Chemical Constituents and Biological Activities of Bruguiera Genus and Its Endophytes: A Review
Abstract
:1. Introduction
2. Chemical Composition of Bruguiera Genus Plants and Their Endophytes
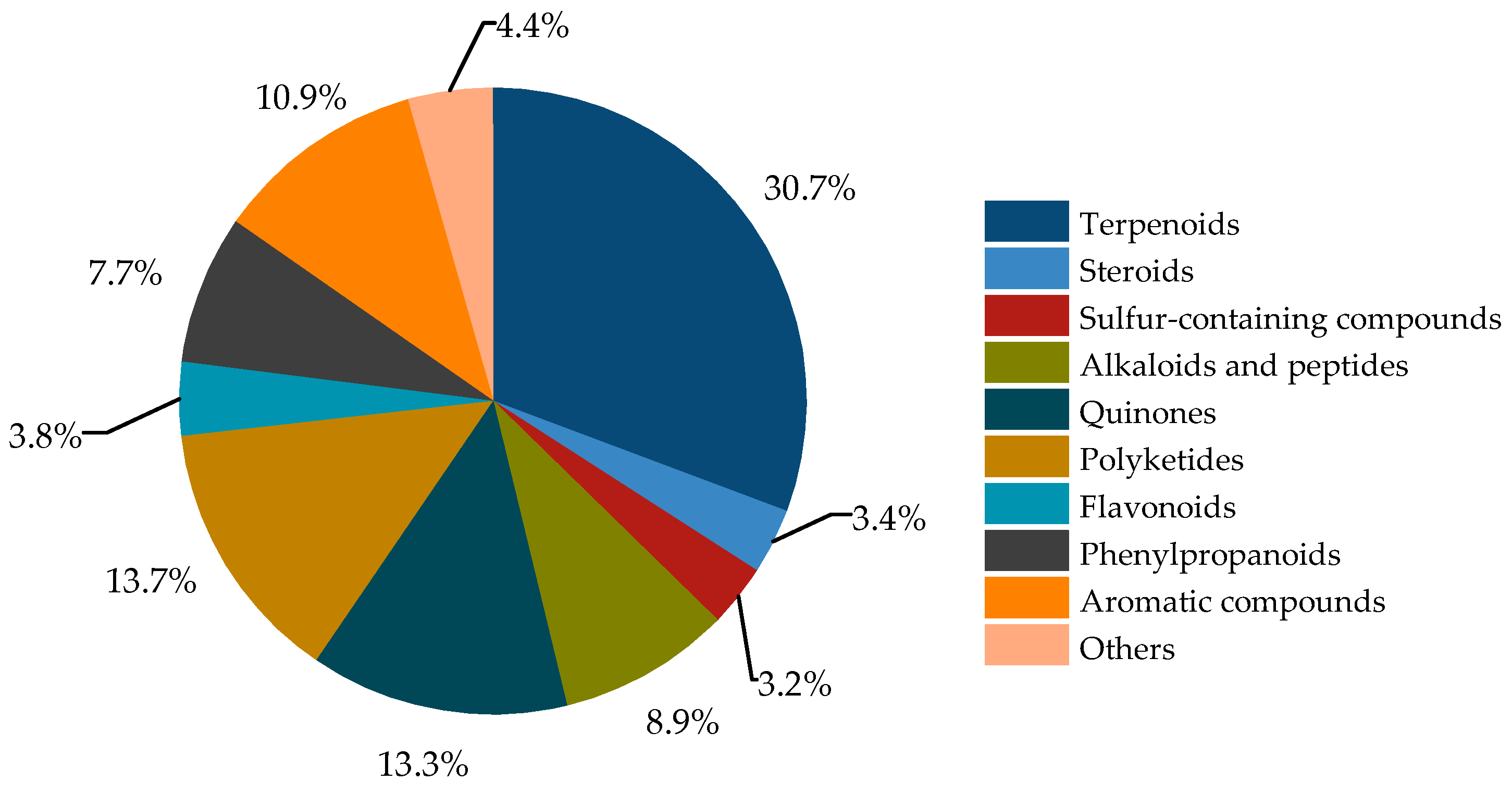
2.1. Terpenoids
2.1.1. Monoterpenes
2.1.2. Sesquiterpenes
| No. | Compound | Source | Reference |
|---|---|---|---|
| 3 | (S)-4-hydroxy-3,5,5-trimethyl-4-((R,E)-3-(((2R,3R,4S,5S,6S)-3,5,6-trihydroxy-4-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)cyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 4 | (S)-4-((R,E)-3-(((2R,3R,4S,5R,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-4-hydroxy-3,5,5-trimethylcyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 5 | (S)-4-((R,E)-3-(((2R,3R,4S,5R,6R)-4-(((2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-3,4-dihydro-2H-pyran-5-yl)oxy)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-4-hydroxy-3,5,5-trimethylcyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 6 | (R)-4-((R,E)-3-(((2R,3R,4S,5R,6R)-4-(((2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-3,4-dihydro-2H-pyran-5-yl)oxy)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-3,5,5-trimethylcyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 7 | (R)-4-((R,E)-3-(((2R,3R,4S,5R,6R)-4-(((2R,3S,4S)-2-((((2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)-3,4-dihydro-2H-pyran-5-yl)oxy)methyl)-3,4-dihydroxy-3,4-dihydro-2H-pyran-5-yl)oxy)-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)but-1-en-1-yl)-3,5,5-trimethylcyclohex-2-en-1-one | B. gymnorrhiza, leaf | [34] |
| 8 | Acaciicolin A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 9 | Acaciicolide A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 10 | Acaciicolide B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 11 | Acaciicolide C | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 12 | Acaciicolinol A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 13 | Acaciicolinol B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 14 | Acaciicolinol C | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 15 | Acaciicolinol D | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 16 | Acaciicolinol E | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 17 | Acaciicolinol F | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 18 | Acaciicolinol G | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 19 | Acaciicolinol H | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 20 | Acaciicolinol I | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 21 | Acaciicolinol J | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 22 | Acaciicolinol K | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 23 | Acaciicolinol L | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 24 | Spiroacaciicolide A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 25 | Spiroacaciicolide B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 26 | Spiroacaciicolide C | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 27 | 3-epi-Steperoxide A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 28 | 3-epi-merulin A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 29 | (3S,4R,6aS,10aR)-4-hydroxy-7,7,10a-trimethyl-5,6,7,10a-tetrahydro-3H-3,6a-methanobenzo[c] [1,2] dioxocin-10(4H)-one | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 30 | Steperoxide A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 31 | Merulin A | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 32 | Merulin B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 33 | Merulin C | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [28] |
| 34 | Merulin D | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 35 | 7-epi-merulin B | P. acaciicola (the root of B. gymnorrhiza, endophytic fungus) | [29] |
| 36 | Petasol | B. gymnorrhiza | [35] |
| 37 | Sporogen AO1 | B. gymnorrhiza | [35] |
| 38 | 6-dehydropetasol | B. gymnorrhiza | [35] |
| 39 | 3α-hydroxy-11-peroxyl-eremophila-6,9-dien-8-one | B. gymnorrhiza | [35] |
| 40 | Botryosphaerin F | Aspergillus terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [36] |
| 41 | 13,14,15,16-tetranorlabd-7-ene-19,6b:12,17-diolide | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [36] |
| 42 | Botryosphaerin B | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [36] |
| 43 | LLZ1271β | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [36] |
| 44 | Xiamycin | Streptomyces sp. GT2002/1503 (the stem of B. gymnorrhiza, endophytic actinomycete) | [30] |
| 45 | Methyl ester of xiamycin | Streptomyces sp. GT2002/1503 (the stem of B. gymnorrhiza, endophytic actinomycete) | [30] |
| 46 | Bacaryolane A | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [31] |
| 47 | Bacaryolane B | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [31] |
| 48 | Bacaryolane C | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [31] |
| 49 | 7R-hydroxygeosmin | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 50 | 3-oxogeosmin | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 51 | 2R-hydroxy-7-oxogeosmin | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 52 | 5-deoxy-7β,9β-dihydroxygeosmin | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 53 | (4S,5S,7R,10S)-4β,10α-eudesmane-5β,11-diol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 54 | (1S,5S,6S,7S,10S)-10α-eudesm-4(15)-ene-1α,6α-diol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 55 | 1(10)E,5E-germacradiene-2,11-diol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [32] |
| 56 | (+)-11-hydroxy-epicubenol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [33] |
| 57 | (+)-12-hydroxy-epicubenol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [33] |
| 58 | 5,11-epoxy-10-cadinanol | Streptomyces sp. JMRC:ST027706 (the stem of B. gymnorrhiza, endophytic actinomycete) | [33] |
2.1.3. Diterpenoids
2.1.4. Sesterpenoids
2.1.5. Triterpenoids
2.1.6. Meroterpenoids
2.2. Steroids
2.3. Sulfides
2.4. Alkaloids and Peptides
2.5. Quinones
2.6. Polyketides
2.7. Flavonoids
2.8. Phenylpropanoids
2.9. Aromatic Compounds
2.10. Other Compounds
3. Bioactivities
3.1. Cytotoxic Activity
3.2. Antimicrobial Activity
3.3. Antioxidant Activity
3.4. Anti-Inflammatory Activity
3.5. Antiviral Activity
3.6. Antidiabetic Activity
3.7. Insecticidal and Mosquito Repellent Activity
3.8. Enzyme Inhibitory Activity
3.9. Other Activities
4. Interactions between Bruguiera Genus and Its Endophytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.S.; Liang, S.C.; Zhang, W.Y.; Zan, Q.J. Mangrove flora of the world. Acta Bot. Sin. 2003, 45, 644–653. [Google Scholar]
- Lin, P. Distribution of mangrove species. Sci. Silvae Sin. 1987, 23, 481–490. [Google Scholar]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zhang, X.; Wang, J.S.; Shen, X.X.; Tang, L.L.; Li, R.L. Utilization Status and Development Countermeasures of Mangrove Medicinal Resources in the Marine-Terrestrial Interlaced Zone. Acta Sci. Nat. Univ. Pekin. 2023, 59, 704–718. [Google Scholar]
- Bibi, S.N.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Kannan, R.R.R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves—A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Sani, W.; Hossain, A.B.M.S.; Taha, R.M.; Monneruzzaman, K.M. Total phenolic contents, antioxidant and antimicrobial activities of Bruguiera gymnorrhiza. J. Med. Plants Res. 2011, 5, 4112–4118. [Google Scholar]
- Sur, T.K.; Hazra, A.; Hazra, A.K.; Bhattacharyya, D. Antioxidant and hepatoprotective properties of Indian Sunderban mangrove Bruguiera gymnorrhiza L. leave. J. Basic Clin. Pharm. 2016, 7, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Karimulla, S.; Kumar, B.P. Anti diabetic and Anti hyperlipidemic activity of bark of Bruguiera gymnorrhiza on streptozotocin induced diabetic rats. Asian J. Pharm. Sci. Technol. 2011, 1, 4–7. [Google Scholar]
- Karim, M.A.; Islam, M.A.; Islam, M.M.; Rahman, M.S.; Sultana, S.; Biswas, S.; Hosen, M.J.; Mazumder, K.; Rahman, M.M.; Hasan, M.N. Evaluation of antioxidant, anti-hemolytic, cytotoxic effects and anti-bacterial activity of selected mangrove plants (Bruguiera gymnorrhiza and Heritiera littoralis) in Bangladesh. Clin. Phytosci. 2020, 6, 8. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Sasikumar, J.M.; Shamna, R.; Pandiarajan, C.; Sofia, P.; Nagarajan, B. Antioxidant activities of bark extract from mangroves, Bruguiera cylindrica (L.) Blume and Ceriops decandra Perr. Indian J. Pharmacol. 2011, 43, 557–562. [Google Scholar]
- Loder, J.W.; Russell, G.B. Tropine 1,2-dithiolane-3-carboxylate, a new alkaloid from Bruguiera sexangula. Tetrahedron Lett. 1966, 51, 6327–6329. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Wang, D.N.; Ming, X.C.; Li, W.; Han, B.; Zhao, J.; Huang, G.L. Recent Advances of Chemical Constituents of Genus Bruguiera. Guangdong Chem. Ind. 2014, 41, 45–46,50. [Google Scholar]
- Xie, L.H.; Hou, X.T.; Deng, J.G.; Wei, L.Y.; Xia, Z.S.; Du, Z.C. Research Progress on Chemical Constituents and Pharmacological Effect of Bruguiera gymnorrhiza. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 225–234. [Google Scholar]
- Chen, S.H.; Cai, R.L.; Liu, Z.M.; Cui, H.; She, Z.G. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2021, 39, 560–595. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, S.; Luo, X.M.; Liu, Y.H. Preparative isolation and purification of two benzoxazinoid glucosides from Acanthus ilicifolius L. by high-speed counter-current chromatography. J. Chromatogr. A 2008, 1205, 177–181. [Google Scholar] [CrossRef]
- Gao, C.H.; Tian, X.P.; Qi, S.H.; Luo, X.M.; Wang, P.; Zhang, S. Antibacterial and antilarval compounds from marine gorgonian-associated bacterium Bacillus amyloliquefaciens SCSIO 00856. J. Antibiot. 2010, 63, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Luo, M.Q.; Xia, M.; Wu, Q.; Long, S.M.; Hu, Y.H.; Gao, G.C.; Yao, X.L.; He, M.; Su, H.X.; et al. Marine Compound Catunaregin Inhibits Angiogenesis through the Modulation of Phosphorylation of Akt and eNOS in vivo and in vitro. Mar. Drugs 2014, 12, 2790–2801. [Google Scholar] [CrossRef]
- Tang, H.; Ge, H.; Chen, Z.B.; Luo, X.M.; Su, F.J.; Liang, Y.B.; Li, Z.Y.; Wu, J.G.; Yang, Q.; Zeng, L.J.; et al. Micrometam C Protects against Oxidative Stress in Inflammation Models in Zebrafish and RAW264.7 Macrophages. Mar. Drugs 2015, 13, 5593–5605. [Google Scholar] [CrossRef]
- Liu, B.; Jin, X.B.; Chen, X.H.; Wang, X.; Zhang, W.B.; Luo, X.M. Two New Lactam Derivatives from Micromelum falcatum (Lour.) Tan. with Brine Shrimp Larvae Toxicity. Molecules 2023, 28, 7157. [Google Scholar] [CrossRef]
- Jia, S.L.; Chi, Z.; Liu, G.L.; Hu, Z.; Chi, Z.M. Fungi in mangrove ecosystems and their potential applications. Crit. Rev. Biotechnol. 2020, 40, 852–864. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Li, X.M.; Yang, S.Q.; Meng, L.H.; Wang, B.G. Thiocladospolides A−D, 12-Membered Macrolides from the Mangrove-Derived Endophytic Fungus Cladosporium cladosporioides MA-299 and Structure Revision of Pandangolide 3. J. Nat. Prod. 2019, 82, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Baunach, M.; Ding, L.; Peng, H.Y.; Franke, J.; Hertweck, C. Biosynthetic Code for Divergolide Assembly in a Bacterial Mangrove Endophyte. Chembiochem 2014, 15, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Nguyen, L.T.; Dhakal, R.; Murphy, B.T. The need to innovate sample collection and library generation in microbial drug discovery: A focus on academia. Nat. Prod. Rep. 2021, 38, 292–300. [Google Scholar] [CrossRef]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How different are marine microbial natural products compared to their terrestrial counterparts? Nat. Prod. Rep. 2022, 39, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, B.Y.; Yang, X.L. Antifungal Monoterpene Derivatives from the Plant Endophytic Fungus Pestalotiopsis foedan. Chem. Biodivers. 2016, 13, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Toyota, M.; Asakawa, Y.; Takaso, T.; Tobe, H. Floral scent chemistry of mangrove plants. J. Plant Res. 2002, 115, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Tricyclic and Spirobicyclic Norsesquiterpenes from the Endophytic Fungus Pseudolagarobasidium acaciicola. Eur. J. Org. Chem. 2014, 2014, 3976–3980. [Google Scholar] [CrossRef]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Cytotoxic sesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Phytochemistry 2016, 122, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Münich, J.; Goerls, H.; Maier, A.; Fiebig, H.H.; Lin, W.H.; Hertweck, C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg. Med. Chem. Lett. 2010, 20, 6685–6687. [Google Scholar] [CrossRef]
- Ding, L.; Goerls, H.; Dornblut, K.; Lin, W.; Maier, A.; Fiebig, H.H.; Hertweck, C. Bacaryolanes A-C, Rare Bacterial Caryolanes from a Mangrove Endophyte. J. Nat. Prod. 2015, 78, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Hertweck, C. Oxygenated Geosmins and Plant-like Eudesmanes from a Bacterial Mangrove Endophyte. J. Nat. Prod. 2020, 83, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Görls, H.; Hertweck, C. Plant-like Cadinane Sesquiterpenes from an Actinobacterial Mangrove Endophyte. Magn. Reson. Chem. MRC 2021, 59, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiao, H.; Sun, D.; Duan, S.S. Investigation of the Inhibitory Effects of Mangrove Leaves and Analysis of Their Active Components on Phaeocystis globosa during Different Stages of Leaf Age. Int. J. Environ. Res. Public Health 2018, 15, 2434. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.S.; Liu, H.L.; Gong, J.X.; Guo, Y.W. Chemical constituents of mangrove plant Bruguiera gymnorrhiza. Chin. J. Mar. Drugs 2011, 30, 15–18. [Google Scholar]
- Deng, C.M.; Huang, C.H.; Wu, Q.L.; Pang, J.Y.; Lin, Y.C. A new sesquiterpene from the mangrove endophytic fungus Aspergillus terreus (No. GX7-3B). Nat. Prod. Res. 2013, 27, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.D.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Sun, Y.W.; Liu, H.L.; Wang, Y. Pathway elucidation and engineering of plant-derived diterpenoids. Curr. Opin. Biotechnol. 2021, 69, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.N.; Sircar, S.M. Gibberellins from mangrove plants. Phytochemistry 1974, 13, 1911–1913. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Rao, B.V.; Ward, R.S.; Hursthouse, M.B.; Hibbs, D.E. Diterpenes from the marine mangrove Bruguiera gymnorhiza. Phytochemistry 1999, 51, 83–90. [Google Scholar] [CrossRef]
- Bao, S.Y.; Deng, Z.W.; Fu, H.Z.; Proksch, P.; Lin, W.H. Diterpenes and Disulfides from the Marine Mangrove Plant Bruguiera sexangula var. rhynchopetala. Helv. Chim. Acta 2005, 88, 2757–2763. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.S.; Sattler, I.; Dahse, H.M.; Fu, H.Z.; Lin, W.H.; Grabley, S. New Diterpenoids from the Marine Mangrove Bruguiera gymnorrhiza. J. Nat. Prod. 2004, 67, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Huang, X.S.; Sattler, I.; Dahse, H.M.; Fu, H.Z.; Grabley, S.; Lin, W.H. Three new pimaren diterpenoids from marine mangrove plant, Bruguiera gymnorrhiza. Pharmazie 2005, 60, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Liu, Y.; Li, S.H. The untapped potential of plant sesterterpenoids: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.; Li, C.; Wang, B. Sesterterpenes and 2H-Pyran-2-ones (=α-Pyrones) from the Mangrove-Derived Endophytic Fungus Fusarium proliferatum MA-84. Helv. Chim. Acta 2013, 96, 437–444. [Google Scholar] [CrossRef]
- Homhual, S.; Bunyapraphatsara, N.; Kondratyuk, T.; Herunsalee, A.; Chaukul, W.; Pezzuto, J.M.; Fong, H.H.S.; Zhang, H.J. Bioactive Dammarane Triterpenes from the Mangrove Plant Bruguiera gymnorrhiza. J. Nat. Prod. 2006, 69, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Li, L.A.; Huang, C.G.; Wang, C.Y.; Guo, Y.W. Sexangulic acid, a new cytotoxic triterpenoid from the Chinese mangrove Bruguiera sexangula. Nat. Prod. Res. 2010, 24, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Huang, G.L.; Tang, X.Z.; Wang, D.N.; Gong, X.L.; Zhang, Q.; Song, X.P.; Chen, G.Y. Secondary Metabolites and Antibacterial Activities of a Bruguiera sexangula var. Rhynchopetala-Derived Fungus Penicillium sp. (J41221). Chin. J. Org. Chem. 2014, 34, 1172–1176. [Google Scholar] [CrossRef]
- Sarkar, A.; Ganguly, S.N. Gymnorhizol, a new triterpene alcohol from Brugiera gymnorhiza. Chem. Informationsdienst 1979, 10, 138. [Google Scholar]
- Ghosh, A.; Misra, S.; Dutta, A.K.; Choudhury, A. Pentacyclic triterpenoids and sterols from seven species of mangrove. Phytochemistry 1985, 24, 1725–1727. [Google Scholar] [CrossRef]
- Luo, D.Q.; Deng, H.Y.; Yang, X.L.; Shi, B.Z.; Zhang, J.Z. Oleanane-Type Triterpenoids from the Endophytic Fungus Pestalotiopsis clavispora Isolated from the Chinese Mangrove Plant Bruguiera sexangula. Helv. Chim. Acta 2011, 94, 1041–1047. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, K.P.T.; Nguyen, P.P.K.; Le, D.T.; Nguyen, T.L. Anti-Inflammatory and α-Glucosidase Inhibitory Activities of Chemical Constituents from Bruguiera parviflora Leaves. J. Chem. 2022, 2022, 3049994. [Google Scholar] [CrossRef]
- Li, F.; Li, X.M.; Wang, B.G. Chemical constituents of marine mangrove plant Bruguiera gymnorrhiza. Mar. Sci. 2010, 34, 24–27. [Google Scholar]
- Chumkaew, P.; Kato, S.; Chantrapromma, K. A New Triterpenoid Ester from the Fruits of Bruguiera parviflora. Chem. Pharm. Bull. 2005, 53, 95–96. [Google Scholar] [CrossRef]
- Karalai, C.; Laphookhieo, S. Triterpenoid Esters from Bruguiera cylindrica. Aust. J. Chem. 2005, 58, 556–559. [Google Scholar] [CrossRef]
- Chantrapromma, S.; Fun, H.-K.; Razak, I.A.; Laphookhieo, S.; Karalai, C. Absolute configuration of 3-feruloyltaraxerol dichloromethane solvate. Acta Cryst. E 2003, 59, o1864–o1866. [Google Scholar] [CrossRef]
- Laphookhieo, S.; Karalai, C.; Ponglimanont, C.; Chantrapromma, K. Pentacyclic Triterpenoid Esters from the Fruits of Bruguiera cylindrica. J. Nat. Prod. 2004, 67, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Kalappurakkal, V.N.; Bhattacharya, D.; Chakravarty, S.; Uppuluri, M.V. Isolation, Synthesis and AChE Inhibitory Potential of Some Novel Cinnamyl Esters of Taraxerol, the Major Metabolite of the Mangrove Bruguiera cylindrica. Chem. Biodivers. 2018, 15, e1800008. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Liu, L.; Chen, S. The chemistry and biology of fungal meroterpenoids (2009–2019). Org. Biomol. Chem. 2020, 19, 1644–1704. [Google Scholar] [CrossRef]
- Huang, G.L.; Zhou, X.M.; Bai, M.; Liu, Y.X.; Zhao, Y.L.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.J.; Chen, G.Y. Dihydroisocoumarins from the Mangrove-Derived Fungus Penicillium citrinum. Mar. Drugs 2016, 14, 177. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Huang, G.L.; Mei, R.Q.; Wang, B.; Luo, Y.P.; Zheng, C.; Niu, Z.G.; Chen, G.Y. Bioactive Meroterpenoids and Isocoumarins from the Mangrove Derived Fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zheng, C.J.; Chen, G.Y. Austins-Type Meroterpenoids from a Mangrove-Derived Penicillium sp. J. Nat. Prod. 2021, 84, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Sofian, F.F.; Suzuki, T.; Supratman, U.; Harneti, D.; Maharani, R.; Salam, S.; Abdullah, F.F.; Koseki, T.; Tanaka, K.; Kimura, K.I.; et al. Cochlioquinone derivatives produced by coculture of endophytes, Clonostachys rosea and Nectria pseudotrichia. Fitoterapia 2021, 155, 105056. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Xiong, B.X.; Xu, J. Chemical Investigation Of Secondary Metabolites Produced By Mangrove Endophytic Fungus Phyllosticta Capitalensis. Nat. Prod. Res. 2019, 35, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G.; Lathe, R. Terpenes, hormones and life: Isoprene rule revisited. J. Endocrinol. 2019, 242, R9–R22. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, Y.; Wang, H.Z.; Wang, S.J.; Zhang, R.Q. The metabolites of a mangrove endophytic fungus, Penicillium thomi. J. Asian Nat. Prod. Res. 2007, 9, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Song, X.M.; Zhou, X.M.; Li, X.B.; Zheng, C.J.; Huang, G.L.; Yu, Z.X.; Song, X.P.; Chen, G.Y. Secondary Metabolites of a Bruguiera sexangula var. Rhynchopetala Derived Fungus Phomopsis longicolla HL-2232. Chin. J. Org. Chem. 2015, 35, 2102–2107. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Jiang, C.S.; Li, G.; Guo, Y.W. (+)-Cyclopenol, a new naturally occurring 7-membered 2,5-dioxopiperazine alkaloid from the fungus Penicillium sclerotiorum endogenous with the Chinese mangrove Bruguiera gymnorrhiza. J. Asian Nat. Prod. Res. 2014, 16, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.M.; Liu, S.X.; Huang, C.H.; Pang, J.Y.; Lin, Y.C. Secondary Metabolites of a Mangrove Endophytic Fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616–2624. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Yang, X.H.; Liu, H.L.; Gu, Y.C.; Ye, B.P.; Guo, Y.W. A New Cyclic Peptide and a New Steroid from the Fungus Penicillium sp. GD6 Isolated from the Mangrove Bruguiera gymnorrhiza. Helv. Chim. Acta 2014, 97, 1564–1570. [Google Scholar] [CrossRef]
- Liu, Z.M.; Li, M.Q.; Wang, S.; Huang, H.B.; Zhang, W.M. Sulfur-Containing Metabolites from Marine and Terrestrial Fungal Sources: Origin, Structures, and Bioactivities. Mar. Drugs 2022, 20, 765. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Shen, X.; Jiang, H.L.; Guo, Y.W. Structural Studies on an Unusual Novel Macrocyclic Polydisulfide from the Chinese Mangrove Bruguiera gymnorrhiza. Chin. J. Org. Chem. 2008, 28, 246–251. [Google Scholar]
- Nwachukwu, I.D.; Slusarenko, A.J.; Gruhlke, M.C. Sulfur and Sulfur Compounds in Plant Defence. Nat. Prod. Commun. 2012, 7, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Dahibhate, N.L.; Shukla, S.K.; Kumar, K. A Cyclic Disulfide Diastereomer From Bioactive Fraction of Bruguiera gymnorhiza Shows Anti–Pseudomonas aeruginosa Activity. Front. Pharmacol. 2022, 13, 890790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Z.; Li, X.M.; Meng, L.H.; Wang, B.G. Cladocladosin A, an unusual macrolide with bicyclo 5/9 ring system, and two thiomacrolides from the marine mangrove-derived endophytic fungus, Cladosporium cladosporioides MA-299. Bioorg Chem. 2020, 101, 103950. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Guo, Y.W. Gymnorrhizol, an unusual macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Tetrahedron Lett. 2004, 45, 5533–5535. [Google Scholar] [CrossRef]
- Kato, A.; Takahashi, J. A new naturally occurring 1,2-dithiolane from Bruguiera cylindrica. Phytochem. Lett. 1976, 15, 220–221. [Google Scholar] [CrossRef]
- Kato, A.; Numata, M. Brugierol and Isobrugierol, trans- and cis-1,2-dithiolane-1-oxide, from Brugiera conjugata. Tetrahedron Lett. 1972, 13, 203–206. [Google Scholar] [CrossRef]
- Homhual, S.; Zhang, H.J.; Bunyapraphatsara, N.; Kondratyuk, T.P.; Santarsiero, B.D.; Mesecar, A.D.; Herunsalee, A.; Chaukul, W.; Pezzuto, J.M.; Fong, H.H. Bruguiesulfurol, A New Sulfur Compound from Bruguiera gymnorrhiza. Planta Med. 2006, 72, 255–260. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, Q.; Liu, H.L.; Zhang, Y.; Xin, G.R.; Shen, X.; Dong, M.L.; Guo, Y.W. Diastereoisomeric macrocyclic polydisulfides from the mangrove Bruguiera gymnorrhiza. Phytochemistry 2009, 70, 2096–2100. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamada, Y. Alkaloid Biogenesis: Molecular Aspects. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 257–285. [Google Scholar] [CrossRef]
- Lei, F.Y.; Liu, X.D.; Huang, H.N.; Fu, S.D.; Zou, K.; Zhang, S.F.; Zhou, L.; Zeng, J.G.; Liu, H.W.; Jiang, L.H.; et al. The Macleaya cordata Symbiont: Revealing the Effects of Plant Niches and Alkaloids on the Bacterial Community. Front. Microbiol. 2021, 12, 681210. [Google Scholar] [CrossRef] [PubMed]
- Arunpanichlert, J.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Tewtrakul, S.; Rungjindamai, N.; Sakayaroj, J. Azaphilone and Isocoumarin Derivatives from the Endophytic Fungus Penicillium sclerotiorum PSU-A13. Chem. Pharm. Bull. 2010, 58, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Bracken, A.; Pocker, A.; Raistrick, H. Studies in the biochemistry of microorganisms. 93. Cyclopenin, a nitrogen-containing metabolic product of Penicillium cyclopium Westling. Biochem. J. 1954, 57, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Qu, C.H.; Lu, J.; Cheng, G.; Gao, C.H. A new antiviral alkaloid from the hypocotyl of Bruguiera gymnorrhiza. Guihaia 2016, 36, 236–239. [Google Scholar]
- Yan, L.L.; Han, N.N.; Zhang, Y.Q.; Yu, L.Y.; Chen, J.; Wei, Y.Z.; Li, Q.P.; Tao, L.; Zheng, G.H.; Yang, S.E.; et al. Antimycin A18 produced by an endophytic Streptomyces albidoflavus isolated from a mangrove plant. J. Antibiot. 2010, 63, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Liao, H.X.; Mei, R.Q.; Huang, G.L.; Yang, L.J.; Zhou, X.M.; Shao, T.M.; Chen, G.Y.; Wang, C.Y. Two new benzophenones and one new natural amide alkaloid isolated from a mangrove-derived Fungus Penicillium citrinum. Nat. Prod. Res. 2018, 33, 1127–1134. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Kurtán, T.; Yang, X.H.; Mándi, A.; Geng, M.Y.; Ye, B.P.; Taglialatela-Scafati, O.; Guo, Y.W. Penibruguieramine A, a Novel Pyrrolizidine Alkaloid from the Endophytic Fungus Penicillium sp. GD6 Associated with Chinese Mangrove Bruguiera gymnorrhiza. Org. Lett. 2014, 16, 1390–1393. [Google Scholar] [CrossRef]
- Kato, A. Brugine from Bruguiera cylindrica. Phytochemistry 1975, 14, 1458. [Google Scholar] [CrossRef]
- Jiang, C.S.; Zhou, Z.F.; Yang, X.H.; Lan, L.F.; Gu, Y.C.; Ye, B.P.; Guo, Y.W. Antibacterial sorbicillin and diketopiperazines from the endogenous fungus Penicillium sp. GD6 associated Chinese mangrove Bruguiera gymnorrhiza. Chin. J. Nat. Med. 2018, 16, 358–365. [Google Scholar] [CrossRef]
- Yi, X.X.; Deng, J.G.; Gao, C.H.; Hou, X.T.; Li, F.; Wang, Z.P.; Hao, E.W.; Xie, Y.; Du, Z.C.; Huang, H.X.; et al. Four New Cyclohexylideneacetonitrile Derivatives from the Hypocotyl of Mangrove (Bruguiera gymnorrhiza). Molecules 2015, 20, 14565–14575. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Chang, M.; Zhang, Q.H.; He, R.F.; Ye, B.P. The endophytic fungus strain ZD6 isolated from the stem of Bruguiera gymnorrhiza and the antibacterial activity of its metabolites. Mycosystema 2010, 29, 739–745. [Google Scholar]
- Li, C.Y.; Chen, M.; Ding, W.J.; Lin, Y.C.; Zhou, S.N. Study on the Metabolites of Endophytic Fungus (Gloesporium sp.) Isolated from Bruguiera gymnoihiza Grown in the South China Sea. J. South. China Agric. Univ. 2008, 29, 122–124. [Google Scholar]
- Zheng, C.J.; Wu, L.Y.; Li, X.B.; Song, X.M.; Niu, Z.G.; Song, X.P.; Chen, G.Y.; Wang, C.Y. Structure and Absolute Configuration of Aspergilumamide A, a Novel Lumazine Peptide from the Mangrove-Derived Fungus Aspergillus Sp. Helv. Chim. Acta 2015, 98, 368–373. [Google Scholar] [CrossRef]
- Laohavisit, A.; Wakatake, T.; Ishihama, N.; Mulvey, H.; Takizawa, K.; Suzuki, T.; Shirasu, K. Quinone perception in plants via leucine-rich-repeat receptor-like kinases. Nature 2020, 587, 92–97. [Google Scholar] [CrossRef]
- Krueger, M.C.; Bergmann, M.; Schlosser, D. Widespread ability of fungi to drive quinone redox cycling for biodegradation. Fems Microbiol. Lett. 2016, 363, fnw105. [Google Scholar] [CrossRef]
- Klaiklay, S.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Buatong, J.; Bussaban, B. Metabolites from the Mangrove-derived Fungus Xylaria cubensis PSU-MA34. Arch. Pharm. Res. 2012, 35, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.S.; Kurtán, T.; Miao, Z.H.; Mándi, A.; Komáromi, I.; Liu, H.L.; Ding, J.; Guo, Y.W. Palmarumycins BG1-BG7 and Preussomerin BG1: Establishment of Their Absolute Configurations Using Theoretical Calculations of Electronic Circular Dichroism Spectra. J. Org. Chem. 2011, 76, 1821–1830. [Google Scholar] [CrossRef]
- Kongyen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Sakayaroj, J. A new hydronaphthalenone from the mangrove-derived Daldinia eschscholtzii PSU-STD57. Nat. Prod. Res. 2015, 29, 1995–1999. [Google Scholar] [CrossRef]
- Yang, L.J.; Liao, H.X.; Bai, M.; Huang, G.L.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.J.; Wang, C.Y. One new cytochalasin metabolite isolated from a mangrove-derived fungus Daldinia eschscholtzii HJ001. Nat. Prod. Res. 2018, 32, 208–213. [Google Scholar] [CrossRef]
- Ukwatta, K.M.; Lawrence, J.L.; Wijayarathna, C.D. The study of antimicrobial, anti-cancer, antiinflammatory and α-glucosidase inhibitory activities of Nigronapthaphenyl, isolated from an extract of Nigrospora sphaerica. Mycology 2019, 10, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Huang, G.L.; Xu, Y.; Song, X.M.; Yao, J.; Liu, H.; Wang, R.P.; Sun, X.P. A new benzopyrans derivatives from a mangrove-derived fungus Penicillium citrinum from the South China Sea. Nat. Prod. Res. 2015, 30, 821–825. [Google Scholar] [CrossRef]
- Liao, H.X.; Shao, T.M.; Mei, R.Q.; Huang, G.L.; Zhou, X.M.; Zheng, C.J.; Wang, C.Y. Bioactive Secondary Metabolites from the Culture of the Mangrove-Derived Fungus Daldinia eschscholtzii HJ004. Mar. Drugs 2019, 17, 710. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Jiao, F.W.; Li, W.; Zhang, X.; Yan, W.; Jiao, R.H. Cytotoxic Xanthone Derivatives from the Mangrove-Derived Endophytic Fungus Peniophora incarnata Z4. J. Nat. Prod. 2020, 83, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- He, K.Y.; Zhang, C.; Duan, Y.R.; Huang, G.L.; Yang, C.Y.; Lu, X.R.; Zheng, C.J.; Chen, G.Y. New chlorinated xanthone and anthraquinone produced by a mangrove-derived fungus Penicillium citrinum HL-5126. J. Antibiot. 2017, 70, 823–827. [Google Scholar] [CrossRef]
- Zhou, X.M.; Zheng, C.J.; Chen, G.Y.; Song, X.P.; Han, C.R.; Li, G.N.; Fu, Y.H.; Chen, W.H.; Niu, Z.G. Bioactive Anthraquinone Derivatives from the Mangrove-Derived Fungus Stemphylium sp. 33231. J. Nat. Prod. 2014, 77, 2021–2028. [Google Scholar] [CrossRef]
- Li, X.B.; Chen, G.Y.; Liu, R.J.; Zheng, C.J.; Song, X.M.; Han, C.R. A new biphenyl derivative from the mangrove endophytic fungus Phomopsis longicolla HL-2232. Nat. Prod. Res. 2017, 31, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Supratman, U.; Suzuki, T.; Nakamura, T.; Yokoyama, Y.; Harneti, D.; Maharani, R.; Salam, S.; Abdullah, F.F.; Koseki, T.; Shiono, Y. New metabolites produced by endophyte Clonostachys rosea B5-2. Nat. Prod. Res. 2019, 35, 1525–1531. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Garrido-Santos, M.Y. Phytotoxic compounds from endophytic fungi. Appl. Microbiol. Biotechnol. 2022, 106, 931–950. [Google Scholar] [CrossRef]
- Su, J.H.; Wang, M.Q.; Li, Y.Z.; Lin, Y.S.; Gu, J.Y.; Zhu, L.P.; Yang, W.Q.; Jiang, S.Q.; Zhao, Z.X.; Sun, Z.H. Rare cytochalasans isolated from the mangrove endophytic fungus Xylaria arbuscula. Fitoterapia 2022, 157, 105124. [Google Scholar] [CrossRef]
- Huang, H.B.; Li, Q.; Feng, X.J.; Chen, B.; Wang, J.; Liu, L.; She, Z.G.; Lin, Y.C. Structural elucidation and NMR assignments of four aromatic lactones from amangrove endophytic fungus (No. GX4-1B). Magn. Reson. Chem. MRC 2010, 48, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Chen, T.; Yu, J.C.; Jia, H.; Chen, C.; Long, Y.H. New Sorbicillinoids from the Mangrove Endophytic Fungus Trichoderma reesei SCNU-F0042. Mar. Drugs 2023, 21, 442. [Google Scholar] [CrossRef]
- Zou, G.; Tan, Q.; Chen, Y.; Yang, W.C.; Zang, Z.M.; Jiang, H.M.; Chen, S.Y.; Wang, B.; She, Z.G. Furobenzotropolones A, B and 3-Hydroxyepicoccone B with Antioxidative Activity from Mangrove Endophytic Fungus Epicoccum nigrum MLY-3. Mar. Drugs 2021, 19, 395. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Li, X.M.; Li, X.; Yang, S.Q.; Meng, L.H.; Wang, B.G. Polyketides from the Mangrove-Derived Endophytic Fungus Cladosporium cladosporioides. Mar. Drugs 2019, 17, 296. [Google Scholar] [CrossRef]
- Deng, P.F.; Luo, Y.P.; Niu, Y.Y.; Zheng, C.J.; Chen, G.Y.; Chen, J.; Ma, W.H. A New Penicitrinone Derivative from the Endophytic Fungus Penicillium sp. from Bruguiera sexangula var. rhynchopetala. Chem. Nat. Compd. 2016, 52, 810–812. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.J.; Cheng, Y.; Gao, H.S.; Chen, X.H. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Qiao, F.; Xu, G.W.; Zhao, J.; Teng, J.F.; Li, C.; Deng, W.J. Neuroprotective metabolites from the endophytic fungus Penicillium citrinum of the mangrove Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 12, 148–152. [Google Scholar] [CrossRef]
- Hu, Y.O.; Ye, B.P.; Wang, D.Y. Study on the bioactivity of 3′,4′,5′-trihydroxy-7-hydroxy-5-methoxyflavone extracted from Bruguiera gymnorrhiza by C.elegans. Chin. J. Mar. Drugs 2007, 26, 20–26. [Google Scholar]
- Panyadee, A.; Sahakitpichan, P.; Ruchirawat, S.; Kanchanapoom, T. 5-Methyl ether flavone glucosides from the leaves of Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 11, 215–219. [Google Scholar] [CrossRef]
- Barik, R.; Sarkar, R.; Biswas, P.; Bera, R.; Sharma, S.; Nath, S.; Karmakar, S.; Sen, T. 5,7-dihydroxy-2-(3-hydroxy-4, 5-dimethoxy-phenyl)-chromen-4-one-a flavone from Bruguiera gymnorrhiza displaying anti-inflammatory properties. Indian. J. Pharmacol. 2016, 48, 304–311. [Google Scholar]
- Yao, J.E.; Shen, M.R.; Yi, X.X.; Yang, Y.; Gao, C.H. A New 8-Hydroxyquercetagetin Glycoside from the Hypocotyls of Mangrove Bruguiera gymnorrhiza. Chem. Nat. Comp. 2017, 53, 33–35. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Stringlis, I.A.; de Jonge, R.; Pieterse, C.M.J. The Age of Coumarins in Plant–Microbe Interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef]
- Yi, X.X.; Gao, C.H.; He, B.J.; Chen, B. Study on phenylpropanoids from hypocotyls of the mangrove plant Bruguiera gymnorrhiza. Guihaia 2013, 33, 191–194. [Google Scholar]
- Han, Z.; Mei, W.L.; Zhao, Y.X.; Deng, Y.Y.; Dai, H.F. A new cytotoxic isocoumarin from endophytic fungus Penicillium sp. 091402 of the mangrove plant Bruguiera sexangula. Chem. Nat. Compd. 2009, 45, 805–807. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.S.; Sattler, I.; Fu, H.Z.; Grabley, S.; Lin, W.H. Two new constituents from mangrove Bruguiera gymnorrhiza. J. Asian Nat. Prod. Res. 2007, 9, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.S.; Long, S.J. Brugnanin, a new syn-2,3-dihydrobenzofuran neolignan dioate from the mangrove Bruguiera gymnorrhiza. Chem. Nat. Compd. 2008, 44, 186–189. [Google Scholar] [CrossRef]
- Bao, S.; Ding, Y.; Deng, Z.; Proksch, P.; Lin, W. Rhyncosides A-F, phenolic constituents from the Chinese mangrove plant Bruguiera sexangula var. rhynchopetala. Chem. Pharm. Bull. 2007, 55, 1175–1180. [Google Scholar] [CrossRef]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal-plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.S.; Sattler, I.; Moellmann, U.; Fu, H.Z.; Lin, W.H.; Grabley, S. New aromatic compounds from the marine mangrove Bruguiera gymnorrhiza. Planta Med. 2005, 71, 160–164. [Google Scholar] [CrossRef]
- Gao, C.H.; Long, B.; Su, Z.W.; Yu, L.; Yan, D.M.; Wen, L.J.; He, B.J. Study on Aromatic Compounds from Hypocotyls of Mangrove Bruguiera gymnorrhiza. J. Guangxi Acad. Sci. 2015, 31, 28–31. [Google Scholar]
- Seshadri, T.R.; Trikha, R.K. Procyanidin of Bruguiera parviflora. Indian. J. Chem. 1971, 9, 302–304. [Google Scholar]
- Chokpaiboon, S.; Choodej, S.; Boonyuen, N.; Teerawatananond, T.; Pudhom, K. Highly oxygenated chromones from mangrove-derived endophytic fungus Rhytidhysteron rufulum. Phytochemistry 2016, 122, 172–177. [Google Scholar] [CrossRef]
- Sriyatep, T.; Maneerat, W.; Sripisut, T.; Cheenpracha, S.; Machan, T.; Phakhodee, W.; Laphookhieo, S. Cowabenzophenones A and B, two new tetracyclo[7.3.3.33,11.03,7]tetradecane-2,12,14-trione derivatives, from ripe fruits of Garcinia cowa. Fitoterapia 2014, 92, 285–289. [Google Scholar] [CrossRef]
- Ukwatta, K.M.; Lawrence, J.L.; Wijayarathne, C.D. Antimicrobial, anti-cancer, anti-filarial and antiinflammatory activities of Cowabenzophenone A extracted from the endophytic fungus Aspergillus terreus isolated from a mangrove plant Bruguiera gymnorrhyza. Mycology 2019, 11, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Habden, X.; Han, N.N.; Yu, S.Y.; Yan, L.L.; Luan, Y.C.; Liu, S.W.; Guo, L.J.; Zhong, K.; Yu, L.Y.; et al. Separation, purification and structural identification of an active nematicidal component from the fermentation broth of Endophytic streptomyces I07A-01824 from Mangrove ecosystem. Chin. Med. Biotechnol. 2012, 7, 5–8. [Google Scholar]
- Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic Effects of Bangladeshi Medicinal Plant Extracts. Evid.-Based Complement. Altern. Med. Ecam. 2011, 2011, 578092. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Q.; Tao, G.L.; Tian, S.H.; Yang, Z.X.; Yao, M.Z.; Huang, L.; Fu, J. Inhibitory effect of Bruguiera sexangula leaves extract on gastric cancer cells proliferation and its acute toxicity in mice. Chin. J. Mar. Drugs 2018, 37, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Qayoom, H.; Alkhanani, M.; Almilaibary, A.; Alsagaby, S.A.; Mir, M.A. A network pharmacology-based investigation of brugine reveals its multi-target molecular mechanism against Breast Cancer. Med. Oncol. 2023, 40, 202. [Google Scholar] [CrossRef]
- Hosen, M.Z.; Biswas, A.; Islam, M.R.; Hossain, S.J. Anti-Bacterial, Anti-Diarrheal, and Cytotoxic Activities of Edible Fruits in the Sundarbans Mangrove Forest of Bangladesh. Prev. Nutr. Food Sci. 2021, 26, 192–199. [Google Scholar] [CrossRef]
- Acharya, S.; Patra, D.K.; Pradhan, C.; Mohapatra, P.K. Anti-bacterial, anti-fungal and anti-oxidative properties of different extracts of Bruguiera gymnorrhiza L. (Mangrove). Eur. J. Integr. Med. 2020, 36, 101140. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Sinan, K.I.; Cziáky, Z.; Jeko, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; Rengasamy, K.R.R.; Mahomoodally, M.F. Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules 2020, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, P.D. Antibacterial Activity of some Medicinal Mangroves against Antibiotic Resistant Pathogenic Bacteria. Indian. J. Pharm. Sci. 2010, 72, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Golder, M.; Sadhu, S.K.; Biswas, B.; Islam, T. Comparative pharmacologic profiles of leaves and hypocotyls of a mangrove plant: Bruguiera gymnorrhiza. Adv. Tradit. Med. 2020, 20, 395–403. [Google Scholar] [CrossRef]
- Tao, G.L.; Yang, F.; Tian, S.H.; Huang, L.; Huang, M.Q. The antioxidative and melanin-inhibitory effects of alcohol extract of Bruguiera sexangula leaves. Chin. J. Mar. Drugs 2019, 38, 59–63. [Google Scholar]
- Liu, X.L.; Chen, T.; Wang, Q.; Liu, J.A.; Lu, Y.H.; Shi, Y. Structure Analysis and Study of Biological Activities of Condensed Tannins from Bruguiera gymnorhiza (L.) Lam and Their Effect on Fresh-Cut Lotus Roots. Molecules 2021, 26, 1369. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Xie, X.G.; Hu, Z.; Xue, J.Y.; Zhang, S.L.; Xie, X.M. (Z)-7,4’-dimethoxy-6-hydroxy-aurone-4-O-β-glucopyranoside attenuates lipoteichoic acid-induced damage in rat cardiomyoblast cells. J. Int. Med. Res. 2020, 48, 300060519889716. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Luo, D.D.; Lin, Y.S.; Liu, Y.H.; Wu, J.Z.; Yi, X.Q.; Wu, Y.; Zhang, Q.; Gao, C.J.; Cai, J.; et al. Aqueous extract of Bruguiera gymnorrhiza leaves protects against dextran sulfate sodium induced ulcerative colitis in mice via suppressing NF-κB activation and modulating intestinal microbiota. J. Ethnopharmacol. 2020, 251, 112554. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Zheng, X.H.; Chen, J.F.; Luo, D.D.; Xie, J.H.; Su, Z.R.; Huang, X.Q.; Yi, X.Q.; Wei, L.; Cai, J.; et al. Protective Effect of Bruguiera gymnorrhiza (L.) Lam. Fruit on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice: Role of Keap1/Nrf2 Pathway and Gut Microbiota. Front. Pharmacol. 2020, 10, 1602. [Google Scholar] [CrossRef]
- Zhang, X.; Mai, J.-H.; Gao, Z.-W.; Wang, L.-L.; Emran, T.B. Bruguiera gymnorrhiza (L.) Lam. Fruit Accelerates Healing in Gastric Injury via the Regulation of the NF-κB Pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 1046712. [Google Scholar] [CrossRef]
- Amalia, R.; Pramono, A.; Afifah, D.N.; Noer, E.R.; Muniroh, M.; Kumoro, A.C. Mangrove fruit (Bruguiera gymnorhiza) increases circulating GLP-1 and PYY, modulates lipid profiles, and reduces systemic inflammation by improving SCFA levels in obese wistar rats. Heliyon 2022, 8, e10887. [Google Scholar] [CrossRef] [PubMed]
- Eldeen, I.M.S.; Ringe, J.; Ismail, N. Inhibition of Pro-inflammatory Enzymes and Growth of an Induced Rheumatoid Arthritis Synovial Fibroblast by Bruguiera cylindrica. Int. J. Pharmacol. 2019, 15, 916–925. [Google Scholar] [CrossRef]
- Hou, S.S.; Liang, C.Y.; Gao, C.H.; Jiang, C.P.; Tang, Q.Q.; Liu, Y.H.; Yi, X.X. Species diversity and anti-hepatitis B virus activity of culturable bacteria isolated from the habitat of Bruguiera gymnorrhiza. Guihaia 2023, 43, 616–625. [Google Scholar]
- Bibi Sadeer, N.; Haddad, J.G.; Oday Ezzat, M.; Desprès, P.; Abdallah, H.H.; Zengin, G.; Uysal, A.; El Kalamouni, C.; Gallo, M.; Montesano, D.; et al. Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections—An In Vitro and In Silico Perspective. Molules 2021, 26, 5768. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Dinesh, D.; Paulpandi, M.; Althbyani, A.D.; Subramaniam, J.; Madhiyazhagan, P.; Wang, L.; Suresh, U.; Kumar, P.M.; Mohan, J.; et al. Nanoparticles in the fight against mosquito-borne diseases: Bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 4349–4361. [Google Scholar] [CrossRef] [PubMed]
- Bibi Sadeer, N.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; AlDhaheri, Y.; Eid, A.H.; Mahomoodally, M.F. Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used. Molules 2022, 27, 2000. [Google Scholar] [CrossRef] [PubMed]
- Pitchaipillai, R.; Ponniah, T. In Vitro Antidiabetic Activity of Ethanolic Leaf Extract of Bruguiera cylindrica L.—Glucose Uptake by Yeast Cells Method. IBBJ 2016, 2, 171–175. [Google Scholar]
- Revathi, P.; Jeyaseelan, S.C.; Subramanian, P.; Manickavasagam, S.; Prabhu, N. A comparative mechanism of antidiabetic role of various extracts of Bruguriera cylindrica L leaves. World J. Pharm. Pharm. Sci. 2015, 4, 1168–1176. [Google Scholar]
- Gong, J.X.; Shen, X.; Yao, L.G.; Jiang, H.L.; Krohn, K.; Guo, Y.W. Total Synthesis of Gymnorrhizol, an Unprecedented 15-Membered Macrocyclic Polydisulfide from the Chinese Mangrove Bruguiera gymnorrhiza. Org. Lett. 2007, 9, 1715–1716. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Gnanadesigan, M. In vitro antiplasmodial activity of ethanolic extracts of mangrove plants from South East coast of India against chloroquine-sensitive Plasmodium falciparum. Parasitol. Res. 2011, 108, 873–878. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Venkatesan, M.; Ramu, A. Mangrove plants as a source of lead compounds for the development of new antiplasmodial drugs from South East coast of India. Parasitol. Res. 2011, 108, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, I.; Zilani, M.N.H.; Biswas, N.N.; Bokshi, B. Bioactivities of Bruguiera gymnorrhiza and profiling of its bioactive polyphenols by HPLC-DAD. Clin. Phytoscience 2017, 3, 11. [Google Scholar] [CrossRef]
- Sun, Z.W.; Tian, F.; An, M.; Duan, S.S. Inhibitive effects of organic solvent extracts from mangrove plant Bruguiera gymnorrhiza on Phaeocystis globosa. Ecol. Sci. 2012, 31, 245–251. [Google Scholar]
- Cui, J.L.; Guo, S.X.; Xiao, P.G. Interaction between endophytes and host plant and the role of endophytes in genuineness analysis of medicinal plant. Acta Pharm. Sin. 2017, 52, 214–221. [Google Scholar]
- Quach, N.T.; Vu, T.H.N.; Bui, T.L.; Le, T.T.X.; Nguyen, T.T.A.; Ngo, C.C.; Phi, Q.-T. Genomic and physiological traits provide insights into ecological niche adaptations of mangrove endophytic Streptomyces parvulus VCCM 22513. Ann. Microbiol. 2022, 72, 27. [Google Scholar] [CrossRef]
- Ma, Q.; Lei, R.F.; Abudourousuli, D.; Aosiman, M.; Rouzi, Z.; An, D.D. Research Progress on the Symbiotic Metabolic of Endophytes and Plants Under Stress. Biotechnol. Bull. 2021, 37, 153–161. [Google Scholar]
- Liu, Y.L.; Qin, Y.Y.; Zheng, H.L. Research Progresses of Water Logging Tolerance and High Saline Adaptation of Mangrove Plants. J. Xiamen Univ. (Nat. Sci.) 2017, 56, 314–322. [Google Scholar]
- Elias, L.M.; Fortlzamp, D.; Sartori, S.B.; Ferreira, M.C.; Gomes, L.H.; Azevedo, J.L.; Montoya, Q.V.; Rodrigues, A.; Ferreira, A.G.; Lira, S.P. The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Braz. J. Microbiol. 2018, 49, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, J.; Sun, Q.Q.; Qin, J.C.; Pescitelli, G.; Gao, J.M. Characterization of Cytochalasins from the Endophytic Xylaria sp. and Their Biological Functions. J. Agric. Food Chem. 2014, 62, 10962–10969. [Google Scholar] [CrossRef]
- Betina, V.; Mičeková, D.; Němec, P. Antimicrobial Properties of Cytochalasins and Their Alteration of Fungal Morphology. Microbiology 1972, 71, 343–349. [Google Scholar] [CrossRef]
- Prasain, J.K.; Ueki, M.; Stefanowicz, P.; Osada, H. Rapid screening and identification of cytochalasins by electrospray tandem mass spectrometry. J. Mass Spectrom. JMS 2002, 37, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Tani, H.; Ichinoe, M.; Nakajima, H. Zygosporin D and Two New Cytochalasins Produced by the Fungus Metarrhizium anisopliae. J. Nat. Prod. 2000, 63, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Kumarihamy, M.; Ferreira, D.; Croom, E.M.; Sahu, R.; Tekwani, B.L.; Duke, S.O.; Khan, S.; Techen, N.; Nanayakkara, N.P.D. Antiplasmodial and Cytotoxic Cytochalasins from an Endophytic Fungus, Nemania sp. UM10M, Isolated from a Diseased Torreya taxifolia Leaf. Molecules 2019, 24, 777. [Google Scholar] [CrossRef] [PubMed]
- Ntemafack, A.; Chouhan, R.; Kapoor, N.; Kumar, A.; Dhiman, S.K.; Manhas, R.S.; Chaubey, A.; Hassan, Q.P.; Gandhi, S.G. Protective effect of Bacillus species associated with Rumex dentatus against postharvest soil borne disease in potato tubers and GC–MS metabolite profile. Arch. Microbiol. 2022, 204, 583. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wakatake, T.; Hashimoto, K.; Saucet, S.B.; Toyooka, K.; Yoshida, S.; Shirasu, K. Haustorial Hairs Are Specialized Root Hairs That Support Parasitism in the Facultative Parasitic Plant Phtheirospermum japonicum. Plant Physiol. 2016, 170, 1492–1503. [Google Scholar] [CrossRef]
- Chong, J.; Baltz, R.; Schmitt, C.; Beffa, R.; Fritig, B.; Saindrenan, P. Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 2002, 14, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Sanier, C.; Macheix, J.J.; D’Auzac, J. Accumulation of scopoletin in Hevea brasiliensis infected by Microcyclus ulei (P. Henn.) V.ARX and evaluation of its fungitoxicity for three leaf pathogens of rubber tree. Physiol. Mol. Plant Pathol. 1995, 47, 213–223. [Google Scholar] [CrossRef]
- Rauckman, B.S.T.; Mary, Y.; Johnson, J.V.; Roth, B. 2,4-Diamino-5-benzylpyrimidines and analogs as antibacterial agents. 10. 2,4-Diamino-5-(6-quinolylmethyl)- and -[(tetrahydro-6-quinolyl)methyl]pyrimidine derivatives. Further specificity studies. J. Med. Chem. 1989, 32, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Segaran, G.; Sathiavelu, M. Fungicidal and plant growth-promoting traits of Lasiodiplodia pseudotheobromae, an endophyte from Andrographis paniculata. Front. Plant Sci. 2023, 14, 1125630. [Google Scholar] [CrossRef]
- Khan, I.H.; Javaid, A. DNA cleavage of the fungal pathogen and production of antifungal compounds are the possible mechanisms of action of biocontrol agent Penicillium italicum against Macrophomina phaseolina. Mycologia 2022, 114, 24–34. [Google Scholar] [CrossRef]
- Patel, T.K.; Williamson, J.D. Mannitol in Plants, Fungi, and Plant–Fungal Interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.B.; Ehrenshaft, M.; Pharr, D.M.; Williamson, J.D. Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc. Natl. Acad. Sci. USA 1998, 95, 15129–15133. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” Tools for Better Understanding the Plant-Endophyte Interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef] [PubMed]








| No. | Compound | Source | Reference |
|---|---|---|---|
| 59 | Gibbererllin A3 | B. gymnorrhiza, fruit | [39] |
| 60 | Gibbererllin A4 | B. gymnorrhiza, fruit | [39] |
| 61 | Gibbererllin A7 | B. gymnorrhiza, fruit | [39] |
| 62 | Steviol | B. gymnorrhiza, root bark | [40] |
| 63 | Methyl-ent-kaur-9(11)-en-13,17-epoxy-16-hydroxy-19-oate | B. gymnorrhiza, root bark; B. sexangula var. rhynchopetala, stem | [40,41] |
| 64 | ent-kaur-16-en-13-hydroxy-19-al | B. gymnorrhiza, root bark and stem | [40,42] |
| 65 | ent-kaur-16-en-13,19-diol | B. gymnorrhiza, root bark and stem | [40,42] |
| 66 | 13,16α,17-trihydroxy-ent-9(11)-kaurene-19-oic acid | B. gymnorrhiza, stem | [42] |
| 67 | 16α,17-dihydroxy-ent-9(11)-kaurene-19-al | B. gymnorrhiza, stem; B. sexangula var. rhynchopetala, stem | [41,42] |
| 68 | 17-chloro-13,16β-dihydroxy-ent-kauran-19-al | B. gymnorrhiza, stem | [42] |
| 69 | Methyl-16α,17-dihydroxy-ent-kauran-19-oate | B. gymnorrhiza, stem | [42] |
| 70 | 16α,17-dihydroxy-ent-9(11)-kauren-19-oic acid | B. gymnorrhiza, stem | [42] |
| 71 | Methyl-16α,17-dihydroxy-ent-9(11)-kauren-19-oate | B. gymnorrhiza, stem; B. sexangula var. rhynchopetala, stem | [41,42] |
| 72 | 16α,17-dihydroxy-ent-kauran-19-al | B. gymnorrhiza, stem | [42] |
| 73 | 16αH-17-hydroxy-ent-kauran-19-oic acid | B. gymnorrhiza, stem | [42] |
| 74 | 16αH-17,19-ent-kaurane-diol | B. gymnorrhiza, stem | [42] |
| 75 | 16-ent-kauren-19-ol | B. gymnorrhiza, stem | [42] |
| 76 | (16R)-13,17-epoxy-16-hydroxy-ent-kaur-9(11)-en-19-al | B. sexangula var. rhynchopetala, stem | [41] |
| 77 | 16,17-dihydroxy-19-nor-ent-kaur-9(11)-en-3-one | B. sexangula var. rhynchopetala, stem | [41] |
| 78 | Ceriopsin F | B. sexangula var. rhynchopetala, stem | [41] |
| 79 | 1β,15(R)-ent-pimar-8(14)-en-1,15,16-triol | B. gymnorrhiza, root bark and stem; B. sexangula var. rhynchopetala, stem | [40,41,43] |
| 80 | ent-8(14)-pimarene-15R,16-diol | B. gymnorrhiza, stem | [43] |
| 81 | ent-8(14)-pimarene-1α,15R,16-triol | B. gymnorrhiza, stem | [43] |
| 82 | (5R,9S,10R,13S,15S)-ent-8(14)-pimarene-1-oxo-15R,16-diol | B. gymnorrhiza, stem | [43] |
| 83 | 15(S)-isopimar-7-en-15,16-diol | B. gymnorrhiza, root bark and stem | [40,43] |
| 84 | Isopimar-7-ene-1β, 15S, 16-triol | B. gymnorrhiza, stem | [43] |
| 85 | (4R,5S,8R,9R,10S,13S)-ent-17-hydroxy-16-oxobeyeran-19-al | B. gymnorrhiza, stem; B. sexangula var. rhynchopetala, stem | [41,42] |
| 86 | 17-hydroxy-16-oxobeyer-9(11)-en-19-al | B. sexangula var. rhynchopetala, stem | [41] |
| No. | Compound | Source | Reference |
|---|---|---|---|
| 91 | Bruguierin A | B. gymnorrhiza, flower | [46] |
| 92 | Bruguierin B | B. gymnorrhiza, flower | [46] |
| 93 | Bruguierin C | B. gymnorrhiza, flower | [46] |
| 94 | Sexangulic acid | B. sexangula, stem | [47] |
| 95 | 11-oxo-12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β-diol | Penicillium sp. J41221 (B. sexangula var. rhynchopetala, endophytic fungus) | [48] |
| 96 | 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-3β,11β-diol | Penicillium sp. J41221 (B. sexangula var. rhynchopetala, endophytic fungus) | [48] |
| 97 | 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-3β,11β-diol | Penicillium sp. J41221 (B. sexangula var. rhynchopetala, endophytic fungus) | [48] |
| 98 | 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β,11β-triol | Penicillium sp. J41221 (B. sexangula var. rhynchopetala, endophytic fungus) | [48] |
| 99 | Gymnorhizol | B. gymnorrhiza, stem and leaf | [49] |
| 100 | β-amyrin | B. gymnorrhiza, leaf | [50] |
| 101 | Oleanolic acid | B. gymnorrhiza, leaf | [50] |
| 102 | (15α)-15-hydroxysoyasapogenol B | Pestalotiopsis clavispora (B. sexangula, endophytic fungus) | [51] |
| 103 | (7β,15α)-7,15-dihydroxysoyasapogenol B | P. clavispora (B. sexangula, endophytic fungus) | [51] |
| 104 | (7β)-7,29-dihydroxysoyasapogenol B | P. clavispora (B. sexangula, endophytic fungus) | [51] |
| 105 | α-amyrin | B. gymnorrhiza, leaf | [50] |
| 106 | Ursolic acid | B. gymnorrhiza, leaf; | [50] |
| 107 | Corosolic acid | B. parviflora, leaf | [52] |
| 108 | Lupeol | B. gymnorrhiza, stem and leaf B. cylindrica, fruit and hypocotyl B. parviflora, fruit and leaf | [50,52,53,54,55] |
| 109 | Lupenone | B. gymnorrhiza, stem and leaf B. cylindrica, fruit and hypocotyl B. parviflora, fruit | [53,54,55] |
| 110 | 3-(Z)-caffeoyllupeol | B. parviflora, fruit | [54] |
| 111 | 3β-E-caffeoyllupeol | B. parviflora, fruit; B. cylindrica, fruit and hypocotyl | [54,55] |
| 112 | 3α-E-coumaroyllupeol | B. cylindrica, fruit and hypocotyl | [55] |
| 113 | 3α-Z-coumaroyllupeol | B. cylindrica, fruit and hypocotyl | [55] |
| 114 | 3β-E-coumaroyllupeol | B. parviflora, fruit; B. cylindrica, fruit and hypocotyl | [54,55] |
| 115 | 3β-Z-coumaroyllupeol | B. cylindrica, fruit and hypocotyl | [55] |
| 116 | 3α-lupeol | B. cylindrica, fruit and hypocotyl | [55] |
| 117 | Betulinic acid | B. parviflora, leaf | [52] |
| 118 | 3α-feruloyltaraxerol dichloromethane solvate | B. cylindrica, fruit | [56] |
| 119 | 3α-E-feruloyltaraxerol | B. cylindrica, fruit | [57] |
| 120 | 3α-Z-feruloyltaraxerol | B. cylindrica, fruit | [57] |
| 121 | 3β-E-feruloyltaraxerol | B. cylindrica, fruit | [57] |
| 122 | 3β-Z-feruloyltaraxerol | B. cylindrica, fruit | [57] |
| 123 | 3α-E-coumaroyltaraxerol | B. cylindrica, fruit | [57] |
| 124 | 3α-Z-coumaroyltaraxerol | B. cylindrica, fruit | [57] |
| 125 | 3α-taraxerol | B. cylindrica, fruit | [57] |
| 126 | 3β-taraxerol | B. cylindrica, fruit and leaf | [57,58] |
| 127 | 14-taraxeren-3-one | B. gymnorrhiza, stem and leaf | [53] |
| 128 | 3α-E-caffeoyltaraxerol | B. cylindrica, fruit and hypocotyl | [55] |
| 129 | 3β-(Z)-coumaroyltaraxerol | B. cylindrica, leaf | [58] |
| 130 | 3β-(E)-coumaroyltaraxerol | B. cylindrica, leaf | [58] |
| No. | Compound | Source | Reference |
|---|---|---|---|
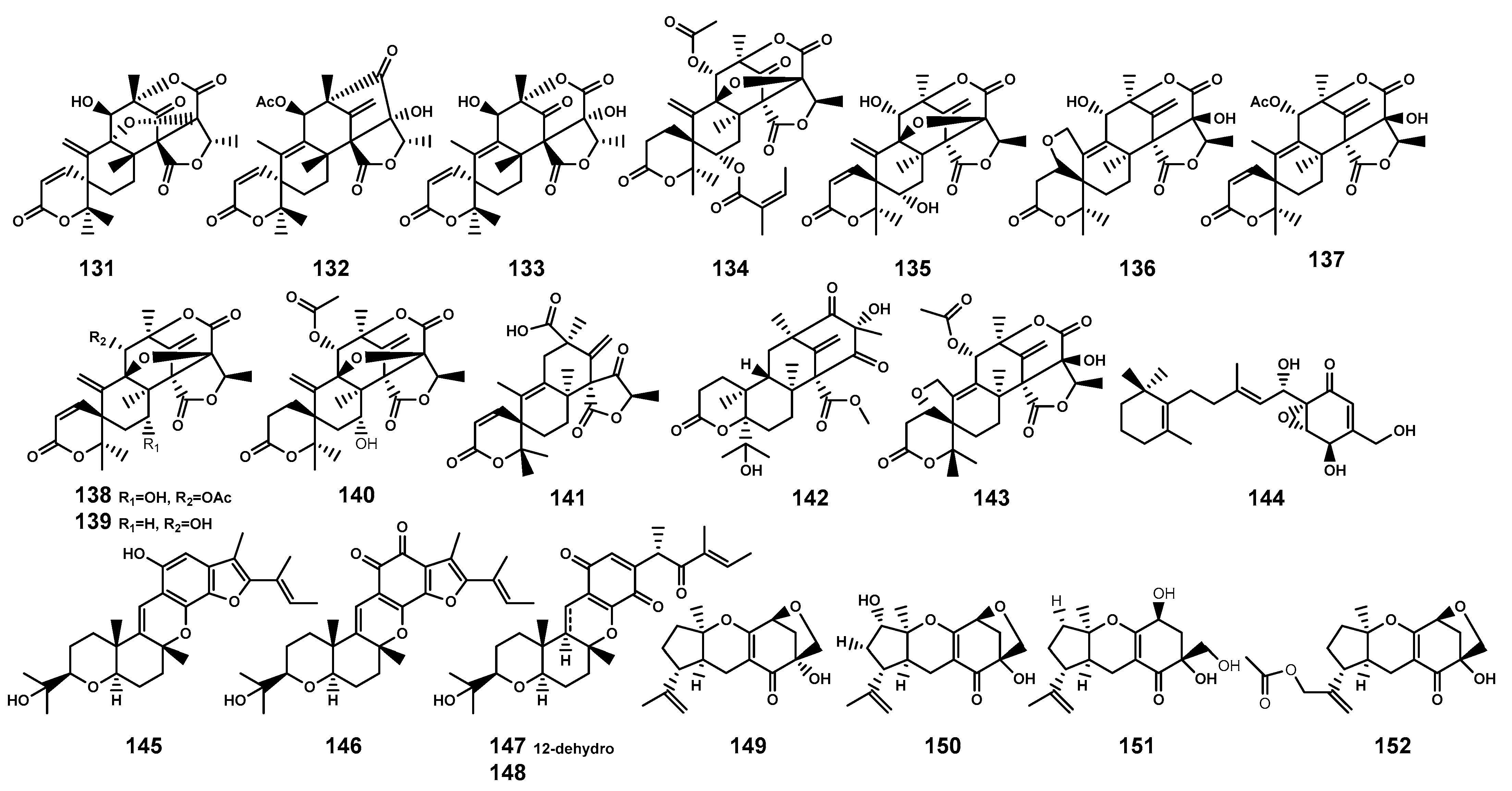
| 131 | Dehydroaustin | Penicillium citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [60] |
| 132 | 11β-acetoxyisoaustinone | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus); | [60,61] |
| 133 | Austinol | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus); Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [60,61] |
| 134 | Penicianstinoid A | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 135 | Penicianstinoid B | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 136 | Furanoaustinol | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 137 | 1,2-dihydro-7-hydroxydehydroaustin | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 138 | 7-hydroxydehydroaustin | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 139 | Dehydroaustinol | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 140 | Austin | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 141 | Penicianstinoid C | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [62] |
| 142 | Penicianstinoid D | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [62] |
| 143 | Penicianstinoid E | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [62] |
| 144 | Nectrianolin D | Clonostachys rosea B5–2 and Nectria pseudotrichia B69–1 (the branch of B. gymnorrhiza, endophytic fungus) | [63] |
| 145 | Furanocochlioquinol | C. rosea B5–2 and N. pseudotrichia B69–1 (the branch of B. gymnorrhiza, endophytic fungus) | [63] |
| 146 | Furanocochlioquinone | C. rosea B5–2 and N. pseudotrichia B69–1 (the branch of B. gymnorrhiza, endophytic fungus) | [63] |
| 147 | Nectripenoid B | C. rosea B5–2 and N. pseudotrichia B69–1 (the branch of B. gymnorrhiza, endophytic fungus) | [63] |
| 148 | Cochlioquinone D | C. rosea B5–2 and N. pseudotrichia B69–1 (the branch of B. gymnorrhiza, endophytic fungus) | [63] |
| 149 | Guignardone A | Phyllosticta capitalensis (the hypocotyl of B. sexangula, endophytic fungus) | [64] |
| 150 | 12-hydroxylated guignardone A | P. capitalensis (the hypocotyl of B. sexangula, endophytic fungus) | [64] |
| 151 | Guignardone J | P. capitalensis (the hypocotyl of B. sexangula, endophytic fungus) | [64] |
| 152 | Guignardone M | P. capitalensis (the hypocotyl of B. sexangula, endophytic fungus) | [64] |
| No. | Compound | Source | Reference |
|---|---|---|---|
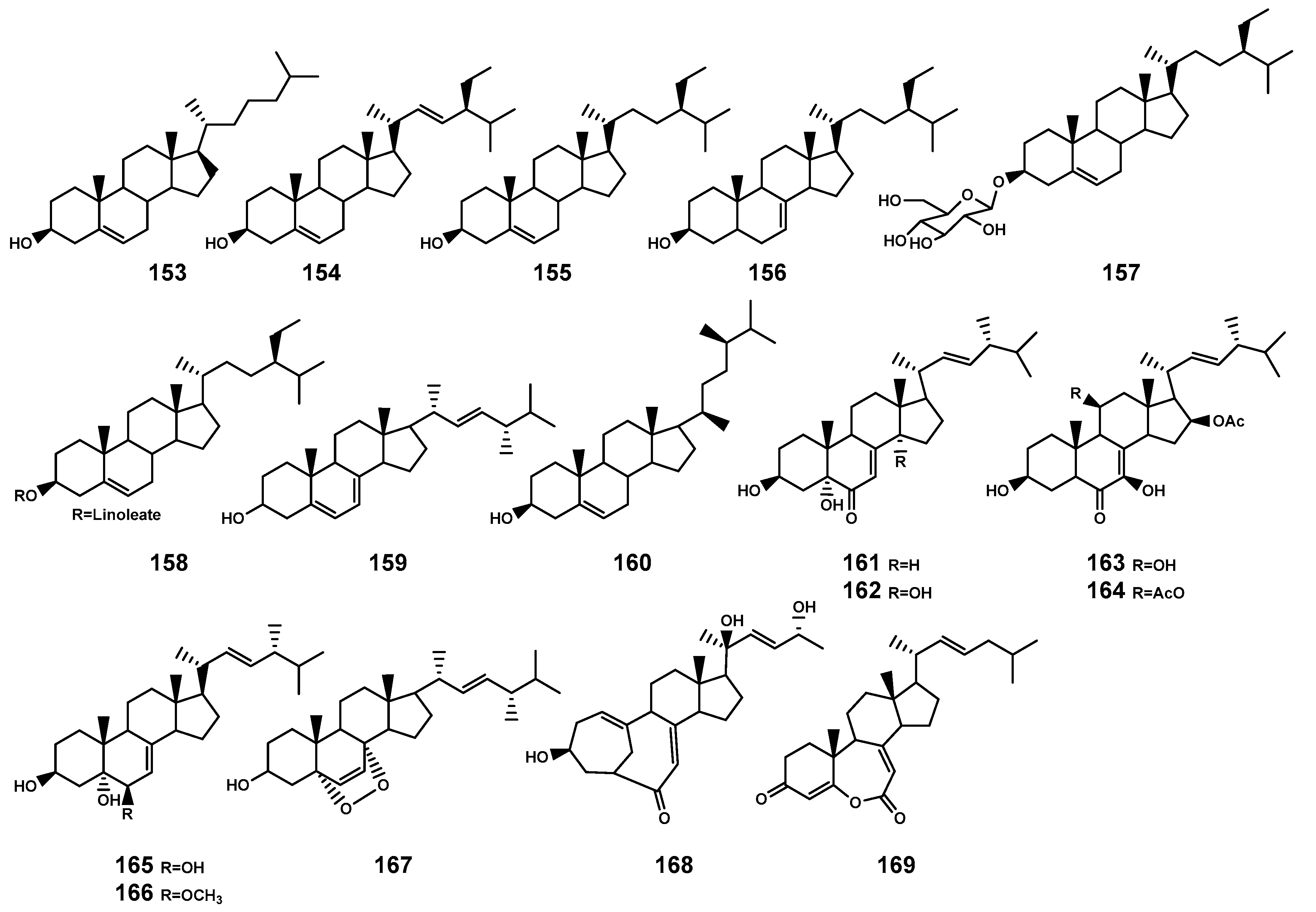
| 153 | Cholesterol | B. gymnorrhiza, leaf; Penicillium thomi (the root of B. gymnorrhiza, endophytic fungus); | [50,66] |
| 154 | Stigmasterol | B. gymnorrhiza, leaf | [50] |
| 155 | Sitosterol | B. gymnorrhiza, leaf; P. thomi (the root of B. gymnorrhiza, endophytic fungus); B. cylindrica, leaf | [50,58,66] |
| 156 | Stigmast-7-en-3β-ol | B. gymnorrhiza, leaf | [50] |
| 157 | β-daucosterol | B. gymnorrhiza, stem and leaf; | [53] |
| 158 | β-sitosteryl linoleate | Phomopsis longicolla HL-2232 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [67] |
| 159 | Ergosterol | Penicillium sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus); | [68] |
| 160 | Campesterol | B. gymnorrhiza, leaf | [50] |
| 161 | 3β,5α-dihydroxy-(22E,24R)-ergosta-7,22-dien-6-one | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 162 | 3β,5α,14α-trihydroxy-(22E,24R)-ergosta-7,22-dien-6-one | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 163 | NGA0187 | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus); Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [69,70] |
| 164 | 11-O-acetyl-NGA0187 | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [70] |
| 165 | Ergosta-7,22-diene-3β,5α,6β-triol | P. thomi (the root of B. gymnorrhiza, endophytic fungus); Penicillium sp. J41221 (B. sexangula var. rhynchopetala, endophytic fungus); P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [48,66,67] |
| 166 | (3β,5α,6β,22E)-6-methoxyergosta-7,22-diene-3,5-diol | Penicillium sp. J41221 (B. sexangula var. rhynchopetala, endophytic fungus); | [48] |
| 167 | (22E)-5α,8α-epidioxyergosta-6,22-dien-3β-ol | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus); P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [67,68] |
| 168 | Cyclocitrinol | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [70] |
| 169 | Fortisterol | P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [67] |
| No. | Compound | Source | Reference |
|---|---|---|---|
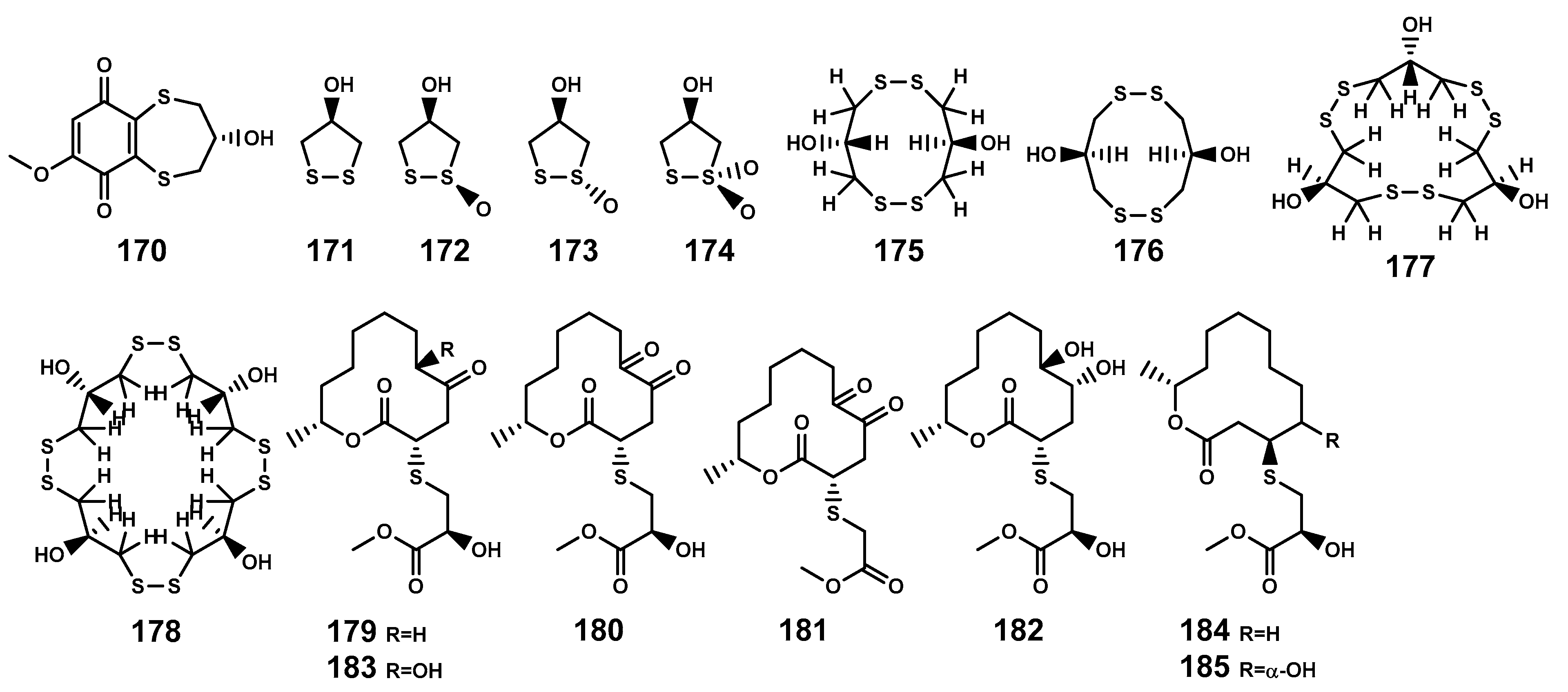
| 170 | (-)-3,4-dihydro-3-hydroxy-7-methoxy-2H-1,5-benzodithiepine-6,9-dione | B. sexangula var. rhynchopetala, stem | [41] |
| 171 | 1,2-dithiolane | B. gymnorrhiza, stem and leaf; B. cylindrica, stem and bark | [76,77] |
| 172 | Brugierol | B. gymnorrhiza, stem, leaf, and flower; B. sexangula var. rhynchopetala, stem; B. cylindrica, stem and bark | [41,72,76,77,78,79] |
| 173 | Isobrugierol | B. gymnorrhiza, stem, leaf and flower; B. sexangula var. rhynchopetala, stem; B. cylindrica, stem and bark | [41,72,76,77,78,79] |
| 174 | Bruguiesulfurol | B. gymnorrhiza, flower | [72,79,80] |
| 175 | Trans-3,3′-dihydroxy-1,5,1′,5′-tetrathiacyclodecane | B. gymnorrhiza, stem and leaf | [80] |
| 176 | Cis-3,3′-dihydroxy-1,5,1′,5′-tetrathiacyclodecane | B. gymnorrhiza, stem and leaf | [80] |
| 177 | Gymnorrhizol | B. gymnorrhiza, stem, leaf and flower; | [72,76,80] |
| 178 | Neogymnorrhizol | B. gymnorrhiza, stem, leaf and flower | [72,80] |
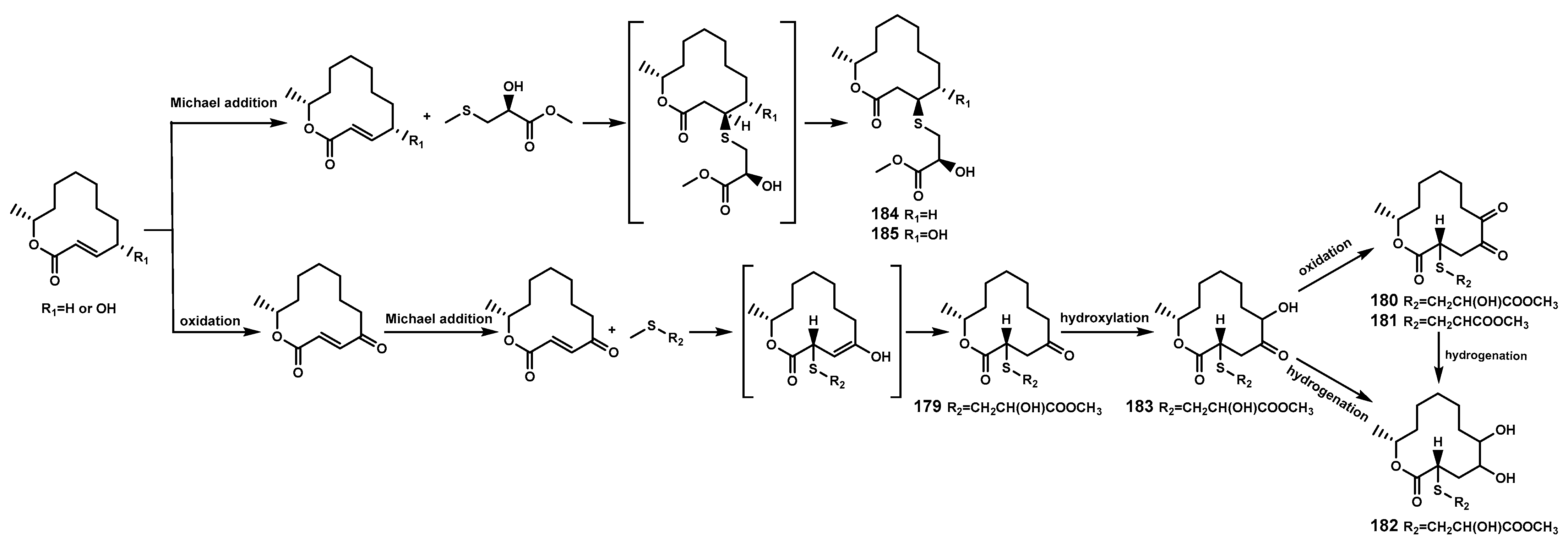
| 179 | Thiocladospolide A | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [21] |
| 180 | Thiocladospolide B | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [21] |
| 181 | Thiocladospolide C | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [21] |
| 182 | Thiocladospolide D | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [21] |
| 183 | Pandangolide 3 | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [21] |
| 184 | Thiocladospolide F | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [75] |
| 185 | Thiocladospolide G | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [75] |
| No. | Compound | Source | Reference |
|---|---|---|---|
| 186 | Gymnorrhizin A | B. gymnorrhiza, hypocotyl | [85] |
| 187 | N-(2-hydroxy-4-methoxyphenyl)acetamide | P. thomi (the root of B. gymnorrhiza, endophytic fungus) | [66] |
| 188 | Antimycin A18 | Streptomyces albidoflavus 107A-01824 (the leaf of B. gymnorrhiza, endophytic actinomycete) | [86] |
| 189 | (2S,2′R,3R,4E,8E,3′E)-2-(2′-hydroxy-3′-octadecenoylamino)-9-methyl-4,8-octadecadiene-l,3-diol | P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [67] |
| 190 | N,N′-diphenyl urea | P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [67] |
| 191 | (E)-tert-butyl(3-cinnamamidopropyl)carbamate | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [87] |
| 192 | 3-(dimethylaminomethyl)-1-(1,1-dimethyl-2-propenyl)indole | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus) | [68] |
| 193 | Meleagrin | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [88] |
| 194 | Uridine | P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [67] |
| 195 | 6-aminopurine-9-carboxylic acid methyl ester | P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [67] |
| 196 | Adenine riboside | P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [67] |
| 197 | Brugine | B. sexangula, stem bark; B. cylindrica, stem and bark | [11,77,89] |
| 198 | Scalusamide A | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [88] |
| 199 | Penibruguieramine A | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [88] |
| 200 | Anacine | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus) | [68] |
| 201 | Aurantiomide C | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus) | [68] |
| 202 | Viridicatol | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus) | [68] |
| 203 | Viridicatin | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus) | [68] |
| 204 | 3-O-methylviridicatin | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus) | [68] |
| 205 | (+)-Cyclopenol | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus) | [68] |
| 206 | Roquefortine F | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [88] |
| 207 | Penilloid A | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [90] |
| 208 | Cyclo(dehydrohistidyl-L-tryptophyl) | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [90] |
| 209 | 5S-hydroxynorvalines-Ile | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [90] |
| 210 | 3S-hydroxylcyclo(S-Pro-S-Phe) | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [90] |
| 211 | Cyclo(S-Phe-S-Gln) | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [90] |
| 212 | Menisdurin B | B. gymnorrhiza, hypocotyl | [91] |
| 213 | Menisdurin C | B. gymnorrhiza, hypocotyl | [91] |
| 214 | Menisdurin D | B. gymnorrhiza, hypocotyl | [91] |
| 215 | Menisdurin E | B. gymnorrhiza, hypocotyl | [91] |
| 216 | Menisdurin | B. gymnorrhiza, hypocotyl | [91] |
| 217 | Coclauril | B. gymnorrhiza, hypocotyl | [91] |
| 218 | Menisdaurilide | B. gymnorrhiza, hypocotyl | [91] |
| 219 | Uracil | P. thomi (the root of B. gymnorrhiza, endophytic fungus) | [66] |
| 220 | Cyclo-(Ala-Gly) | P. thomi (the root of B. gymnorrhiza, endophytic fungus); Penicillium citrinum ZD6 (the stem of B. gymnorrhiza, endophytic fungus) | [66,92] |
| 221 | Cyclo-(Pro-Gly) | P. thomi (the root of B. gymnorrhiza, endophytic fungus) | [66] |
| 222 | Cyclo-(Ala-Pro) | P. thomi (the root of B. gymnorrhiza, endophytic fungus) | [66] |
| 223 | 3-benzylpiperazine-2,5-dione | Gloesporium sp. (B. gymnorrhiza, endophytic fungus) | [93] |
| 224 | 3-benzyl-6-(4-hydroxybenzyl) piperazine-2,5-dione | Gloesporium sp. (B. gymnorrhiza, endophytic fungus) | [93] |
| 225 | 3-(2-methylpropyl)-2,5-piperazinedione | Gloesporium sp. (B. gymnorrhiza, endophytic fungus) | [93] |
| 226 | Beauvericin | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 227 | 5,5′-epoxy-MKN-349A | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [70] |
| 228 | Aspergilumamide A | Aspergillus sp. 33241 (B. sexangula var. rhynchopetala, fungus) | [94] |
| 229 | Penilumamide | Aspergillus sp. 33241 (B. sexangula var. rhynchopetala, fungus) | [94] |
| No. | Compound | Source | Reference |
|---|---|---|---|
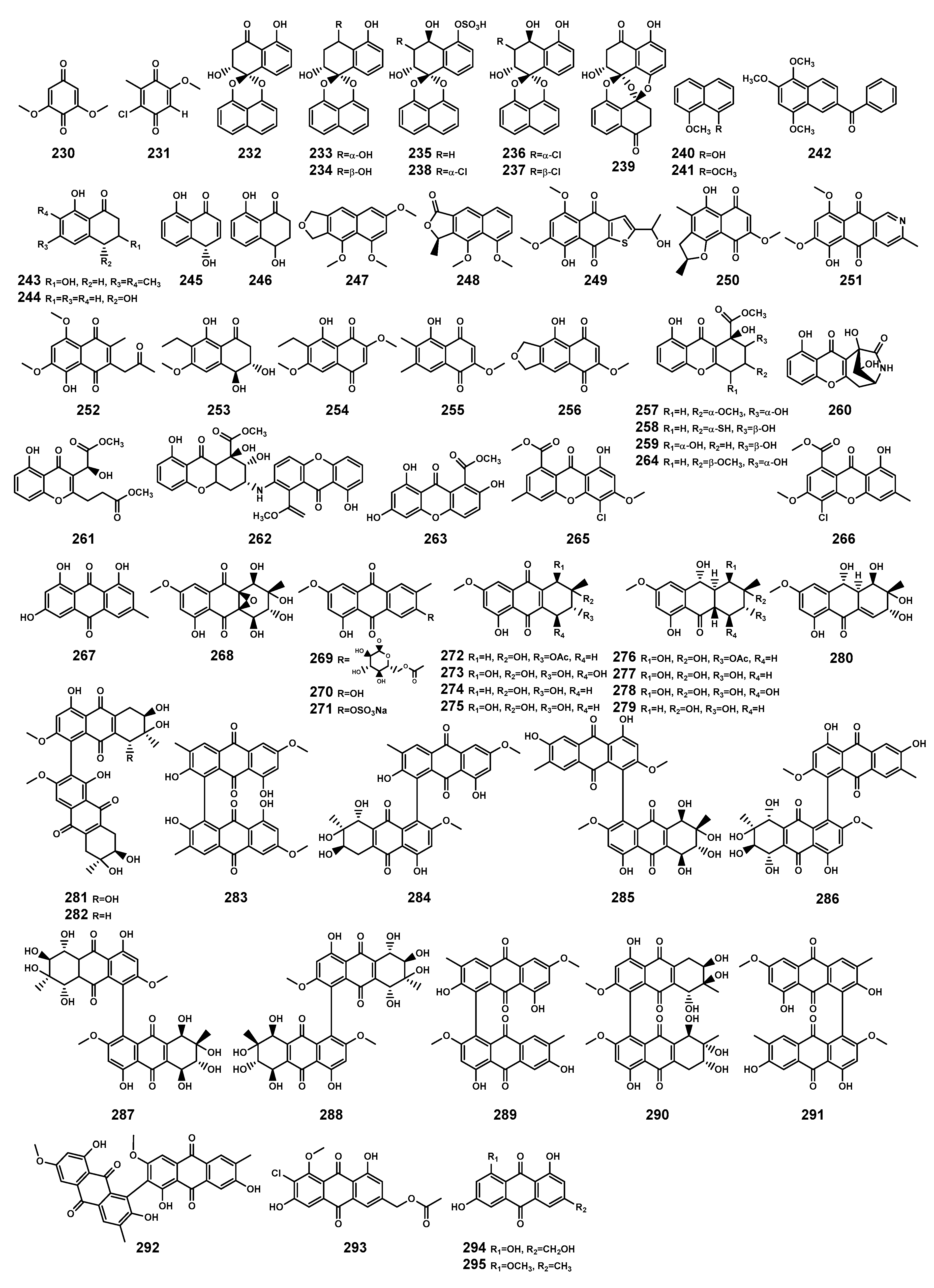
| 230 | 2,6-dimethoxy-1,4-benzoquinone | B. sexangula var. rhynchopetala, stem | [41] |
| 231 | 2-chloro-5-methoxy-3-methylcyclohexa-2,5-diene-1,4-dione | Xylaria cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [97] |
| 232 | Palmarumycins BG1 | B. gymnorrhiza, stem and leaf | [98] |
| 233 | Palmarumycins BG2 | B. gymnorrhiza, stem and leaf | [98] |
| 234 | Palmarumycins BG3 | B. gymnorrhiza, stem and leaf | [98] |
| 235 | Palmarumycins BG4 | B. gymnorrhiza, stem and leaf | [98] |
| 236 | Palmarumycins BG5 | B. gymnorrhiza, stem and leaf | [98] |
| 237 | Palmarumycins BG6 | B. gymnorrhiza, stem and leaf | [98] |
| 238 | Palmarumycins BG7 | B. gymnorrhiza, stem and leaf | [98] |
| 239 | Preussomerin BG1 | B. gymnorrhiza, stem and leaf | [98] |
| 240 | 8-methoxy-1-naphthol | Daldinia eschscholtzii PSU-STD57 (the leaf of B. gymnorrhiza, endophytic fungus) | [99] |
| 241 | 1,8-dimethoxynaphthalene | D. eschscholtzii PSU-STD57 (the leaf of B. gymnorrhiza, endophytic fungus); Daldinia eschscholtzii HJ001 (B. sexangula var. rhynchopetala, endophytic fungus) | [99,100] |
| 242 | Nigronatthaphenyl | Nigrospora sphaerica (the mature leaf of B. gymnorrhiza, endophytic fungus) | [101] |
| 243 | (3S)-3,8-dihydroxy-6,7-dimethyl-α-tetralone | D. eschscholtzii PSU-STD57 (the leaf of B. gymnorrhiza, endophytic fungus) | [99] |
| 244 | Isosclerone | D. eschscholtzii PSU-STD57 (the leaf of B. gymnorrhiza, endophytic fungus); X. cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [97,99] |
| 245 | (4S)-3,4-dihydro-4,8-dihydroxy-l(2H)-naphthalenoe | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [102] |
| 246 | Regiolone | P. capitalensis (the hypocotyl of B. sexangula, endophytic fungus) | [64] |
| 247 | 1,3,8-trimethoxynaphtho[9–c]furan | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 248 | 4-O-methyl eleutherol | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 249 | 8-hydroxy-2-[1-hydroxyethyl]-5,7-dimethoxynaphtho[2,3-b] thiophene-4,9-dione | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 250 | Anhydrojavanicin | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 251 | 8-O-methylbostrycoidin | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 252 | 8-O-methyljavanicin | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 253 | Botryosphaerone D | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 254 | 6-ethyl-5-hydroxy-3,7-dimethoxynaphthoquinone | A. terreus No. GX7-3B (the branch of B. gymnorrhiza, endophytic fungus) | [69] |
| 255 | 5-hydroxy-2-methoxy-6,7-dimethyl-1,4-naphthoquinone | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 256 | 5-hydroxy-2-methoxynaphtho[9–c] furan-1,4-dione | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 257 | Incarxanthone A | Peniophora incarnata Z4 (B. gymnorrhiza, endophytic fungus) | [104] |
| 258 | Incarxanthone B | P. incarnata Z4 (B. gymnorrhiza, endophytic fungus) | [104] |
| 259 | Incarxanthone C | P. incarnata Z4 (B. gymnorrhiza, endophytic fungus) | [104] |
| 260 | Incarxanthone D | P. incarnata Z4 (B. gymnorrhiza, endophytic fungus) | [104] |
| 261 | Incarxanthone E | P. incarnata Z4 (B. gymnorrhiza, endophytic fungus) | [104] |
| 262 | Incarxanthone F | P. incarnata Z4 (B. gymnorrhiza, endophytic fungus) | [104] |
| 263 | 2,8-Dihydroxyvertixanthone | P. incarnata Z4 (B. gymnorrhiza, endophytic fungus) | [104] |
| 264 | Globosuxanthone B | P. incarnata Z4 (B. gymnorrhiza, endophytic fungus) | [104] |
| 265 | 4-chloro-1-hydroxy-3-methoxy-6-methyl-8-methoxycarbonyl-xanthen-9-one | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [105] |
| 266 | Chloroisosulochrin dehydrate | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [105] |
| 267 | Emodin | P. citrinum ZD6 (the stem of B. gymnorrhiza, endophytic fungus) | [92] |
| 268 | Auxarthrol C | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 269 | Macrosporin-2-O-(6′-acetyl)-α-D-glucopyranoside | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 270 | Macrosporin | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 271 | Macrosporin-7-O-sulfate | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 272 | 2-O-acetylaltersolanol B | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 273 | Altersolanol A | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 274 | Altersolanol B | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus); P. longicolla HL-2232 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [106,107] |
| 275 | Altersolanol C | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 276 | 2-O-acetylaltersolanol L | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 277 | Altersolanol L | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 278 | Ampelanol | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 279 | Tetrahydroaltersolanol B | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 280 | Dihydroaltersolanol A | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 281 | Alterporriol T | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 282 | Alterporriol U | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 283 | Alterporriol V | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 284 | Alterporriol W | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 285 | Alterporriol A | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 286 | Alterporriol B | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 287 | Alterporriol D | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 288 | Alterporriol E | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 289 | Alterporriol C | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 290 | Alterporriol N | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 291 | Alterporriol R | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 292 | Alterporriol Q | Stemphylium sp. 33231 (B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 293 | 2′-acetoxy-7-chlorocitreorosein | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [105] |
| 294 | Citreorosein | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [105] |
| 295 | MT-1 | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [105] |
| No. | Compound | Source | Reference |
|---|---|---|---|
| 296 | Cytochalasin D | B. gymnorrhiza; Xylaria arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) X. cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [35,97,110] |
| 297 | Zygosporin D | B. gymnorrhiza; X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [35,110] |
| 298 | [11]-cytochalasa-5(6),13-diene-1,21-dione-7,18-dihydroxy-16,18-dimethyl-10-phenyl(7S*,13E,16S*,18R*) | D. eschscholtzii HJ001 (B. sexangula var. rhynchopetala, endophytic fungus) | [100] |
| 299 | [11]-cytochalasa-6(12),13-diene-1,21-dione-7,18-dihydroxy16,18-dimethyl-10-phenyl-(7S*,13E,16S*,18R*) | D. eschscholtzii HJ001 (B. sexangula var. rhynchopetala, endophytic fungus) | [100] |
| 300 | Arbuschalasins A | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 301 | Arbuschalasins B | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 302 | Arbuschalasins C | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 303 | Arbuschalasins D | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 304 | Cytochalasin Q | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 305 | 12-hydroxylcytochalasin Q | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 306 | Cytochalasin D-13,14-epoxid | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 307 | 19,20-epoxycytochalasin D | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 308 | Cytochalasin O | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 309 | Cytochalasin P | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 310 | Cytochalasin C | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 311 | Deacetylcytochalasin C | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 312 | 6,7-dihydro-7-oxo-cytochalasin C | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 313 | 6,7-dihydro-7-oxo-deacetylcytochalasin C | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 314 | 19, 20-epoxycytochalasin C | X. arbuscula GZS74 (the fruit of B. gymnorrhiza, endophytic fungus) | [110] |
| 315 | 1,10-dihydroxy-8-methyldibenz[b, e] oxepin-6,11-dione | No. GX4-1B (the branch of B. gymnorrhiza, endophytic fungus) | [111] |
| 316 | 1,10-dihydroxy-dibenz[b, e]oxepin-6,11-dione | No. GX4-1B (the branch of B. gymnorrhiza, endophytic fungus) | [111] |
| 317 | 2-deoxy-sohirnone C | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [90] |
| 318 | Sohirnone A | Penicillium sp. GD6 (the stem bark of B. gymnorrhiza, endophytic fungus) | [90] |
| 319 | 3,4-dihydroxybenzoic acid | P. capitalensis (the hypocotyl of B. sexangula, endophytic fungus) | [64] |
| 320 | Penibenzophenone A | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [87] |
| 321 | Penibenzophenone B | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [87] |
| 322 | Sulochrin | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [87] |
| 323 | Sorbicillin | Trichoderma reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 324 | Asterric acid | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [87] |
| 325 | N-butyl asterrate | P. citrinum HL-5126 (B. sexangula var. rhynchopetala, endophytic fungus) | [87] |
| 326 | 8-O-methylnodulisporin F | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 327 | Nodulisporin H | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 328 | Epicoccolide A | Epicoccum nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 329 | Divergolide A | Streptomyces sp. (B. gymnorrhiza, endophytic bacteria) | [22] |
| 330 | Divergolide B | Streptomyces sp. (B. gymnorrhiza, endophytic bacteria) | [22] |
| 331 | Divergolide C | Streptomyces sp. (B. gymnorrhiza, endophytic bacteria) | [22] |
| 332 | Divergolide D | Streptomyces sp. (B. gymnorrhiza, endophytic bacteria) | [22] |
| 333 | Divergolide E | Streptomyces sp. (B. gymnorrhiza, endophytic bacteria) | [22] |
| 334 | Divergolide F | Streptomyces sp. (B. gymnorrhiza, endophytic bacteria) | [22] |
| 335 | Divergolide G | Streptomyces sp. (B. gymnorrhiza, endophytic bacteria) | [22] |
| 336 | Divergolide H | Streptomyces sp. (B. gymnorrhiza, endophytic bacteria) | [22] |
| 337 | Seco-patulolide C | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [21] |
| 338 | 5R-hydroxyrecifeiolide | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [114] |
| 339 | 5S-hydroxyrecifeiolide | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [114] |
| 340 | Pandangolide 1 | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [114] |
| 341 | Cladocladosin A | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [75] |
| 342 | Verrucosidin | P. sclerotiorum (the inner bark of B. gymnorrhiza, endophytic fungus) | [68] |
| 343 | Acetylchrysopyrone B | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 344 | Saturnispol H | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 345 | Infectopyrone A | Stemphylium sp. 33231 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 346 | Infectopyrone B | Stemphylium sp. 33231 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [106] |
| 347 | 6,8-dihydroxy-5-methoxy-3-methyl-1H-isochromen-1-one | P. capitalensis (the hypocotyl of B. sexangula, endophytic fungus) | [64] |
| 348 | ent-cladospolide F | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [114] |
| 349 | Cladospolide G | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [114] |
| 350 | Cladospolide H | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [114] |
| 351 | Iso-cladospolide B | C. cladosporioides MA-299 (the leaf of B. gymnorrhiza, endophytic fungus) | [114] |
| 352 | (-)-dihydrovertinolide | C. rosea B5-2 (the branch of B. gymnorrhiza, endophytic fungus) | [108] |
| 353 | (-)-vertinolide | C. rosea B5-2 (the branch of B. gymnorrhiza, endophytic fungus) | [108] |
| 354 | Xenofuranone B | P. capitalensis (the hypocotyl of B. sexangula, endophytic fungus) | [64] |
| 355 | 14-hydroxybislongiquinolide | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 356 | 20-hydroxybislongiquinolide | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 357 | 14, 20-dihydroxybislongiquinolide | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 358 | Bislongiquinolide | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 359 | Trichodimerol | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 360 | Bisorbicillinolide | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 361 | Saturnispol B | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 362 | Bisvertinolone | T. reesei SCNU-F0042 (the fresh bark of B. gymnorrhiza, endophytic fungus) | [112] |
| 363 | Penicitrinone acetate | Penicillium sp. B21 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [115] |
| No. | Compound | Source | Reference |
|---|---|---|---|
| 364 | 3′,4′,5′-trihydroxy-7-hydroxy-5-methoxyflavone | B. gymnorrhiza, leaf | [118,119] |
| 365 | Gramrione | B. gymnorrhiza, stem and leaf | [53,119] |
| 366 | 3′,4′,5,7-tetrahydroxy methylflavone | B. gymnorrhiza, stem and leaf | [53] |
| 367 | 5,7-dihydroxy-2-[3-hydroxy-4,5-dimethoxy-phenyl]-chromen-4-one | B. gymnorrhiza, leaf | [120] |
| 368 | Taxifolin | B. parviflora, leaf | [52] |
| 369 | Quercetin | B. parviflora, leaf | [52] |
| 370 | Myricetin | B. parviflora, leaf | [52] |
| 371 | Kaempferol | B. parviflora, leaf | [52] |
| 372 | Luteolin 5-methyl ether 7-O-β-D-glucopyranoside | B. gymnorrhiza, leaf | [119] |
| 373 | 7,4′-dihydroxy-5,3′-dimethoxyflavone 7-O-β-D-glucopyranoside | B. gymnorrhiza, leaf | [119] |
| 374 | 7,4′,5′-trihydroxy-5,3′-dimethoxyflavone 7-O-β-D- glucopyranoside | B. gymnorrhiza, leaf | [119] |
| 375 | 7,4′-dihydroxy-5-methoxyflavone 7-O-β-D-glucopyranoside | B. gymnorrhiza, leaf | [119] |
| 376 | Quercetin-3-O-β-D-glucopyranoside | B. gymnorrhiza, stem and leaf | [53,119] |
| 377 | Astragalin | B. gymnorrhiza, stem and leaf | [53] |
| 378 | Rutin | B. gymnorrhiza, stem and leaf; B. parviflora, leaf | [52,53,119] |
| 379 | Kaempferol 3-O-rutinoside | B. gymnorrhiza, leaf | [119] |
| 380 | Myricetin 3-O-rutinoside | B. gymnorrhiza, leaf | [119] |
| 381 | Brugymnoside A | B. gymnorrhiza, hypocotyl | [121] |
| 382 | (Z)-7,4’-dimethoxy-6-hydroxy-aurone-4-O-β-glucopyranoside | P. citrinum (B. gymnorrhiza, endophytic fungus) | [117] |
| No. | Compound | Source | Reference |
|---|---|---|---|
| 383 | 3-phenylpropanoic acid | Gloesporium sp. (B. gymnorrhiza, endophytic fungus) | [93] |
| 384 | Scopoletin | B. gymnorrhiza, hypocotyl | [124] |
| 385 | 3-hydroxymethyl-6,8-dimethoxycoumarin | No. GX4-1B (the branch of B. gymnorrhiza, endophytic fungus) | [111] |
| 386 | 6-hydroxy-4-hydroxymethyl-8-methoxy-3- methylisocoumarin | No. GX4-1B (the branch of B. gymnorrhiza, endophytic fungus) | [111] |
| 387 | (3R*,4S*)-6,8-dihydroxy-3,4,7-trimethylisocoumarin | Penicillium sp. 091402 (the root of B. sexangula, endophytic fungus) | [125] |
| 388 | (3R,4S)-6,8-dihydroxy-3,4,5-trimethylisocoumarin | Penicillium sp. 091402 (the root of B. sexangula, endophytic fungus) | [125] |
| 389 | €-6-hydroxymellein | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus | [102] |
| 390 | Penicimarin G | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus | [60] |
| 391 | Penicimarin H | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus | [60] |
| 392 | Penicimarin I | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus | [60] |
| 393 | Aspergillumarin A | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus; Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [60,61] |
| 394 | Peniciisocoumarin A | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 395 | Peniciisocoumarin B | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 396 | Peniciisocoumarin C | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 397 | Peniciisocoumarin D | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 398 | Peniciisocoumarin E | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 399 | Peniciisocoumarin F | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 400 | Peniciisocoumarin G | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 401 | Peniciisocoumarin H | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 402 | (R)-3-(3-hydroxypropyl)-8-hydroxy-3,4-dihydroisocoumarin | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 403 | Penicimarin C | Penicillium sp. TGM112 (B. sexangula var. rhynchopetala, fungus) | [61] |
| 404 | (-)-Orthosporin | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 405 | Diaporthin | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 406 | 6-hydroxymellein | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 407 | (R)-(-)-5-Carbonylmellein | X. cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [97] |
| 408 | (R)-(-)-5-Methoxycarbonylmellein | X. cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [97] |
| 409 | (R)-(-)-Mellein methyl ether | X. cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [97] |
| 410 | Brugunin A | B. gymnorrhiza, branch | [126] |
| 411 | Brugnanin | B. gymnorrhiza, stem bark | [127] |
| 412 | Balanophonin | B. gymnorrhiza, hypocotyl | [124] |
| 413 | Secoisolariciresinol | B. gymnorrhiza, hypocotyl | [124] |
| 414 | Cleomiscosin A | B. gymnorrhiza, hypocotyl | [124] |
| 415 | Pinoresinol | B. gymnorrhiza, hypocotyl | [124] |
| 416 | Medioresinol | B. gymnorrhiza, hypocotyl | [124] |
| 417 | Lyoniresinol-3α-O-β-D-glucopyranosides | B. gymnorrhiza, hypocotyl | [124] |
| 418 | Aryl-tetralin lignan rhamnoside | B. gymnorrhiza, leaf | [119] |
| 419 | Rhyncosides E | B. sexangula var. rhynchopetala, stem | [128] |
| 420 | Rhyncosides F | B. sexangula var. rhynchopetala, stem | [128] |
| No. | Compound | Source | Reference |
|---|---|---|---|
| 421 | Bruguierol A | B. gymnorrhiza, stem | [130] |
| 422 | Bruguierol B | B. gymnorrhiza, stem | [130] |
| 423 | Bruguierol C | B. gymnorrhiza, stem | [130] |
| 424 | 1-(3-hydroxyphenyl)-hexane-2,5-diol | B. gymnorrhiza, stem | [130] |
| 425 | Bruguierol D | B. gymnorrhiza, branch | [126] |
| 426 | 2,3-dimethoxy-5-propylphenol | B. gymnorrhiza, branch | [126] |
| 427 | Phenol A | Penicillium sp. 091402 (the root of B. sexangula, endophytic fungus) | [125] |
| 428 | 3,4,5-trimethyl-1,2-benzenediol | Penicillium sp. 091402 (the root of B. sexangula, endophytic fungus) | [125] |
| 429 | Tyrosol | D. eschscholtzii PSU-STD57 (the leaf of B. gymnorrhiza, endophytic fungus) | [99] |
| 430 | 3-hydroxy-4-methoxybenzoic acid | B. gymnorrhiza, hypocotyl | [131] |
| 431 | 4-hydroxybenzoic acid | B. gymnorrhiza, hypocotyl | [131] |
| 432 | 4-methoxybenylacetic acid | B. gymnorrhiza, hypocotyl | [131] |
| 433 | Di-(2-entylhexyl) phthalate | B. gymnorrhiza, hypocotyl | [131] |
| 434 | Dibutylphthalate | B. gymnorrhiza, hypocotyl; P. thomi (the root of B. gymnorrhiza, endophytic fungus) | [66,131] |
| 435 | Methyl caffeate | B. gymnorrhiza, hypocotyl | [131] |
| 436 | Methyl 3,5-dihydroxybenzoate | B. gymnorrhiza, hypocotyl | [131] |
| 437 | (S)-3-(3′,5′-dihydroxy-2′,4′-methylphenyl) butan-2-one | Penicillium sp. 091402 (the root of B. sexangula, endophytic fungus) | [125] |
| 438 | 1-(2,6-dihydroxyphenyl)butan-1-one | D. eschscholtzii PSU-STD57 (the leaf of B. gymnorrhiza, endophytic fungus); P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus); D. eschscholtzii HJ001 (B. sexangular var. rhynchopetala, endophytic fungus) | [99,100,102] |
| 439 | 1-(2-methoxyphenyl)butan-1-one | P. longicolla HL-2232 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [107] |
| 440 | Deoxyphomalone | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 441 | Phomalone | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 442 | Procyanidin | B. parviflora, bark | [132] |
| 443 | 3-(3,4-dihydroxyphenyl)-7,8-dihydroxyhexahydro-6H-pyrano[2,3-b][1,4]dioxine-6-carboxylic acid | B. gymnorrhiza, leaf | [34] |
| 444 | 2-(((3,4-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)-6-(3,4-dihydroxybenzyl)tetrahydro-2H-pyran-3,4,5-triol | B. gymnorrhiza, leaf | [34] |
| 445 | 7,8-dihydroxy-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)hexahydro-6H-pyrano[2,3-b][1,4]dioxine-6-carboxylic acid | B. gymnorrhiza, leaf | [34] |
| 446 | 3-(3,4-dimethoxyphenyl)-7,8-dihydroxy-2-(hydroxymethyl)hexahydro-6H-pyrano[2,3-b][1,4]dioxine-6-carboxylic acid | B. gymnorrhiza, leaf | [34] |
| 447 | Rhyncosides A | B. sexangula var. rhynchopetala, stem | [128] |
| 448 | Rhyncosides B | B. sexangula var. rhynchopetala, stem | [128] |
| 449 | Rhyncosides C | B. sexangula var. rhynchopetala, stem | [128] |
| 450 | Rhyncosides D | B. sexangula var. rhynchopetala, stem | [128] |
| 451 | 4′,5-dihydroxy-2,3-dimethoxy-4-(hydroxypropyl)-biphenyl | P. thomi (the root of B. gymnorrhiza, endophytic fungus) | [66] |
| 452 | 5,5′-dimethoxybiphenyl-2,2′-diol | P. longicolla HL-2232 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [107] |
| 453 | 6,6′-dimethoxybiphenyl-2,2′-diol | P. longicolla HL-2232 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [107] |
| 454 | 3-(3-hydro-xybutyl)-1,1-dimethylisochroman-6,8-diol | B. gymnorrhiza, stem | [130] |
| 455 | (3R,4S)-6,8-dihydroxy-3,4,5,7-tetramethylisochroman | Penicillium sp. 091402 (the root of B. sexangula, endophytic fungus) | [125] |
| 456 | (1S,3R,4S)-1-(4′-hydroxylphenyl)-3,4-dihydro-3,4,5-trimethyl-1H-2-benzopyran-6,8-diol | P. citrinum (B. gymnorrhiza, endophytic fungus) | [117] |
| 457 | (2R*,4R*)-3,4-dihydro-5-methoxy-2-methyl-2H-1-benzopyran-4-ol | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [102] |
| 458 | (2R*,4R*)-3,4-dihydro-4-methoxy-2-methyl-2H-1-benzopyran-4-ol | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [102] |
| 459 | 2-(2’S-hydroxypropyl)-5-methyl-7-hydroxychromone | P. longicolla HL-2232 (the leaf B. sexangula var. rhynchopetala, endophytic fungus) | [67] |
| 460 | 5,7-dihydroxy-2-propylchromone | P. citrinum HL-5126 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [102] |
| 461 | Rhytidchromone A | R. rufulum (the leaf of B. gymnorrhiza, endophytic fungus) | [133] |
| 462 | Rhytidchromone B | R. rufulum (the leaf of B. gymnorrhiza, endophytic fungus) | [133] |
| 463 | Rhytidchromone C | R. rufulum (the leaf of B. gymnorrhiza, endophytic fungus) | [133] |
| 464 | Rhytidchromone D | R. rufulum (the leaf of B. gymnorrhiza, endophytic fungus) | [133] |
| 465 | Rhytidchromone E | R. rufulum (the leaf of B. gymnorrhiza, endophytic fungus) | [133] |
| 466 | Alternariol 5-O-methyl ether | P. longicolla HL-2232 (the leaf of B. sexangula var. rhynchopetala, endophytic fungus) | [107] |
| 467 | 2-acetyl-7-methoxybenzofuran | D. eschscholtzii HJ004 (the stem of B. sexangula var. rhynchopetala, endophytic fungus) | [103] |
| 468 | 4,6-dihydroxy-5-methoxy-7-methyl-1,3-dihydroisobenzofuran | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 469 | Furobenzotropolone A | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 470 | Furobenzotropolone B | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 471 | 3-hydroxyepicoccone B | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 472 | 4,6-dihydroxy5-methoxy-7-methylphthalide | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 473 | 4,5,6-trihydroxy-7-methyl-3H-isobenzofuran-1-one | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| 474 | Sparalide C | E. nigrum MLY-3 (the leaf of B. gymnorrhiza, endophytic fungus) | [113] |
| No. | Compound | Source | Reference |
|---|---|---|---|
| 475 | (9Z,12Z,15Z)-6,8,11-trihydroxyoctadeca-9,12,15-trienoic acid | B. gymnorrhiza, leaf | [34] |
| 476 | (9E,12Z)-6,8,11-trihydroxyoctadeca-9,12-dienoic acid | B. gymnorrhiza, leaf | [34] |
| 477 | (E)-6,8,12-trihydroxyoctadec-9-enoic acid | B. gymnorrhiza, leaf | [34] |
| 478 | 8,12-dihydroxyhexadecanoic acid | B. gymnorrhiza, leaf | [34] |
| 479 | Tetradeca-5,8-dienoic acid | S. albidoflavus 107A-01824 (the leaf of B. gymnorrhiza, endophytic actinomycete) | [136] |
| 480 | Clonostach acids A | C. rosea B5-2 (the branch of B. gymnorrhiza, endophytic fungus) | [108] |
| 481 | Clonostach acids B | C. rosea B5-2 (the branch of B. gymnorrhiza, endophytic fungus) | [108] |
| 482 | Clonostach acids C | C. rosea B5-2 (the branch of B. gymnorrhiza, endophytic fungus) | [108] |
| 483 | Succinic acid | P. thomi (the root of B. gymnorrhiza, endophytic fungus) | [66] |
| 484 | Xylacinic acid A | X. cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [97] |
| 485 | Xylacinic acid B | X. cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [97] |
| 486 | 2-Hexylidene-3-methyl succinic acid 4-methyl ester | X. cubensis PSU-MA34 (the branch of B. parviflora, endophytic fungus) | [97] |
| 487 | Prolipyrone A | Fusarium proliferatum MA-84 (the inner tissue of B. sexangula, endophytic fungus) | [45] |
| 488 | Prolipyrone B | F. proliferatum MA-84 (the inner tissue of B. sexangula, endophytic fungus) | [45] |
| 489 | Prolipyrone C | F. proliferatum MA-84 (the inner tissue of B. sexangula, endophytic fungus) | [45] |
| 490 | Gibepyrone D | F. proliferatum MA-84 (the inner tissue of B. sexangula, endophytic fungus) | [45] |
| 491 | Erythritol | P. citrinum ZD6 (the stem of B. gymnorrhiza, endophytic fungus) | [92] |
| 492 | Mannitol | P. citrinum ZD6 (the stem of B. gymnorrhiza, endophytic fungus) | [92] |
| 493 | Eicosanol | B. cylindrica, leaf | [58] |
| 494 | (2R,3R,4S,5R,6R)-4-(((4R,5S,6R)-4-hydroxy-5-methoxy-6-methyltetrahydro-2H-pyran-3-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-2,3,5-triol | B. gymnorrhiza, leaf | [34] |
| 495 | Cowabenzophenone A | A. terreus (B. gymnorrhiza, endophytic fungus) | [135] |
| 496 | (±)-1-monopalmitin | P. thomi (the root of B. gymnorrhiza, endophytic fungus) | [66] |
| Compound | Cell Lines/Brine Shrimp | Effects | References |
|---|---|---|---|
| Acaciicolide B (10) | cytotoxic activity against MOLT-3 and HL-60 cancer cells; | IC50 values of 165.04 and 159.05 μM | [29] |
| 3-epi-Steperoxide A (27) | cytotoxic activity against MOLT-3, HuCCA-1, A549, HepG2, HL-60, MDA-MB-231, T47D, and HeLa cancer cell lines; | IC50 values range of 0.68–3.71 μg/mL | [28] |
| Steperoxide A (30) | cytotoxic activity against MOLT-3, HuCCA-1, A549, HepG2, HL-60, MDA-MB-231, T47D, and HeLa cancer cell lines; | IC50 values range of 0.67–5.25 μg/mL | [28] |
| Merulin A (31) | cytotoxic activity against HuCCA-1, A549, MOLT-3, HepG2, HL-60, MDA-MB-231, T47D, Hela and MRC-5 cell lines; | IC50 values range of 15.20–76.97 μM | [29] |
| Merulin B (32) | cytotoxic activity against MOLT-3, A549, HepG2, HL-60, MDA-MB-231, and T47D cell lines; | IC50 values range of 11.94–49.08 μg/mL | [28] |
| Merulin C (33) | cytotoxic activity against HL-60 cancer cells; cytotoxic activity against MOLT-3, HuCCA-1, A549, HepG2, MDA-MB-231, T47D, and HeLa cell lines; | IC50 value of 0.08 μg/mL; IC50 values range of 0.19–3.75 μg/mL | [28] |
| Merulin D (34) | cytotoxic activity against HuCCA-1, A549, MOLT-3, HepG2, HL-60, MDA-MB-231, T47D, Hela and MRC-5 cell lines; | IC50 values range of 18.31–154.51 μM | [29] |
| 7-epi-merulin B (35) | cytotoxic activity against HuCCA-1, A549, MOLT-3, HepG2, HL-60, MDA-MB-231, T47D, and MRC-5 cell lines; | IC50 values range of 0.28–37.46 μM | [29] |
| Botryosphaerin F (40) | cytotoxic activity against MCF-7 and HL-60 cancer cells; | IC50 values of 4.49 and 3.43 μM | [36] |
| 13,14,15,16-tetranorlabd-7-ene-19,6b:12,17-diolide (41) | cytotoxic activity against MCF-7 and HL-60 cancer cells; | IC50 values of 2.79 and >30 μM | [36] |
| Botryosphaerin B (42) | cytotoxic activity against MCF-7 and HL-60 cancer cells; | IC50 values of 17.60 and >30 μM | [36] |
| LLZ1271β (43) | cytotoxic activity against MCF-7 and HL-60 cancer cells; | IC50 values of >30 and 0.60 μM | [36] |
| Ent-kaur-16-en-13-hydroxy-19-al (64) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 11.5, 10.5 and 42.3 μg/mL | [42] |
| Ent-kaur-16-en-13,19-diol (65) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 50.0, 50.0 and 50.0 μg/mL | [42] |
| 17-chloro-13,16β-dihydroxy-ent-kauran-19-al (68) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 50.0, 29.2 and 38.2 μg/mL | [42] |
| Methyl-16α,17-dihydroxy-ent-kauran-19-oate (69) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 39.5, 27.7 and 40.5 μg/mL | [42] |
| Methyl-16α,17-dihydroxy-ent-9(11)-kauren-19-oate (71) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 41.9, 26.7 and 38.7 μg/mL | [42] |
| 16α,17-dihydroxy-ent-kauran-19-al (72) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 50.0, 50.0 and 50.0 μg/mL | [42] |
| 16αH-17-hydroxy-ent-kauran-19-oic acid (73) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 42.4, 32.8 and 43.0 μg/mL | [42] |
| 16αH-17,19-ent-kaurane-diol (74) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 12.8, 13.6 and 35.7 μg/mL | [42] |
| 16-ent-kauren-19-ol (75) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 18.2, 6.8 and 32.8 μg/mL | [42] |
| (5R,9S,10R,13S,15S)-ent-8(14)-pimarene-1-oxo-15R,16-diol (82) | cytotoxic activities against L-929 cell lines; | IC50 value of 9.8 μg/mL | [43] |
| 15(S)-isopimar-7-en-15,16-diol (83) | cytotoxic activities against K562 cell lines; | IC50 value of 7.0 μg/mL | [43] |
| (4R,5S,8R,9R,10S,13S)-ent-17-hydroxy-16-oxobeyeran-19-al (85) | cytotoxic activities against L-929, K562 and HeLa cell lines; | GI50/CC50 values of 45.4, 50.0 and 37.7 μg/mL | [42] |
| Sexangulic acid (94) | anti-tumour activity against A-549 and HL60 cell lines; | moderate in vitro cytotoxicity at 5 μg/mL | [47] |
| 3α-Z-feruloyltaraxerol (120) | cytotoxic activities against NCI-H187 cell lines; | IC50 value of 12.2 μg/mL | [57] |
| 3α-Z-coumaroyltaraxerol (124) | cytotoxic activities against NCI-H187 cell lines; | IC50 value of 20.0 μg/mL | [57] |
| 3β-(Z)-coumaroyltaraxerol (129) | cytotoxic against Neuro 2A cell lines | IC50 value of 75.76 μM | [58] |
| Nectrianolin D (144) | cytotoxic against HL-60 cell lines; | IC50 value of 10.16 μM | [63] |
| Furanocochlioquinol (145) | cytotoxic against HL-60 cell lines; | IC50 value of 0.47 μM | [63] |
| Furanocochlioquinone (146) | cytotoxic against HL-60 cell lines; | IC50 value of 0.63 μM | [63] |
| Nectripenoid B (147) | cytotoxic against HL-60 cell lines; | IC50 value of 0.93 μM | [63] |
| Cochlioquinone D (148) | cytotoxic against HL-60 cell lines; | IC50 value of 1.61 μM | [63] |
| 3β,5α-dihydroxy-(22E,24R)-ergosta-7,22-dien-6-one (161) | cytotoxic against MCF-7, A549, Hela and KB cell lines; | IC50 values of 4.98, 1.95, 0.68 and 1.50 μM | [69] |
| 3β,5α,14α-trihydroxy-(22E,24R)-ergosta-7,22-dien-6-one (162) | cytotoxic against MCF-7, A549, Hela and KB cell lines; | IC50 values of 25.4, 27.1, 24.4 and 19.4 μM | [69] |
| Meleagrin (193) | cytotoxic against MCF-7, A549, Hela and KB cell lines; | IC50 values of 9.7 and 8.3 μM | [88] |
| Uridine (194) | cytotoxic against B16F10, A549, HL60 and MCF-7 cell lines; | IC50 values of 16.7, 8.6, 15.9 and 31.9 μmol/L | [67] |
| 6-aminopurine-9-carboxylic acid methyl ester (195) | cytotoxic against B16F10, A549, HL60 and MCF-7 cell lines; | IC50 values of 29.0, 21.2, 4.1 and 14.9 μmol/L | [67] |
| Adenine riboside (196) | cytotoxic against B16F10, A549, HL60 and MCF-7 cell lines; | IC50 values of 18.2, 12.5, 14.4 and 26.2 μmol/L | [67] |
| Cyclo-(Ala-Gly) (220) | cytotoxic against K562, HepG2 and HT29 cell lines; | IC50 values of 18.1, 9.5, and 10.3 μM | [66] |
| Cyclo-(Pro-Gly) (221) | cytotoxic against K562, HepG2 and HT29 cell lines; | IC50 values of 17.6, >50 and 10.8 μM | [66] |
| Cyclo-(Ala-Pro) (222) | cytotoxic against K562, HepG2 and HT29 cell lines; | IC50 values of 9.6, 13.6, and 20.1 μM | [66] |
| Beauvericin (226) | cytotoxic against HL60 and A549 cell lines; | IC50 values of 2.02, 0.8, 1.14 and 1.10 μM | [69] |
| Palmarumycins BG5 (236) | cytotoxic against MCF-7 and HL-60 cell lines; | IC50 values of 7.6 and 1.9 μM | [98] |
| Nigronatthaphenyl (242) | cytotoxic against HCT 116 colon cell line; | IC50 value of 9.62 μM | [101] |
| Incarxanthone B (258) | cytotoxicity against A375, MCF-7 and HL-60 cell lines; | IC50 values of 8.6, 6.5, and 4.9 μM | [104] |
| Cytochalasin D (296) | cytotoxic against KB cell lines; | IC50 value of 3.99 μg/mL | [97] |
| Zygosporin D (297) | cytotoxic against HCT15 cell lines; | IC50 value of 13.5 μM | [110] |
| 12-hydroxylcytochalasin Q (305) | cytotoxic against HCT15 cell lines; | IC50 value of 13.4 μM | [110] |
| Penibenzophenone B (321) | cytotoxic against A549 cell lines; | IC50 value of 15.7 μg/mL | [87] |
| Asterric acid (324) | cytotoxic against Hela cell lines; | IC50 value of 21.6 μg/mL | [87] |
| Scopoletin (384) | cytotoxic against Hela, A435, A549 and K562 cell lines; | IC50 values of 764.7, 593.4, 290.2 and 487.7 μg/mL | [124] |
| (3R*,4S*)-6,8-dihydroxy-3,4,7-trimethylisocoumarin (387) | cytotoxic against K562 cell lines; | IC50 value of 18.9 μg/mL | [125] |
| Brugnanin (411) | cytotoxic against CNE-1 nasopharyngeal carcinoma cell lines; | IC50 value of 5.72 × 10−4 M | [127] |
| Secoisolariciresinol (413) | cytotoxic against Hela, A435, A549 and K562 cell lines; | IC50 values of 571.5, 397.5, 323.0 and 768.8 μg/mL | [124] |
| Lyoniresinol-3α-O-β-D-glucopyranosides (417) | cytotoxic against Hela, A435, A549 and K562 cell lines; | IC50 values of 328.8, 455.5, 209.3 and 361.9 μg/mL | [124] |
| 3,4,5-trimethyl-1,2-benzenediol (428) | cytotoxic against SGC-7901 cell lines; | IC50 value of 36.0 μg/mL | [125] |
| Dibutylphthalate (434) | cytotoxic against K562, HepG2 and HT29 cell lines; | IC50 values of 17.3, 15.2, and 11.1 μM | [66] |
| 4′,5-dihydroxy-2,3-dimethoxy-4-(hydroxypropyl)-biphenyl (451) | cytotoxic against K562, HepG2 and HT29 cell lines; | IC50 values of 10.1, 12.2 and 8.9 μM | [66] |
| Rhytidchromone A (461) | cytotoxic against MCF-7 and Kato-3 cell lines; | IC50 values of 19.3 and 23.3 μM | [133] |
| Rhytidchromone B (462) | cytotoxic against Kato-3 cell lines; | IC50 value of 21.4 μM | [133] |
| Rhytidchromone D (464) | cytotoxic against Kato-3 cell lines; | IC50 value of 16.8 μM | [133] |
| Rhytidchromone E (465) | cytotoxic against MCF-7 and Kato-3 cell lines; | IC50 values of 17.7 and 16.0 μM | [133] |
| Cowabenzophenone A (495) | cytotoxic against HCT 116 colon cell line; | IC50 value of 10.1 μM | [135] |
| Fusaprolifin A (87) | The lethality activity against brine shrimp; | lethality rate of 49.5%, at 100 mg/mL | [45] |
| Fusaprolifin B (88) | The lethality activity against brine shrimp; | lethality rate of 9.6%, at 100 mg/mL | [45] |
| Macrosporin-2-O-(6′-acetyl)-α-D-glucopyranoside (269) | The lethality activity against brine shrimp; | LD50 value of 10 μM | [106] |
| Compound | Pathogen | Effect | Reference |
|---|---|---|---|
| (3R,4R,6R,7S)-7-hydroxyl-3,7-dimethyl-oxabicyclo[3.3.1]nonan-2-one (1) | B. cinerea, and P. nicotianae | 3.1 and 6.3 μg/mL | [26] |
| (3R,4R)-3-(7-methylcyclohexenyl)-propanoic acid (2) | C. albicans, B. cinerea, and P. nicotianae | 50, 3.1 and 6.3 μg/mL | [26] |
| 7R-hydroxygeosmin (49) | B. subtilis, E. coli and P. aeruginosa | weak activities | [32] |
| 3-oxogeosmin (50) | B. subtilis, P. aeruginosa and VRE | weak activities | [32] |
| (4S,5S,7R,10S)-4β,10α-eudesmane-5β,11-diol (53) | B. subtilis, S. aureus, E. coli, P. aeruginosa, MRSA, VRE, M. vaccae, S. salmonicolor and P. notatum | broad antimicrobial activities | [32] |
| 11-oxo-12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β-diol (95) | S. aureus, B. subtilis, E. coli, M. luteus, M. tetragenus, S. albus and B. cereus | >20, >20, 9.76, >20, >20, 9.76 and 9.76 μmol/L | [48] |
| 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-3β,11β-diol (96) | S. aureus, B. subtilis, E. coli, M. luteus, M. tetragenus, S. albus and B. cereus | 5, 10, 5, >20, 5, 10 and 10 μmol/L | [48] |
| 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β,11β-triol (98) | S. aureus, B. subtilis, E. coli, M. luteus, M. tetragenus, S. albus and B. cereus | 4.86, 9.72, 4.86, 9.72, 4.86, >20 and >20 μmol/L | [48] |
| Dehydroaustin (131) | S. epidermidis, S. aureus, E. coli, B. cereus and V. alginolyticus | 20, >20, >20, >20 and >20 µM | [60] |
| 11β-acetoxyisoaustinone (132) | S. epidermidis, S. aureus, E. coli, B. cereus and V. alginolyticus | 20, >20, >20, >20 and >20 µM | [60] |
| Austinol (133) | S. epidermidis, S. aureus, E. coli, B. cereus and V. alginolyticus | 10, >20, >20, 20 and >20 µM | [60] |
| Guignardone A (149) | P. aeruginosa, and MRSA | 25, and 25 μg/mL | [64] |
| Guignardone J (151) | P. aeruginosa, and MRSA | 50, and 50 μg/mL | [64] |
| BG138 (a mixture of brugierol 172 and isobrugierol 173) | P. aeruginosa | 32 μg/mL | [74] |
| Thiocladospolide A (179) | E. tarda, E. ictarda and C. glecosporioides | 1, 8 and 2 µg/mL | [21] |
| Thiocladospolide B (180) | C. glecosporioides, P. piricola Nose and F. oxysporum f. sp. Cucumerinum | 2, 32 and 1 µg/mL | [21] |
| Thiocladospolide C (181) | C. glecosporioides, P. piricola Nose and F. oxysporum f. sp. Cucumerinum | 1, 32 and 32 µg/mL | [21] |
| Thiocladospolide D (182) | E. ictarda, C. glecosporioides, P. piricola Nose and F. oxysporum f. sp. Cucumerinum | 1, 1, 32 and 1 µg/mL | [21] |
| Pandangolide 3 (183) | C. glecosporioides and B. sorokiniana | 2 and 8 µg/mL | [21] |
| Thiocladospolide F (184) | E. coli, E. tarda, P. aeruginosa, V. anguillarum, F. oxysporum f. sp. Momodicae, P. digitatum and H. maydis | 16, 1.0, 4.0, 2.0, 32, 32 and 8.0 µg/mL | [75] |
| Thiocladospolide G (185) | E. coli, E. tarda, V. anguillarum, F. oxysporum f. sp. Momodicae, P. digitatum and H. maydis | 16, 2.0, 2.0, 16, 16 and 4.0 µg/mL | [75] |
| Antimycin A18 (188) | plant pathogenic fungi: C. lindemuthianum, B. cinerea, A. solani and M. grisea | the minimum concentration values to show inhibition zone on plates were 0.01, 0.06, 0.03 and 0.20 μg/mL | [86] |
| Penibruguieramine A (199) | S. aureus | 20 µg/mL | [88] |
| Cyclo-(Ala-Gly) (220) | B. subtilis | 400 µg/mL | [92] |
| 2-Chloro-5-methoxy-3-methylcyclohexa-2,5-diene-1,4-dione (231) | SA and MRSA | equal MIC values of 128 µg/mL | [97] |
| 8-methoxy-1-naphthol (240) | S. aureus, MRSA and M. gypseum | 200 µg/mL | [99] |
| 1,8-dimethoxynaphthalene (241) | S. aureus, MRSA and M. gypseum | 200 µg/mL | [99] |
| Nigronatthaphenyl (242) | B. subtilis, B. subtilis TISTR 088, B. cereus TISTR 688, S. aureus, E. coli, MRSA, C. albicans, P. aeruginosa, C. gloeosporioides and A. niger | 4, 4, 2, 4, 2, 2, 2, 2, 4 and 8 μg/mL | [101] |
| (3S)-3,8-Dihydroxy-6,7-dimethyl-α-tetralone (243) | S. aureus, MRSA and M. gypseum | 200 µg/mL | [99] |
| Isosclerone (244) | S. aureus, MRSA and M. gypseum | 200 µg/mL | [99] |
| 1,3,8-trimethoxynaphtho[9-c]furan (247) | S. aureus, MRSA and B. cereus | >25, >25 and 12.5 µg/mL | [103] |
| 5-hydroxy-2-methoxy-6,7-dimethyl-1,4-naphthoquinone (255) | B. cereus | 12.5 µg/mL | [103] |
| Chloroisosulochrin dehydrate (266) | MRSA, S. aureus, E. coli, B. cereus, V. alginolyticus and V. parahaemolyticus | with the same MIC value of 50 Μm | [105] |
| Emodin (267) | B. subtilis and P. aeruginosa | 25 and 100 µg/mL | [92] |
| Auxarthrol C (268) | E. coli | 9.8 µM | [106] |
| Macrosporin (270) | M. tetragenus, E. coli, S. albus, S. aureus and B. subtilis | 7.8, 3.9, 3.9, 3.9 and 3.9 µM | [106] |
| 2-O-acetylaltersolanol B (272) | M. tetragenus, E. coli and S. aureus | 4.6, 4.6 and 9.2 µM | [106] |
| Altersolanol A (273) | M. tetragenus, E. coli, S. aureus and B. subtilis | 8.2, 4.1, 2.07 and 4.1 µM | [106] |
| Altersolanol B (274) | V. parahaemolyticus and V. anguillarum; M. tetragenus, S. aureus, K. rhizophila, and B. subtilis | 2.5 and 5 µg/mL; 7.8, 7.8, 7.8 and 7.8 µM | [106,107] |
| Altersolanol C (275) | B. subtilis | 8.8 µM | [106] |
| Tetrahydroaltersolanol B (279) | E. coli | 7.3 µM | [106] |
| Alterporriol U (282) | B. cereus | 8.3 µM | [106] |
| Alterporriol V (283) | B. cereus | 8.1 µM | [106] |
| Alterporriol B (286) | B. cereus | 7.9 µM | [106] |
| Alterporriol D (287) | E. coli, B. cereus and S. aureus | 7.5, 10.0 and 5.0 µM | [106] |
| Alterporriol E (288) | E. coli and B. cereus | 5.0 and 2.50 µM | [106] |
| Alterporriol C (289) | B. cereus | 8.9 µM | [106] |
| 2′-acetoxy-7-chlorocitreorosein (293) | S. aureus and V. parahaemolyticus | 22.8 and 10 µM | [105] |
| Citreorosein (294) | S. aureus, E. coli, B. cereus, V. alginolyticus and V. parahaemolyticus | 22.8, 50, 50, 50 and 50 µM | [105] |
| MT-1 (295) | MRSA, S. aureus, E. coli, B. cereus, V. alginolyticus and V. parahaemolyticus | with the same MIC value of 50 Μm | [105] |
| [11]-cytochalasa-5(6),13-diene-1,21-dione-7,18-dihydroxy-16,18-dimethyl-10-phenyl(7S*,13E,16S*,18R*) (298) | E. coli, S. aureus, B. cereus, V. parahaemolyticus and V. alginolyticus | with the same MIC value of 50 μg/mL | [100] |
| 2-deoxy-sohirnone C (317) | MRSA | 80 μg/mL | [90] |
| 3,4-dihydroxybenzoic acid (319) | P. aeruginosa, E. faecalis, MRSA, E. coli and C. albicans | 25, 25, 25, 12.5 and 25 μg/mL | [64] |
| 8-O-methylnodulisporin F (326) | S. aureus, MRSA and B. cereus | 6.25, 12.5 and 6.25 µg/mL | [103] |
| Nodulisporin H (327) | S. aureus, MRSA and B. cereus | 12.5, 12.5 and 6.25 µg/mL | [103] |
| 5R-hydroxyrecifeiolide (338) | E. ictarda and P. aeruginosa | 32 and 32 µg/mL | [114] |
| 5S-hydroxyrecifeiolide (339) | G. cingulate | 16 µg/mL | [114] |
| Pandangolide 1 (340) | S. aureus, E. ictarda, G. cingulate and P. aeruginosa | 32, 4.0, 1.0 and 32 µg/mL | [114] |
| Cladocladosin A (341) | E. tarda, V. anguillarum, F. oxysporum f. sp. Momodicae, P. digitatum and H. maydis | 2.0, 4.0, 32, 32 and 8.0 µg/mL | [75] |
| Infectopyrone A (345) | S. albus, E. coli, B. subtilis, M. tetragenus and M. luteus | 5.0, 2.5, 10.0, 10.0 and 10.0 µg/mL | [106] |
| Infectopyrone B (346) | S. albus, E. coli, M. tetragenus and M. luteus | 10.0, 2.5, 10.0 and 10.0 µg/mL | [106] |
| 6,8-dihydroxy-5-methoxy-3-methyl-1H-isochromen-1-one (347) | P. aeruginosa, and MRSA | 50, and 50 μg/mL | [64] |
| Ent-cladospolide F (348) | S. aureus, E. ictarda and P. aeruginosa | 8.0, 16 and 64 µg/mL | [114] |
| Cladospolide G (349) | E. coli, G. cingulate, B. sorokiniana and F. oxysporum f. sp. Cucumerinum | 32, 1.0, 32 and 1.0 µg/mL | [114] |
| Iso-cladospolide B (351) | E. coli, E. tarda, E. ictarda and G. cingulate | 32, 32, 16 and 64 µg/mL | [114] |
| Penicimarin G (390) | S. epidermidis, S. aureus, E. coli, B. cereus and V. alginolyticus | 20, 20, 20, 20 and 20 µM | [60] |
| Penicimarin H (391) | S. epidermidis, S. aureus, E. coli, B. cereus and V. alginolyticus | 10, 20, >20, 20 and 20 µM | [60] |
| Bruguierol A (421) | M. vaccae and C. albicans | 25 and 50 µg/mL | [130] |
| Bruguierol B (422) | M. vaccae and C. albicans | 25 and 50 µg/mL | [130] |
| Bruguierol C (423) | S. aureus, M. luteus, E. faecalis, E. coli, M. vaccae and C. albicans | 12.5, 12.5, 12.5, 12.5, 12.5 and 50 µg/mL | [130] |
| Tyrosol (429) | S. aureus, MRSA and M. gypseum | 200 µg/mL | [99] |
| 1-(2,6-dihydroxyphenyl)butan-1-one (438) | S. aureus, MRSA and M. gypseum; B. subtilis, B. cereus and M. tetragenus | 200 µg/mL; 6.94 µM | [99] |
| 5,5′-dimethoxybiphenyl-2,2′-diol (452) | V. parahaemolyticus | 10 µg/mL | [107] |
| 2-(2′S-hydroxypropyl)-5-methyl-7-hydroxychromone (459) | E. coli | 5 µg/mL | [67] |
| Erythritol (491) | B. subtilis | 50 µg/mL | [58] |
| Cowabenzophenone A (495) | B. subtilis, S. aureus, E. coli, MRSA, C. albicans, P. aeruginosa and C. gloeosporioides | 1, 2, 4, 4, 4, 2 and 2 μg/mL | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Chen, X.; Zhang, L.; Liu, B.; Xie, L.; Ma, Y.; Zhang, M.; Jin, X. Chemical Constituents and Biological Activities of Bruguiera Genus and Its Endophytes: A Review. Mar. Drugs 2024, 22, 158. https://doi.org/10.3390/md22040158
Luo X, Chen X, Zhang L, Liu B, Xie L, Ma Y, Zhang M, Jin X. Chemical Constituents and Biological Activities of Bruguiera Genus and Its Endophytes: A Review. Marine Drugs. 2024; 22(4):158. https://doi.org/10.3390/md22040158
Chicago/Turabian StyleLuo, Xiongming, Xiaohong Chen, Lingli Zhang, Bin Liu, Lian Xie, Yan Ma, Min Zhang, and Xiaobao Jin. 2024. "Chemical Constituents and Biological Activities of Bruguiera Genus and Its Endophytes: A Review" Marine Drugs 22, no. 4: 158. https://doi.org/10.3390/md22040158
APA StyleLuo, X., Chen, X., Zhang, L., Liu, B., Xie, L., Ma, Y., Zhang, M., & Jin, X. (2024). Chemical Constituents and Biological Activities of Bruguiera Genus and Its Endophytes: A Review. Marine Drugs, 22(4), 158. https://doi.org/10.3390/md22040158






