Genetic Engineering and Innovative Cultivation Strategies for Enhancing the Lutein Production in Microalgae
Abstract
:1. Lutein as One of the Important Carotenoids
2. Microalgae as Producers of Lutein and Other Carotenoids
3. Random Mutagenesis to Increase the Lutein Production
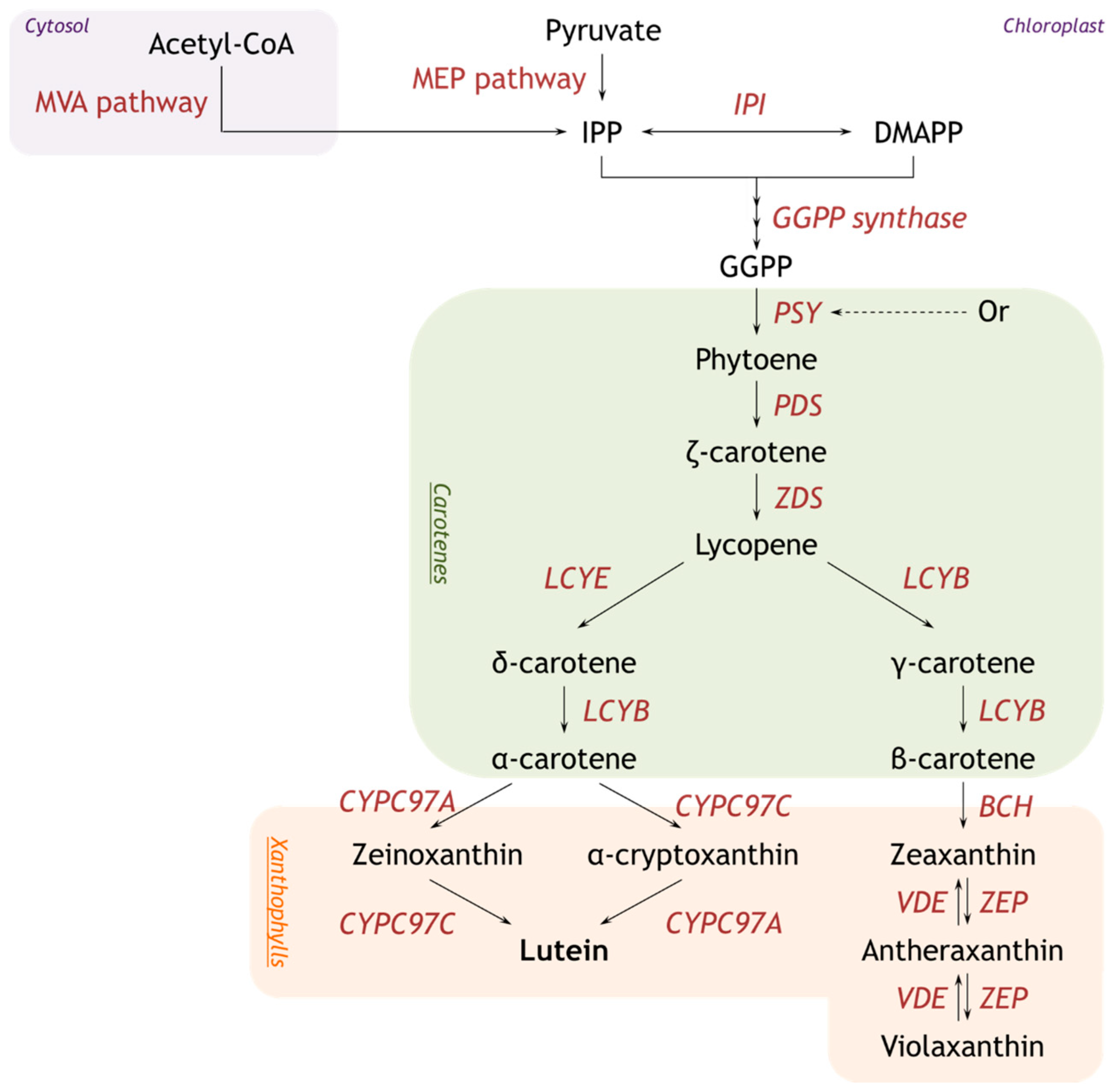
4. Metabolic Engineering for Enhanced Production of Lutein
| Source Species | Host Strain | Target Gene | Technique | Carotenoid | Reference |
|---|---|---|---|---|---|
| C. sorokiniana | / | / | Random mutagenesis (MNNG treatment) | Increased lutein content up to 7.25 mg/g DW with a productivity of 2.56 mg/L/day. | [29] |
| C. zofingiensis | / | / | Random mutagenesis (MNNG treatment) | Increased lutein content up to 6.25 mg/g DW with a productivity of 10.57 mg/L/day | [30] |
| C. sorokiniana | / | / | Random mutagenesis (MNNG treatment) | Increased lutein content up to 7.0 mg/g DW and 6.4 mg/L/day | [32] |
| C. zofingiensis | / | / | Random mutagenesis (MNNG treatment) | Increased zeaxanthin (up to 7.0 mg/g DW), lutein (up to 13.81 mg/g DW) and β-carotene (7.18 mg/g DW). | [33] |
| C. reinhardtii | Endogenous | Or | Overexpression Or gene using dual-promotor system | Lutein production increase from 0.69 mg/L to 1.04 mg/L and from 0.18 mg/L to 0.24 mg/L | [45] |
| C. reinhardtiii | Endogenous | Or | Overexpression Or gene | Increased α-carotene (1.9-fold higher), lutein (2-fold higher), β-carotene (2.1-fold higher) and violaxanthin (2.1-fold higher) content compared to WT. | [46] |
| C. reinhardtii | Endogenous | Or | Overexpression Or gene with single amino acid substitution using site-directed mutagenesis | Increased α-carotene (4-fold higher), lutein (3.1-fold higher), β-carotene (3.2-fold higher) and violaxanthin (3.1-fold higher) content compared to WT. | [46] |
| C. reinhardtii | Brassica oleracea | Or | Heterologous expression Or gene. | Increased lutein (1.5-fold higher: 112.4 pg/cell to 73.0 pg/cell lutein (WT)) and astaxanthin content (2-fold higher: 0.41 pg/cell to 0.2 pg/cell (WT)) | [50] |
| C. reinhardtii | Mesorhizobium loti and Sulfurihydrogenibium yellowstonense | CA | Heterologous expression of CA gene | Increased lutein concentration from 4.41 mg/L (WT) to 8.89 mg/L (CA from Ml) and 7.07 mg/L (CA from SY). | [51] |
| D. salina | Endogenous | PSY | Overexpression of PSY gene. | Increased lutein (7.6-fold higher) and β-carotene (5.4-fold higher) content compared to WT. | [47] |
| D. salina | H. pluvialis | PSY | Heterologous expression of PSY gene. | Increased lutein (7.2-fold higher) and β-carotene (2.4-fold higher) conten compared to WT. | [47] |
| C. reinhardtii | D. salina | PSY | Heterologous expression LCYE gene. | Increased lutein (2.6-fold higher) content compared to WT. | [32] |
| Scenedesmus | / | PSY | Expression of synthetic PSY gene. | Increased β-carotene content from 10.8 mg/g (WT) cell to 30 mg/g cell. | [28] |
| C. reinhardtii | Endogenous | LCYE | Overexpression of LCYE gene. | Increased lutein (at least 2-fold higher) content. | [48] |
| C. reinhardtii | C. vulgaris | LCYE | Heterologous of LCYE gene. | Increased lutein content (2.3-fold higher) compared to WT. | [49] |
| H. pluvialis | Endogenous | PDS | Overexpression of PDS gene with single amino acid substitution using site-directed mutagenesis. | Increased lutein (1.5 µg/g DW to 1.9 µg/g DW), zeaxanthin (142 µg/g DW to 214 µg/g DW), β-carotene (532 µg/g DW to 728 µg/g DW) and astaxanthin content compared to WT. | [52] |
| C. zofingiensis | Endogenous | PDS | Overexpression of PDS gene with single amino acid substitution using site-directed mutagenesis. | Increased total carotenoid content with 32.1% and astaxanthin with 54.1%. | [53] |
| C. reinhardtii | / | LCYE | Knock-out of the LCYE gene using CRISPR/Cas (NHEJ) | Increased zeaxantin content (up to 60%). | [54] |
| C. reinhardtii | / | LCYE | Knock-out of the LCYE gene using CRISPR/Cas (HDR) | Increased zeaxantin (0.31 mg/L (WT) to 0.59 mg/L), antheraxanthin (0.28 mg/L (WT) to 0.63 mg/L) and violaxanthin (1.3 mg/L (WT) to 2.3 mg/L) content. | [55] |
5. Cultivation Strategies to Increase Microalgal Lutein Production
5.1. Cultivation Strategies for Chlorella Species
| Microalgae | Cultivation Mode | Reactor Volume (L) | Strategy * | Lutein Content (mg/g DW) | Lutein Production (mg/L) | Lutein Productivity (mg/L/day) | Reference |
|---|---|---|---|---|---|---|---|
| C. sorokiniana FZU60 | Two-stage semi-continuous (5 cycles) | 1 | 1st Fed-batch Mixo (BG-11 with 1 g/L NaAc every 12 h and 150 μmol/m2/s) After 1.5 days, 92.5% medium replacement which is transferred to 2nd stage. 7.5% to new cycle 1st stage. 2nd Batch Photo (150 μmol/m2/s) | 9.57 (Day 3, average of the 5 cycles) | 17.35 (Day 3, average of the 5 cycles) | 11.57 (average of the 5 cycles) | [65] |
| C. sorokiniana FZU60 | Two-stage | 50 | 1st Fed-batch Mixo (acetate and 350 μmol/m2/s) 2nd Fed-batch Photo (BG11 and 350 μmol/m2/s) | 9.51 (Day 7) | 33.55 (Day 7) | 4.67 (average over 7 days) | [64] |
| C. sorokiniana FZU60 | Fed-batch | 1 | Mixo (acetate and 750 μmol/m2/s) | 8.29 (Day 7) | 32.16 (Day 4) | 8.04 (average over 4 days) | [66] |
| C. sorokiniana FZU60 | Two-stage | 1 | 1st Fed-batch Mixo (acetate and 750 μmol/m2/s) 2nd Batch Photo (750 μmol/m2/s) | 11.22 (Day 8) | 65.96 (Day 8) | 8.25 (average over 8 days) | [66] |
| C. sorokiniana FZU60 | Fed-batch | 5 | Hetero (Mann and Myer’s with glucose and urea) | 2.57 (Day 6) | 415.93 (Day 6) | 82.50 (average) | [59] |
| C. sorokiniana MB-1-M12 | Semi-continuous | 1 | Batch Mixo (acetate and 150 μmol/m2/s) After glucose depletion, 75% medium replacement | 4.98 (Day 7 in 2nd cycle) | 11.95 (Day 7 in 2nd cycle) | 6.61 (2nd cycle average) | [67] |
| C. sorokiniana MB-1-M12 | Batch | 1 | Batch Photo (150 μmol/m2/s) | 6.01 (Day 4) | 16.40 (Day 5) | 3.56 (average) | [68] |
| C. sorokiniana MB-1-M12 | Batch | 1 | Batch Mixo (acetate and 150 μmol/m2/s) | 7.00 (Day 5) | 18.04 (Day 5) | 5.15 (average) | [68] |
| C. sorokiniana MB-1-M12 | Batch | 1 | Batch Hetero (glucose) | 2.31 (Day 7) | 7.71 (Day 4) | 1.88 (average) | [68] |
| C. sorokiniana MB-1-M12 | Two-stage | 1 | 1st Batch Photo (150 μmol/m2/s) 2nd Batch Hetero (glucose) | 4.75 (Day 10) | 24.97 (Day 10) 20.5 (after day 6) | 1.75 (average) | [68] |
| C. sorokiniana MB-1-M12 | Two-stage | 1 | 1st Batch Hetero (glucose) 2nd Batch Photo (150 μmol/m2/s) | 6.52 (Day 6) | 34.62 (Day 9) | 2.86 (average) | [68] |
| C. sorokiniana MB-1-M12 | Two-stage | 1 | 1st Batch Mixo (acetate and 150 μmol/m2/s) 2nd Batch Hetero (glucose) | 3.50 (Day 10) | 19.07 (Day 10) 17.5 (after day 7) | 1.3 (average) | [68] |
| C. sorokiniana MB-1-M12 | Two-stage | 1 | 1st Batch Hetero (glucose) 2nd Batch Mixo (acetate and 150 μmol/m2/s) | 6.17 (Day 10) | 33.64 (Day 10) | 3.42 (average) | [68] |
| C. sorokiniana MB-1-M12 | Fed-batch | 1 | Fed-batch Hetero (glucose) | 3.40 (Day 11) | 39.50 (Day 11) | 3.24 (average) | [69] |
| C. sorokiniana MB-1-M12 | Two-stage semi-continuous (3 cycles) | 1 | 1st Fed-batch Hetero (glucose and urea) After highest biomass accumulation, 75% medium replacement which is transferred to 2nd stage. 25% to new cycle 1st stage. 2nd Batch Mixo (acetate and 150 μmol/m2/s) | 6.77 (1ste cycle; day 11) 6.61 (2nd cycle, day 17) 6.53 (3th cycle, day 23) | 76.00 (1ste cycle; day 11) 80.88 (2nd cycle, day 17) 81.77 (3th cycle, day 23) | 1st stage (±6.17 average) 2nd stage (±2.86 average) | [69] |
| C. sorokiniana MB-1-M12 | Two-stage semi-continuous (3 cycles) | 5 | 1st Fed-batch Hetero (glucose and urea) After highest biomass accumulation, 75% medium replacement which is transferred to 2nd stage. 25% to new cycle 1st stage. 2nd Batch Mixo (acetate and 150 μmol/m2/s) | 8.19 (1ste cycle; day 14) 8.09 (2nd cycle, day 18) 8.71 (3th cycle, day 21) | 181.11 (1ste cycle; day 14) 153.60 (2nd cycle, day 18) 169.17 (3th cycle, day 21) | 1st stage (±20.02 average) 2nd stage (±5.55 average) | [69] |
| Chlorella protothecoides CS-41 | Two-stage | 30 | 1st Fed-batch Hetero (glucose and urea) After 10 days temperature shifted from 28 °C to 32 °C 2nd Batch (nutrient limited phase) | 5.35 (Day 14) 3.8 (after 10 days) | 209.08 (Day 14) 200 (after 10 days) | 19.18 (average) | [70] |
| Chlorella minutissima MCC-27 | Batch | 2 | Batch Photo (Constant 260 µmol/m s) | 6.37 (Day 5) | 22.1 (Day 5) | 4.32 (average) | [71] |
| Chlorella minutissima MCC-27 | Batch | 2 | Batch Photo (linear increase from 75 µmol/m s to 260 µmol/m s) | 8.24 (Day 5) | 26.75 (Day 5) | 5.35 (average) | [71] |
| Chlorella vulgaris | Fed-batch | 5 | Hetero (glucose and urea) | 5.32 (Day 5) | 252.75 (Day 5) | 67.4 (average) | [72] |
5.2. Cultivation Strategies for Other Microalgal Species
| Microalgae | Cultivation Mode | Reactor Volume (L) | Strategy * | Lutein Content (mg/g DW) | Lutein Production (mg/L) | Lutein Productivity (mg/L/day) | Reference |
|---|---|---|---|---|---|---|---|
| Scenedesmus almeriensis | Batch | 2 | Photo (1625 μE/m2/s) | 5.5 | / | 4.77 | [78] |
| Scenedesmus almeriensis | continuous mode (dilution rate 0.3 L/day) | 2 | Photo (1625 μE/m2/s) | 5.4 | / | 3.8 | [79] |
| Scenedesmus obliquus FSP-3 | Batch | 1 | Photo (white TL5 fluorescent 300 µmol/m2/s) | 4.80 (Day 5) | 20.5 (Day 5) | 4.08 (average) | [61] |
| Scenedesmus incrassatulus CLHE-Si01 | Two-stage | 6 | 1st Batch Hetero (glucose) After glucose was consumed 2nd Batch Photo (150 μmol/m2/s) | 1.49 (Day 7) | / | 3.10 (average) | [73] |
| Scenedesmus obliquus CWL-1 | Fed-batch | 7 | Mixo (glucose and 150 μmol/m2/s 12 h/12 h) | 2.55 (Day 9) | 27.3 (Day 9) | 4.96 (Day 5) | [74] |
| Chlamydomonas sp. JSC4 | Batch | 1 | Photo (625 μmol/m2/s) | 3.82 | / | 5.08 | [62] |
| Desmodesmus sp. F51 | Fed-batch | 1 | Photo (nitrate and 150 µmol/m2/s) | 5.05 (Day 6) | 16.5 (Day 6) | 3.56 (Day 6) | [75] |
| Desmodesmus sp. | Fed-batch | 1 | Photo (nitrate, succinic acid and salicylic acid) | 7.5 (Day 4) | 18.9 (Day 6) | 5.78 | [76] |
| Coccomyxa onubensis | Batch | Photo (100 mM NaCl) | 6.7 (Day 3) | 1.63 | [80] |
6. Future Directions and Challenges
6.1. Conclusion and Future Directions in Metabolic Engineering for Microalgal Lutein Production
6.2. Comparing the Optimal Cultivation Strategies for Lutein Production
6.3. Comparing Microalgae and Marigold for Lutein Production
6.3.1. Advantages of Microalgal Production for Lutein Compared to Marigold
6.3.2. Disadvantages of Microalgal Production for Lutein Compared to Marigold
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, L. Toward the ‘Golden’ Era: The Status in Uncovering the Regulatory Control of Carotenoid Accumulation in Plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef] [PubMed]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic Uses of Natural Astaxanthin: An Evidence-Based Review Focused on Human Clinical Trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xie, C.; Hill, D.R.A.; Barrow, C.J.; Dunshea, F.R.; Martin, G.J.O.; Suleria, H.A.R. Bioaccessibility, Bioavailability and Bioactivities of Carotenoids in Microalgae: A Review. Food Rev. Int. 2024, 40, 230–259. [Google Scholar] [CrossRef]
- Cao, K.; Cui, Y.; Sun, F.; Zhang, H.; Fan, J.; Ge, B.; Cao, Y.; Wang, X.; Zhu, X.; Wei, Z.; et al. Metabolic Engineering and Synthetic Biology Strategies for Producing High-Value Natural Pigments in Microalgae. Biotechnol. Adv. 2023, 68, 108236. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from Fungi and Microalgae: A Review on Their Recent Production, Extraction, and Developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review and Industry and Market Trend Analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Li, Y.; Yang, S.; Di, D.; Tu, Y.; Liu, J. Synthetic Biology in Microalgae towards Fucoxanthin Production for Pharmacy and Nutraceuticals. Biochem. Pharmacol. 2024, 220, 115958. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Microbial Platforms to Produce Commercially Vital Carotenoids at Industrial Scale: An Updated Review of Critical Issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an Alternate Biofactory for Carotenoid Production: A Review of Its Applications, Opportunities and Challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Elissen, H.J.; van der Wiede, R.Y. Productie van Zoutwateralgen Voor Toepassingen in Food (En Feed) Deelrapport II: Werkpakketten 1&2 van Project Foodgrade Productie van Zoutwateralgen: Deelrapport II: Batchexperimenten Met Zoute Reststromen En Verschillende Mariene Algensoorten; ACRRES: Lelystad, The Netherlands, 2018. [Google Scholar]
- Carvalho, J.C.M.; Matsudo, M.C.; Bezerra, R.P.; Ferreira-Camargo, L.S.; Sato, S. Microalgae Bioreactors. In Algal Biorefineries: Volume 1: Cultivation of Cells and Products; Bajpai, R., Prokop, A., Zappi, M., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 83–126. ISBN 978-94-007-7494-0. [Google Scholar]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the Microalgae Cultivation Technology and Harvesting Process for Biofuel Production: A Review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.; Lee, D. Heterotrophic Cultivation of Microalgae for Pigment Production: A Review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using Green Alga Haematococcus Pluvialis for Astaxanthin and Lipid Co-Production: Advances and Outlook. Bioresour. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef]
- Shi, T.Q.; Wang, L.R.; Zhang, Z.X.; Sun, X.M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus Pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An Economic Assessment of Astaxanthin Production by Large Scale Cultivation of Haematococcus Pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Monte, J.; Ribeiro, C.; Parreira, C.; Costa, L.; Brive, L.; Casal, S.; Brazinha, C.; Crespo, J.G. Biorefinery of Dunaliella Salina: Sustainable Recovery of Carotenoids, Polar Lipids and Glycerol. Bioresour. Technol. 2020, 297, 122509. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein Production from Biomass: Marigold Flowers versus Microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Kee, M.; Wei, J.; Shi, I.; Lai, B.; Chin, F.; Hoong, S.; Lim, S.; Ling, Y.; Loo, P. Abiotic Stress as a Dynamic Strategy for Enhancing High Value Phytochemicals in Microalgae: Critical Insights, Challenges and Future Prospects. Biotechnol. Adv. 2024, 70, 108280. [Google Scholar] [CrossRef] [PubMed]
- Zohra, F.; Medjekal, S. Microalgal Carotenoids: A Promising Alternative to Synthetic Dyes. Algal Res. 2022, 66, 102823. [Google Scholar] [CrossRef]
- Bleisch, R.; Freitag, L.; Ihadjadene, Y.; Sprenger, U.; Steingröwer, J.; Walther, T.; Krujatz, F. Strain Development in Microalgal Biotechnology—Random Mutagenesis Techniques. Life 2022, 12, 961. [Google Scholar] [CrossRef] [PubMed]
- Trovão, M.; Schüler, L.M.; Machado, A.; Bombo, G.; Navalho, S.; Barros, A.; Pereira, H.; Silva, J.; Freitas, F.; Varela, J. Random Mutagenesis as a Promising Tool for Microalgal Strain Improvement towards Industrial Production. Mar. Drugs 2022, 20, 440. [Google Scholar] [CrossRef] [PubMed]
- Dasan, Y.K.; Lam, M.K.; Chai, Y.H.; Lim, J.W.; Ho, Y.C.; Tan, I.S.; Lau, S.Y.; Show, P.L.; Lee, K.T. Unlocking the Potential of Microalgae Bio-Factories for Carbon Dioxide Mitigation: A Comprehensive Exploration of Recent Advances, Key Challenges, and Energy-Economic Insights. Bioresour. Technol. 2023, 380, 129094. [Google Scholar] [CrossRef] [PubMed]
- Fathy, W.A.; Techen, N.; Elsayed, K.N.M.; Essawy, E.A.; Tawfik, E.; Abdelhameed, M.S.; Hammouda, O.; Ross, S.A. Insights into Random Mutagenesis Techniques to Enhance Biomolecule Production in Microalgae: Implications for Economically Viable Bioprocesses. Int. Aquat. Res. 2023, 15, 85–102. [Google Scholar]
- Chen, J.-H.; Chen, C.-Y.; Chang, J.-S. Lutein Production with Wild-Type and Mutant Strains of Chlorella Sorokiniana MB-1 under Mixotrophic Growth. J. Taiwan Inst. Chem. Eng. 2017, 79, 66–73. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, J.; Lin, Y.; Huang, J.; Chen, F. Developing a Chromochloris Zofingiensis Mutant for Enhanced Production of Lutein under CO2 Aeration. Mar. Drugs 2022, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, J.; Zhu, C.; Sandmann, G. Bleaching Herbicide Norflurazon Inhibits Phytoene Desaturase by Competition with Cofactors. J. Agric. Food Chem. 2001, 49, 5270–5272. [Google Scholar] [CrossRef]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of Lutein Production in Chlorella Sorokiniana (Chorophyta) by Improvement of Culture Conditions and Random Mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein and Β-carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 31, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome Engineering Using the CRISPR-Cas9 System. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D. Genome Engineering with Zinc-Finger Nucleases. Genetics 2011, 188, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhao, H. Transcription Activator-like Effector Nucleases (TALENs): A Highly Efficient and Versatile Tool for Genome Editing. Biotechnol. Bioeng. 2013, 110, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.G.; Bjelic, J.; Malhotra, G.; Huang, C.; Alsaffar, S.H. Comparison of the Feasibility, Efficiency, and Safety of Genome Editing Technologies. Int. J. Mol. Sci. 2021, 22, 10355. [Google Scholar] [CrossRef]
- Boettcher, M.; McManus, M.T. Choosing the Right Tool for the Job: RNAi, TALEN, or CRISPR. Mol. Cell 2015, 58, 575–585. [Google Scholar] [CrossRef]
- Ghavami, S.; Pani, A. CRISPR Interference and Its Applications. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 123–140. [Google Scholar]
- Velmurugan, A.; Muthukaliannan, G.K. Genetic Manipulation for Carotenoid Production in Microalgae an Overview. Curr. Res. Biotechnol. 2022, 4, 221–228. [Google Scholar] [CrossRef]
- Sirohi, P.; Verma, H.; Singh, S.K.; Singh, V.K.; Pandey, J.; Khusharia, S.; Kumar, D.; Kaushalendra; Teotia, P.; Kumar, A. Microalgal Carotenoids: Therapeutic Application and Latest Approaches to Enhance the Production. Curr. Issues Mol. Biol. 2022, 44, 6257–6279. [Google Scholar] [CrossRef]
- Liang, M.-H.; Li, X.-Y. Involvment of Transcription Factors and Regulatory Proteins in the Regulation of Carotenoid Accumulation in Plants and Algae. J. Agric. Food Chem. 2023, 71, 18660–18673. [Google Scholar] [CrossRef]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse Biosynthetic Pathways and Protective Functions against Environmental Stress of Antioxidants in Microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef]
- Chayut, N.; Yuan, H.; Ohali, S.; Meir, A.; Sa’ar, U.; Tzuri, G.; Zheng, Y.; Mazourek, M.; Gepstein, S.; Zhou, X.; et al. Distinct Mechanisms of the ORANGE Protein in Controlling Carotenoid Flux. Plant Physiol. 2017, 173, 376–389. [Google Scholar] [CrossRef]
- Morikawa, T.; Uraguchi, Y.; Sawayama, S. Overexpression of DnaJ-Like Chaperone Enhances Carotenoid Synthesis in Chlamydomonas Reinhardtii. Appl. Biochem. Biotechnol. 2018, 184, 80–91. [Google Scholar] [CrossRef]
- Yazdani, M.; Croen, M.G.; Fish, T.L.; Thannhauser, T.W.; Ahner, B.A. Overexpression of Native ORANGE (OR) and OR Mutant Protein in Chlamydomonas Reinhardtii Enhances Carotenoid and ABA Accumulation and Increases Resistance to Abiotic Stress. Metab. Eng. 2021, 68, 94–105. [Google Scholar] [CrossRef]
- Velmurugan, A.; Muthukaliannan, G.K. Homologous and Heterologous Expression of Phytoene Synthase Gene in Marine Microalgae Dunaliella Salina and Its Potential as Aquaculture Feed. Aquac. Int. 2023, 31, 3125–3144. [Google Scholar] [CrossRef]
- Tokunaga, S.; Morimoto, D.; Koyama, T.; Sawayama, S. Enhanced Lutein Production in Chlamydomonas Reinhardtii by Overexpression of the Lycopene Epsilon Cyclase Gene. Appl. Biochem. Biotechnol. 2021, 193, 1967–1978. [Google Scholar] [CrossRef]
- Lou, S.; Lin, X.; Liu, C.; Anwar, M.; Li, H.; Hu, Z. Molecular Cloning and Functional Characterization of CvLCYE, a Key Enzyme in Lutein Synthesis Pathway in Chlorella Vulgaris. Algal Res. 2021, 55, 102246. [Google Scholar] [CrossRef]
- Kumari, S.; Vira, C.; Lali, A.M.; Prakash, G. Heterologous Expression of a Mutant Orange Gene from Brassica Oleracea Increases Carotenoids and Induces Phenotypic Changes in the Microalga Chlamydomonas Reinhardtii. Algal Res. 2020, 47, 101871. [Google Scholar]
- Lin, J.Y.; Effendi, S.S.W.; Ng, I.S. Enhanced Carbon Capture and Utilization (CCU) Using Heterologous Carbonic Anhydrase in Chlamydomonas Reinhardtii for Lutein and Lipid Production. Bioresour. Technol. 2022, 351, 127009. [Google Scholar] [CrossRef]
- Steinbrenner, J.; Sandmann, G. Transformation of the Green Alga Haematococcus Pluvialis with a Phytoene Desaturase for Accelerated Astaxanthin Biosynthesis. Appl. Environ. Microbiol. 2006, 72, 7477–7484. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Huang, J.; Jiang, Y.; Chen, F. Genetic Engineering of the Green Alga Chlorella Zofingiensis: A Modified Norflurazon-Resistant Phytoene Desaturase Gene as a Dominant Selectable Marker. Appl. Microbiol. Biotechnol. 2014, 98, 5069–5079. [Google Scholar] [CrossRef]
- Song, I.; Kim, J.; Baek, K.; Choi, Y.; Shin, B.; Jin, E. The Generation of Metabolic Changes for the Production of High-Purity Zeaxanthin Mediated by CRISPR-Cas9 in Chlamydomonas Reinhardtii. Microb. Cell Fact. 2020, 19, 220. [Google Scholar] [CrossRef]
- Kneip, J.S.; Kniepkamp, N.; Jang, J.; Mortaro, M.G.; Jin, E.; Kruse, O.; Baier, T. CRISPR/Cas9-Mediated Knockout of the Lycopene ε-Cyclase for Efficient Astaxanthin Production in the Green Microalga Chlamydomonas Reinhardtii. Plants 2024, 13, 1393. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein Production from Microalgae: A Review. Bioresour. Technol. 2023, 376, 128875. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.; Chang, J. Recent Advances in Lutein Production from Microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Shi, X.-M.; Zhang, X.-W.; Chen, F. Heterotrophic Production of Biomass and Lutein by Chlorella Protothecoides on Various Nitrogen Sources. Enzyme Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Ma, R.; Liu, X.; Miao, M.; Ho, S.-H.; Chen, J.; Leong, Y.K.; Chang, J.-S. High-Cell-Density Heterotrophic Cultivation of Microalga Chlorella Sorokiniana FZU60 for Achieving Ultra-High Lutein Production Efficiency. Bioresour. Technol. 2022, 365, 128130. [Google Scholar] [CrossRef]
- Qu, L.; Ren, L.-J.; Huang, H. Scale-up of Docosahexaenoic Acid Production in Fed-Batch Fermentation by Schizochytrium Sp. Based on Volumetric Oxygen-Transfer Coefficient. Biochem. Eng. J. 2013, 77, 82–87. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing Lutein Productivity of an Indigenous Microalga Scenedesmus Obliquus FSP-3 Using Light-Related Strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.-H.; Chen, J. Enhancing Lutein Productivity of Chlamydomonas Sp. via High-Intensity Light Exposure with Corresponding Carotenogenic Genes Expression Profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Vigara, J.; Funk, C. Exploring Nordic Microalgae as a Potential Novel Source of Antioxidant and Bioactive Compounds. New Biotechnol. 2023, 73, 1–8. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Ho, S.-H.; Ma, R.; Shi, X.; Liu, L.; Chen, J. Pilot-Scale Cultivation of Chlorella Sorokiniana FZU60 with a Mixotrophy/Photoautotrophy Two-Stage Strategy for Efficient Lutein Production. Bioresour. Technol. 2020, 314, 123767. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Ma, R.; Ho, S.-H.; Shi, X.; Liu, L.; Chen, J. Bioprocess Operation Strategies with Mixotrophy/Photoinduction to Enhance Lutein Production of Microalga Chlorella Sorokiniana FZU60. Bioresour. Technol. 2019, 290, 121798. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, Z.; Ho, S.-H.; Ruan, C.; Li, J.; Xie, Y.; Shi, X.; Liu, L.; Chen, J. Two-Stage Bioprocess for Hyper-Production of Lutein from Microalga Chlorella Sorokiniana FZU60: Effects of Temperature, Light Intensity, and Operation Strategies. Algal Res. 2020, 52, 102119. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, C.-H.; Ng, I.-S.; Chang, J.-S. Enhancing Lutein Production with Mixotrophic Cultivation of Chlorella Sorokiniana MB-1-M12 Using Different Bioprocess Operation Strategies. Bioresour. Technol. 2019, 278, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Kato, Y.; Matsuda, M.; Chen, C.-Y.; Nagarajan, D.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Lutein Production with Chlorella Sorokiniana MB-1-M12 Using Novel Two-Stage Cultivation Strategies—Metabolic Analysis and Process Improvement. Bioresour. Technol. 2021, 334, 125200. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Nagarajan, D.; Huang, Y.; Zhu, X.; Liao, Q.; Chang, J.-S. A Novel and Effective Two-Stage Cultivation Strategy for Enhanced Lutein Production with Chlorella Sorokiniana. Biochem. Eng. J. 2022, 188, 108688. [Google Scholar] [CrossRef]
- Shi, X.-M.; Chen, F. High-Yield Production of Lutein by the Green Microalga Chlorella Protothecoidesin Heterotrophic Fed-Batch Culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, G.; Dash, S.K.; Sen, R. Development of an Optimal Light-Feeding Strategy Coupled with Semi-Continuous Reactor Operation for Simultaneous Improvement of Microalgal Photosynthetic Efficiency, Lutein Production and CO2 Sequestration. Biochem. Eng. J. 2016, 113, 47–56. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Kwon, J.-S.; Kang, S.T.; Kim, B.-R.; Jung, Y.; Han, J.G.; Park, J.H.; Hwang, J.K. Optimization of Culture Media for Large-Scale Lutein Production by Heterotrophic Chlorella Vulgaris. Biotechnol. Prog. 2014, 30, 736–743. [Google Scholar] [CrossRef]
- Flórez-Miranda, L.; Cañizares-Villanueva, R.O.; Melchy-Antonio, O.; Martínez-Jerónimo, F.; Ortíz, C.M.F. Two Stage Heterotrophy/Photoinduction Culture of Scenedesmus Incrassatulus: Potential for Lutein Production. J. Biotechnol. 2017, 262, 67–74. [Google Scholar] [CrossRef]
- Chen, W.-C.; Hsu, Y.-C.; Chang, J.-S.; Ho, S.-H.; Wang, L.-F.; Wei, Y.-H. Enhancing Production of Lutein by a Mixotrophic Cultivation System Using Microalga Scenedesmus Obliquus CWL-1. Bioresour. Technol. 2019, 291, 121891. [Google Scholar] [CrossRef]
- Xie, Y.; Ho, S.-H.; Chen, C.-N.N.; Chen, C.-Y.; Ng, I.-S.; Jing, K.-J.; Chang, J.-S.; Lu, Y. Phototrophic Cultivation of a Thermo-Tolerant Desmodesmus Sp. for Lutein Production: Effects of Nitrate Concentration, Light Intensity and Fed-Batch Operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef]
- Ahmed, N.R.; Manirafasha, E.; Pan, X.; Chen, B.-Y.; Lu, Y.; Jing, K. Exploring Biostimulation of Plant Hormones and Nitrate Supplement to Effectively Enhance Biomass Growth and Lutein Production with Thermo-Tolerant Desmodesmus Sp. F51. Bioresour. Technol. 2019, 291, 121883. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Ho, S.-H.; Shi, X.; Liu, L.; Xie, Y.; Chen, J.; Lu, Y. Co-Production of Lutein and Fatty Acid in Microalga Chlamydomonas Sp. JSC4 in Response to Different Temperatures with Gene Expression Profiles. Algal Res. 2020, 47, 101821. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández-Sevilla, J.M.; Acién, F.G.; Cerón, M.C.; Pérez-Parra, J.; Molina-Grima, E. Biomass and Lutein Productivity of Scenedesmus Almeriensis: Influence of Irradiance, Dilution Rate and Temperature. Appl. Microbiol. Biotechnol. 2008, 79, 719–729. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of Culture Conditions on the Productivity and Lutein Content of the New Strain Scenedesmus Almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Bermejo, E.; Ruiz-Domínguez, M.C.; Cuaresma, M.; Vaquero, I.; Ramos-Merchante, A.; Vega, J.M.; Vílchez, C.; Garbayo, I. Production of Lutein, and Polyunsaturated Fatty Acids by the Acidophilic Eukaryotic Microalga Coccomyxa Onubensis under Abiotic Stress by Salt or Ultraviolet Light. J. Biosci. Bioeng. 2018, 125, 669–675. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M. Life Cycle Assessment of the Production of the Red Antioxidant Carotenoid Astaxanthin by Microalgae: From Lab to Pilot Scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.-M.; Sun, Z.; Liu, S.-F.; Qin, Z.-H.; Mou, J.-H.; Zhou, Z.-G.; Lin, C.S.K. Sustainable Lipid and Lutein Production from Chlorella Mixotrophic Fermentation by Food Waste Hydrolysate. J. Hazard. Mater. 2020, 400, 123258. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.E.S.; Martini, M.; Altomonte, I.; Salari, F.; Nardoni, S.; Sorce, C.; da Silva, F.L.H.; Andreucci, A. Production of Chlorella Protothecoides Biomass, Chlorophyll and Carotenoids Using the Dairy Industry by-Product Scotta as a Substrate. Biocatal. Agric. Biotechnol. 2017, 11, 207–213. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Zhong, Y.; Gerken, H.; Huang, J.; Chen, F. Utilization of Cane Molasses towards Cost-Saving Astaxanthin Production by a Chlorella Zofingiensis Mutant. J. Appl. Phycol. 2013, 25, 1447–1456. [Google Scholar] [CrossRef]
- Tran, D.; Doan, N.; Louime, C.; Giordano, M.; Portilla, S. Growth, Antioxidant Capacity and Total Carotene of Dunaliella Salina DCCBC15 in a Low Cost Enriched Natural Seawater Medium. World J. Microbiol. Biotechnol. 2014, 30, 317–322. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-Conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Gille, A.; Trautmann, A.; Posten, C.; Briviba, K. Bioaccessibility of Carotenoids from Chlorella Vulgaris and Chlamydomonas Reinhardtii. Int. J. Food Sci. Nutr. 2016, 67, 507–513. [Google Scholar] [CrossRef]
- Coleman, B.; Van Poucke, C.; Dewitte, B.; Ruttens, A.; Moerdijk-Poortvliet, T.; Latsos, C.; De Reu, K.; Blommaert, L.; Duquenne, B.; Timmermans, K.; et al. The Potential of Microalgae as Flavoring Agent for Plant-Based Seafood Alternatives. Futur. Foods 2022, 5, 100139. [Google Scholar] [CrossRef]

| Carotenoid | Health Benefits | Natural Sources | Recommended Dose |
|---|---|---|---|
| Astaxanthin | Strong anti-oxidant property | Shrimp; Salmon; Crabs; Microalgae (Haematococcus pluvialis) Phaffia rhodozyma | 4–12 mg/day |
| Anti-inflammatory effects | |||
| Anti-cancer | |||
| Cardiovascular health | |||
| β-Carotene | Prevent night blindness | Pumpkin; Mango; Carrots; Microalgae (Dunaliella salina) | 600 µg RE */day |
| Anti-oxidant property | |||
| Prevents liver fibrosis | |||
| Lutein | Prevents cataract and age-related | Marigold flower; Yolk; Broccoli; Microalgae; Orange-yellow fruits; Leafy green vegetables | 6 mg/day |
| macular degeneration | |||
| Anti-oxidant property | |||
| Anti-cancer | |||
| Prevents cardiovascular diseases | |||
| Zeaxanthin | Anti-cancer | Marigold flower; Maize; Orange peppers; Microalgae; Scallions | 2 mg/day |
| Anti-inflammatory | |||
| Anti-allergy | |||
| Against UV, skin redness | |||
| Fucoxanthin | Anti-obesity | Macroalgae; Microalgae | / |
| Anti-oxidant property |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coleman, B.; Vereecke, E.; Van Laere, K.; Novoveska, L.; Robbens, J. Genetic Engineering and Innovative Cultivation Strategies for Enhancing the Lutein Production in Microalgae. Mar. Drugs 2024, 22, 329. https://doi.org/10.3390/md22080329
Coleman B, Vereecke E, Van Laere K, Novoveska L, Robbens J. Genetic Engineering and Innovative Cultivation Strategies for Enhancing the Lutein Production in Microalgae. Marine Drugs. 2024; 22(8):329. https://doi.org/10.3390/md22080329
Chicago/Turabian StyleColeman, Bert, Elke Vereecke, Katrijn Van Laere, Lucie Novoveska, and Johan Robbens. 2024. "Genetic Engineering and Innovative Cultivation Strategies for Enhancing the Lutein Production in Microalgae" Marine Drugs 22, no. 8: 329. https://doi.org/10.3390/md22080329
APA StyleColeman, B., Vereecke, E., Van Laere, K., Novoveska, L., & Robbens, J. (2024). Genetic Engineering and Innovative Cultivation Strategies for Enhancing the Lutein Production in Microalgae. Marine Drugs, 22(8), 329. https://doi.org/10.3390/md22080329







