Integr. Med. 2020, 36, 101140. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Sinan, K.I.; Cziáky, Z.; Jeko, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; Rengasamy, K.R.R.; Mahomoodally, M.F. Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules 2020, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, P.D. Antibacterial Activity of some Medicinal Mangroves against Antibiotic Resistant Pathogenic Bacteria. Indian. J. Pharm. Sci. 2010, 72, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Golder, M.; Sadhu, S.K.; Biswas, B.; Islam, T. Comparative pharmacologic profiles of leaves and hypocotyls of a mangrove plant: Bruguiera gymnorrhiza. Adv. Tradit. Med. 2020, 20, 395–403. [Google Scholar] [CrossRef]
- Tao, G.L.; Yang, F.; Tian, S.H.; Huang, L.; Huang, M.Q. The antioxidative and melanin-inhibitory effects of alcohol extract of Bruguiera sexangula leaves. Chin. J. Mar. Drugs 2019, 38, 59–63. [Google Scholar]
- Liu, X.L.; Chen, T.; Wang, Q.; Liu, J.A.; Lu, Y.H.; Shi, Y. Structure Analysis and Study of Biological Activities of Condensed Tannins from Bruguiera gymnorhiza (L.) Lam and Their Effect on Fresh-Cut Lotus Roots. Molecules 2021, 26, 1369. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Xie, X.G.; Hu, Z.; Xue, J.Y.; Zhang, S.L.; Xie, X.M. (Z)-7,4’-dimethoxy-6-hydroxy-aurone-4-O-β-glucopyranoside attenuates lipoteichoic acid-induced damage in rat cardiomyoblast cells. J. Int. Med. Res. 2020, 48, 300060519889716. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Luo, D.D.; Lin, Y.S.; Liu, Y.H.; Wu, J.Z.; Yi, X.Q.; Wu, Y.; Zhang, Q.; Gao, C.J.; Cai, J.; et al. Aqueous extract of Bruguiera gymnorrhiza leaves protects against dextran sulfate sodium induced ulcerative colitis in mice via suppressing NF-κB activation and modulating intestinal microbiota. J. Ethnopharmacol. 2020, 251, 112554. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Zheng, X.H.; Chen, J.F.; Luo, D.D.; Xie, J.H.; Su, Z.R.; Huang, X.Q.; Yi, X.Q.; Wei, L.; Cai, J.; et al. Protective Effect of Bruguiera gymnorrhiza (L.) Lam. Fruit on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice: Role of Keap1/Nrf2 Pathway and Gut Microbiota. Front. Pharmacol. 2020, 10, 1602. [Google Scholar] [CrossRef]
- Zhang, X.; Mai, J.-H.; Gao, Z.-W.; Wang, L.-L.; Emran, T.B. Bruguiera gymnorrhiza (L.) Lam. Fruit Accelerates Healing in Gastric Injury via the Regulation of the NF-κB Pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 1046712. [Google Scholar] [CrossRef]
- Amalia, R.; Pramono, A.; Afifah, D.N.; Noer, E.R.; Muniroh, M.; Kumoro, A.C. Mangrove fruit (Bruguiera gymnorhiza) increases circulating GLP-1 and PYY, modulates lipid profiles, and reduces systemic inflammation by improving SCFA levels in obese wistar rats. Heliyon 2022, 8, e10887. [Google Scholar] [CrossRef] [PubMed]
- Eldeen, I.M.S.; Ringe, J.; Ismail, N. Inhibition of Pro-inflammatory Enzymes and Growth of an Induced Rheumatoid Arthritis Synovial Fibroblast by Bruguiera cylindrica. Int. J. Pharmacol. 2019, 15, 916–925. [Google Scholar] [CrossRef]
- Hou, S.S.; Liang, C.Y.; Gao, C.H.; Jiang, C.P.; Tang, Q.Q.; Liu, Y.H.; Yi, X.X. Species diversity and anti-hepatitis B virus activity of culturable bacteria isolated from the habitat of Bruguiera gymnorrhiza. Guihaia 2023, 43, 616–625. [Google Scholar]
- Bibi Sadeer, N.; Haddad, J.G.; Oday Ezzat, M.; Desprès, P.; Abdallah, H.H.; Zengin, G.; Uysal, A.; El Kalamouni, C.; Gallo, M.; Montesano, D.; et al. Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections—An In Vitro and In Silico Perspective. Molules 2021, 26, 5768. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Dinesh, D.; Paulpandi, M.; Althbyani, A.D.; Subramaniam, J.; Madhiyazhagan, P.; Wang, L.; Suresh, U.; Kumar, P.M.; Mohan, J.; et al. Nanoparticles in the fight against mosquito-borne diseases: Bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 4349–4361. [Google Scholar] [CrossRef] [PubMed]
- Bibi Sadeer, N.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; AlDhaheri, Y.; Eid, A.H.; Mahomoodally, M.F. Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used. Molules 2022, 27, 2000. [Google Scholar] [CrossRef] [PubMed]
- Pitchaipillai, R.; Ponniah, T. In Vitro Antidiabetic Activity of Ethanolic Leaf Extract of Bruguiera cylindrica L.—Glucose Uptake by Yeast Cells Method. IBBJ 2016, 2, 171–175. [Google Scholar]
- Revathi, P.; Jeyaseelan, S.C.; Subramanian, P.; Manickavasagam, S.; Prabhu, N. A comparative mechanism of antidiabetic role of various extracts of Bruguriera cylindrica L leaves. World J. Pharm. Pharm. Sci. 2015, 4, 1168–1176. [Google Scholar]
- Gong, J.X.; Shen, X.; Yao, L.G.; Jiang, H.L.; Krohn, K.; Guo, Y.W. Total Synthesis of Gymnorrhizol, an Unprecedented 15-Membered Macrocyclic Polydisulfide from the Chinese Mangrove Bruguiera gymnorrhiza. Org. Lett. 2007, 9, 1715–1716. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Gnanadesigan, M. In vitro antiplasmodial activity of ethanolic extracts of mangrove plants from South East coast of India against chloroquine-sensitive Plasmodium falciparum. Parasitol. Res. 2011, 108, 873–878. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Venkatesan, M.; Ramu, A. Mangrove plants as a source of lead compounds for the development of new antiplasmodial drugs from South East coast of India. Parasitol. Res. 2011, 108, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, I.; Zilani, M.N.H.; Biswas, N.N.; Bokshi, B. Bioactivities of Bruguiera gymnorrhiza and profiling of its bioactive polyphenols by HPLC-DAD. Clin. Phytoscience 2017, 3, 11. [Google Scholar] [CrossRef]
- Sun, Z.W.; Tian, F.; An, M.; Duan, S.S. Inhibitive effects of organic solvent extracts from mangrove plant Bruguiera gymnorrhiza on Phaeocystis globosa. Ecol. Sci. 2012, 31, 245–251. [Google Scholar]
- Cui, J.L.; Guo, S.X.; Xiao, P.G. Interaction between endophytes and host plant and the role of endophytes in genuineness analysis of medicinal plant. Acta Pharm. Sin. 2017, 52, 214–221. [Google Scholar]
- Quach, N.T.; Vu, T.H.N.; Bui, T.L.; Le, T.T.X.; Nguyen, T.T.A.; Ngo, C.C.; Phi, Q.-T. Genomic and physiological traits provide insights into ecological niche adaptations of mangrove endophytic Streptomyces parvulus VCCM 22513. Ann. Microbiol. 2022, 72, 27. [Google Scholar] [CrossRef]
- Ma, Q.; Lei, R.F.; Abudourousuli, D.; Aosiman, M.; Rouzi, Z.; An, D.D. Research Progress on the Symbiotic Metabolic of Endophytes and Plants Under Stress. Biotechnol. Bull. 2021, 37, 153–161. [Google Scholar]
- Liu, Y.L.; Qin, Y.Y.; Zheng, H.L. Research Progresses of Water Logging Tolerance and High Saline Adaptation of Mangrove Plants. J. Xiamen Univ. (Nat. Sci.) 2017, 56, 314–322. [Google Scholar]
- Elias, L.M.; Fortlzamp, D.; Sartori, S.B.; Ferreira, M.C.; Gomes, L.H.; Azevedo, J.L.; Montoya, Q.V.; Rodrigues, A.; Ferreira, A.G.; Lira, S.P. The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Braz. J. Microbiol. 2018, 49, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, J.; Sun, Q.Q.; Qin, J.C.; Pescitelli, G.; Gao, J.M. Characterization of Cytochalasins from the Endophytic Xylaria sp. and Their Biological Functions. J. Agric. Food Chem. 2014, 62, 10962–10969. [Google Scholar] [CrossRef]
- Betina, V.; Mičeková, D.; Němec, P. Antimicrobial Properties of Cytochalasins and Their Alteration of Fungal Morphology. Microbiology 1972, 71, 343–349. [Google Scholar] [CrossRef]
- Prasain, J.K.; Ueki, M.; Stefanowicz, P.; Osada, H. Rapid screening and identification of cytochalasins by electrospray tandem mass spectrometry. J. Mass Spectrom. JMS 2002, 37, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Tani, H.; Ichinoe, M.; Nakajima, H. Zygosporin D and Two New Cytochalasins Produced by the Fungus Metarrhizium anisopliae. J. Nat. Prod. 2000, 63, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Kumarihamy, M.; Ferreira, D.; Croom, E.M.; Sahu, R.; Tekwani, B.L.; Duke, S.O.; Khan, S.; Techen, N.; Nanayakkara, N.P.D. Antiplasmodial and Cytotoxic Cytochalasins from an Endophytic Fungus, Nemania sp. UM10M, Isolated from a Diseased Torreya taxifolia Leaf. Molecules 2019, 24, 777. [Google Scholar] [CrossRef] [PubMed]
- Ntemafack, A.; Chouhan, R.; Kapoor, N.; Kumar, A.; Dhiman, S.K.; Manhas, R.S.; Chaubey, A.; Hassan, Q.P.; Gandhi, S.G. Protective effect of Bacillus species associated with Rumex dentatus against postharvest soil borne disease in potato tubers and GC–MS metabolite profile. Arch. Microbiol. 2022, 204, 583. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wakatake, T.; Hashimoto, K.; Saucet, S.B.; Toyooka, K.; Yoshida, S.; Shirasu, K. Haustorial Hairs Are Specialized Root Hairs That Support Parasitism in the Facultative Parasitic Plant Phtheirospermum japonicum. Plant Physiol. 2016, 170, 1492–1503. [Google Scholar] [CrossRef]
- Chong, J.; Baltz, R.; Schmitt, C.; Beffa, R.; Fritig, B.; Saindrenan, P. Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 2002, 14, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Sanier, C.; Macheix, J.J.; D’Auzac, J. Accumulation of scopoletin in Hevea brasiliensis infected by Microcyclus ulei (P. Henn.) V.ARX and evaluation of its fungitoxicity for three leaf pathogens of rubber tree. Physiol. Mol. Plant Pathol. 1995, 47, 213–223. [Google Scholar] [CrossRef]
- Rauckman, B.S.T.; Mary, Y.; Johnson, J.V.; Roth, B. 2,4-Diamino-5-benzylpyrimidines and analogs as antibacterial agents. 10. 2,4-Diamino-5-(6-quinolylmethyl)- and -[(tetrahydro-6-quinolyl)methyl]pyrimidine derivatives. Further specificity studies. J. Med. Chem. 1989, 32, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Segaran, G.; Sathiavelu, M. Fungicidal and plant growth-promoting traits of Lasiodiplodia pseudotheobromae, an endophyte from Andrographis paniculata. Front. Plant Sci. 2023, 14, 1125630. [Google Scholar] [CrossRef]
- Khan, I.H.; Javaid, A. DNA cleavage of the fungal pathogen and production of antifungal compounds are the possible mechanisms of action of biocontrol agent Penicillium italicum against Macrophomina phaseolina. Mycologia 2022, 114, 24–34. [Google Scholar] [CrossRef]
- Patel, T.K.; Williamson, J.D. Mannitol in Plants, Fungi, and Plant–Fungal Interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.B.; Ehrenshaft, M.; Pharr, D.M.; Williamson, J.D. Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc. Natl. Acad. Sci. USA 1998, 95, 15129–15133. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” Tools for Better Understanding the Plant-Endophyte Interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).