New Secondary Metabolites of Mangrove-Associated Strains
Abstract
:1. Introduction
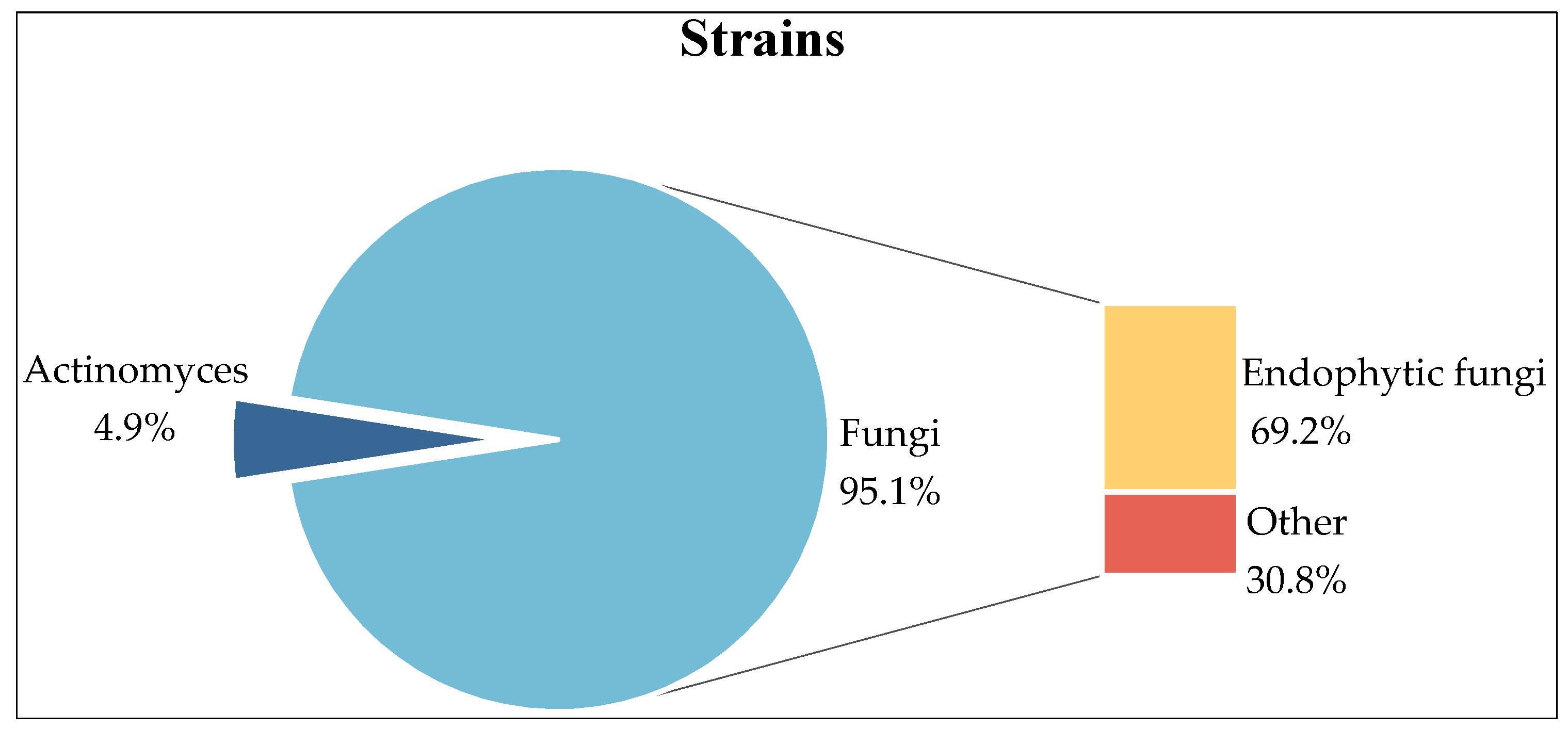
2. Strains
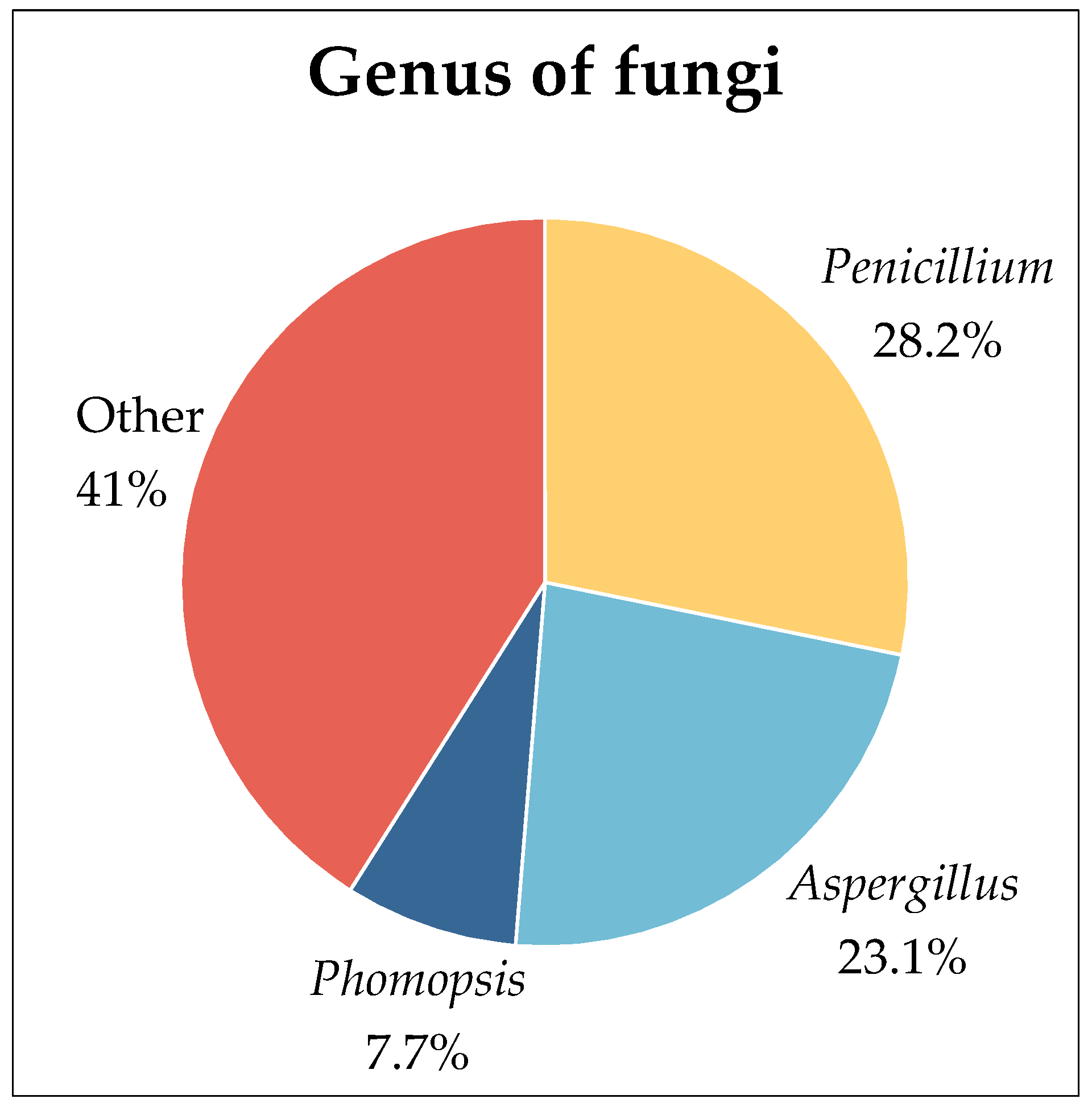
2.1. Fungi
2.2. Actinomycetes
2.3. Endophytic Fungi
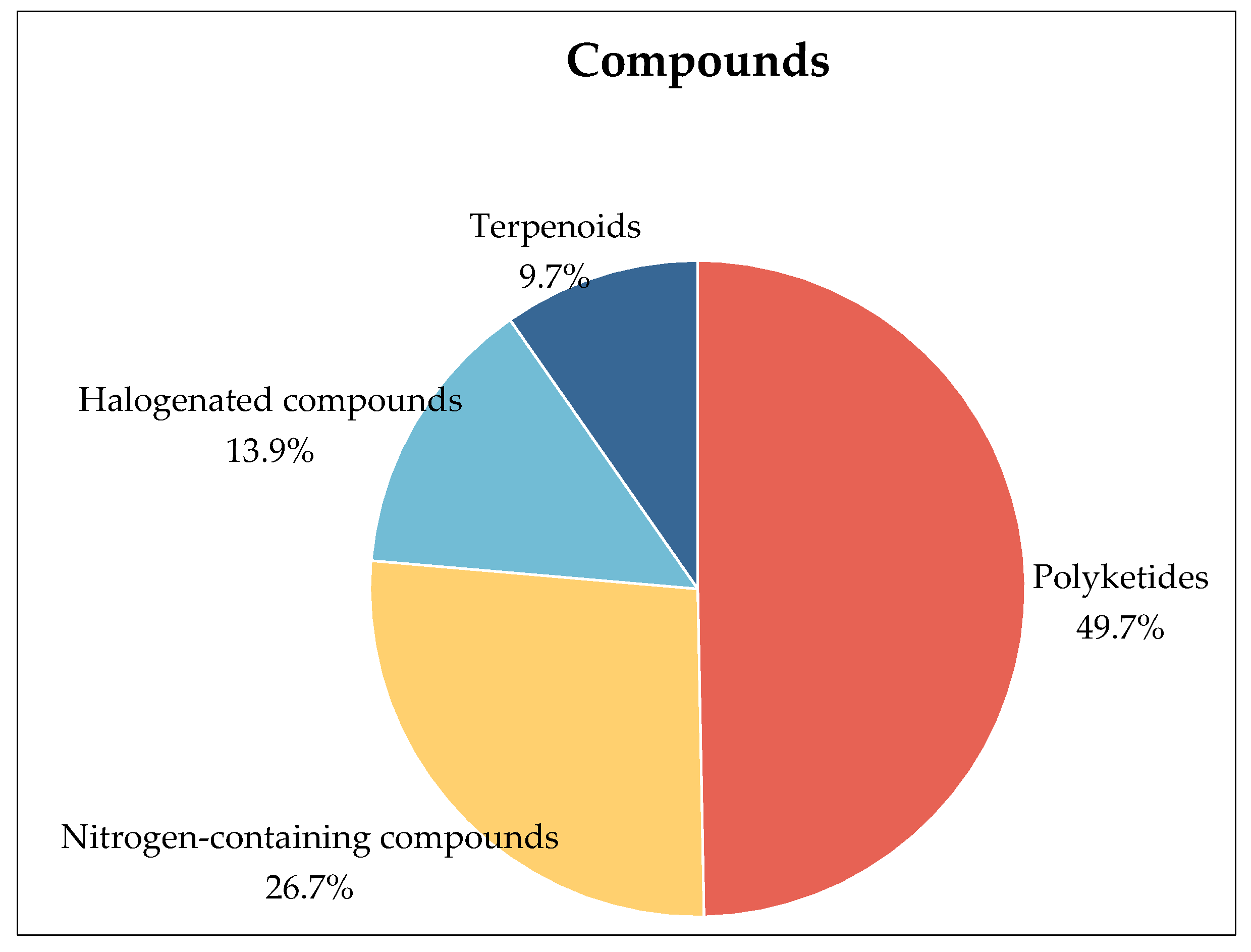
3. Compounds
3.1. Polyketides
3.2. Nitrogen-Containing Compounds
3.3. Terpenoids
3.4. Halogenated Compounds
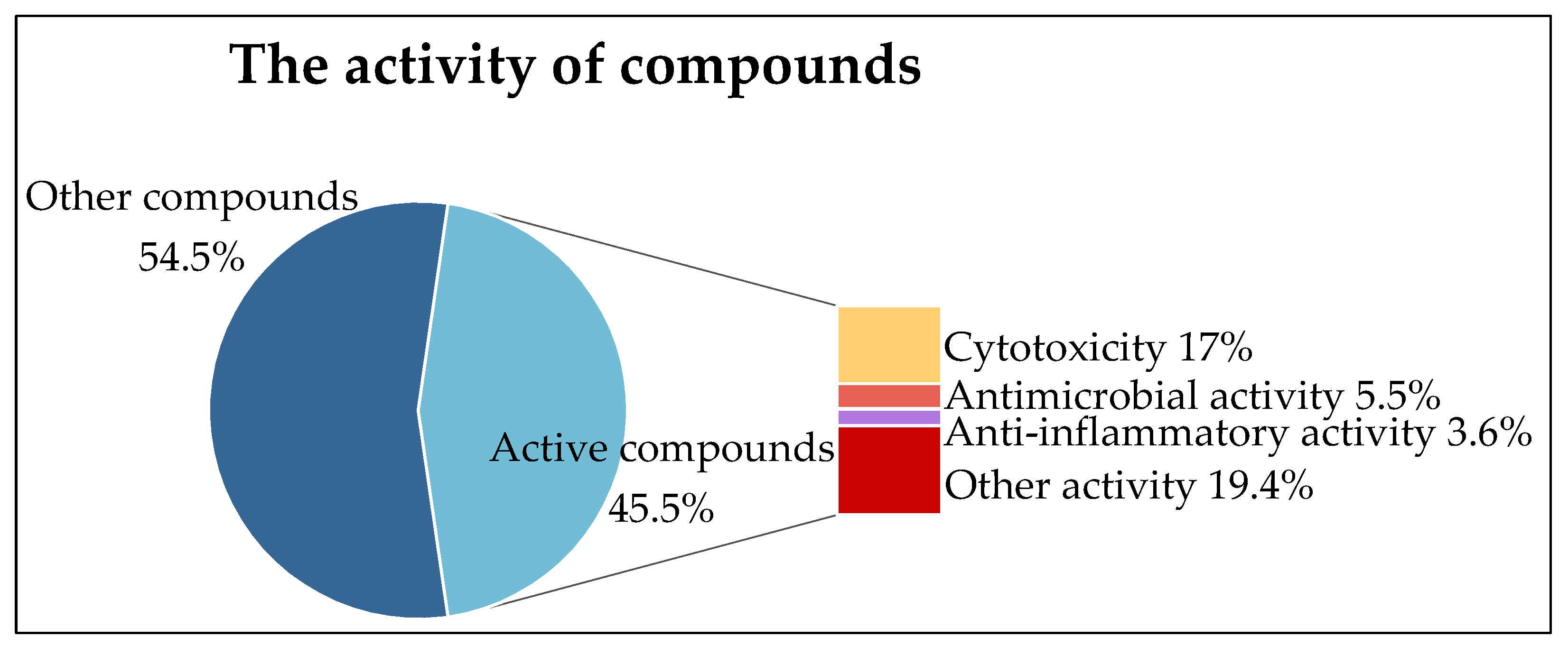
4. Bioactivity
4.1. Cytotoxicity
4.2. Antimicrobial Activity
4.3. Anti-Inflammatory Activity
4.4. Antioxidant Activity
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann. Microbiol. 2013, 63, 1–19. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef]
- Hastilestari, B.R.; Syahidah, D.; Riyanti, E.I.; Alfiansah, Y.R. Below tropical coastal land ecosystems: Composition and functional diversity of microbial community across mangroves and paddy field. In Microbiome Drivers of Ecosystem Function; Academic Press: Cambridge, MA, USA, 2024; pp. 281–309. [Google Scholar]
- Pietra, F. Secondary metabolites from marine microorganisms: Bacteria, protozoa, algae and fungi. Achievements and prospects. Nat. Prod. Rep. 1997, 14, 453–464. [Google Scholar] [CrossRef]
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Yue, J.M. Frontier studies on natural products: Moving toward paradigm shifts. Sci. China Chem. 2023, 66, 928–942. [Google Scholar] [CrossRef]
- Hong, K.; Gao, A.-H.; Xie, Q.-Y.; Gao, H.G.; Zhuang, L.; Lin, H.-P.; Yu, H.-P.; Li, J.; Yao, X.-S.; Goodfellow, M.; et al. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef]
- Dias, A.C.F.; Andreote, F.D.; Dini-Andreote, F.; Lacava, P.T.; Sá, A.L.B.; Melo, I.S.; Azevedo, J.L.; Araújo, W.L. Diversity and biotechnological potential of culturable bacteria from Brazilian mangrove sediment. World J. Microbiol. Biotechnol. 2009, 25, 1305–1311. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munroa, M.H.; Prinsepd, M.R. Natural product reports. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef]
- Braga, A.F.V.; Rosário, M.S.D.; Gomes, J.B.N.; Monteiro, C.D.A.; Farias, F.A.; Rodrigues Filho, E.; Cantanhede Filho, A.J. Antimicrobial Potential of Soil/Sediment Mangrove Associated Fungi: A Review. J. Braz. Chem. Soc. 2024, 35, e-20240032. [Google Scholar] [CrossRef]
- Wu, M.J.; Xu, B.; Guo, Y.W. Unusual secondary metabolites from the mangrove ecosystems: Structures, bioactivities, chemical, and bio-syntheses. Mar. Drugs 2022, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.-F.; Law, L.N.-S.; Letchumanan, V.; Tan, L.T.-H.; Wong, S.H.; Chan, K.-G.; Ab Mutalib, N.-S.; Lee, L.-H. Anticancer drug discovery from microbial sources: The unique mangrove streptomycetes. Molecules 2020, 25, 5365. [Google Scholar] [CrossRef] [PubMed]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanová, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Assami Doi, S.; Bartelochi Pinto, A.; Carolina Canali, M.; Raquel Polezel, D.; Merguizo Chinellato, R.A.; de Oliveira, A.J.F.C. Density and diversity of filamentous fungi in the water and sediment of Araçá bay in São Sebastião, São Paulo, Brazil. Biota Neotrop. (Ed. Em Iingles) 2018, 18, e20170416. [Google Scholar]
- Thatoi, H.; Behera, B.C.; Mishra, R.R. Ecological role and biotechnological potential of mangrove fungi: A review. Mycology 2013, 4, 54–71. [Google Scholar] [CrossRef]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.L.; Ali, L.; Rizvi, T.S.; Khan, S.A.; Hussain, J.; Hamayun, M.; Al-Harrasi, A. Enzyme inhibitory metabolites from endophytic Penicillium citrinum isolated from Boswellia sacra. Arch. Microbiol. 2017, 199, 691–700. [Google Scholar] [CrossRef]
- Chávez, R.; Fierro, F.; García-Rico, R.O.; Vaca, I. Filamentous fungi from extreme environments as a promising source of novel bioactive secondary metabolites. Front. Microbiol. 2015, 6, 903. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, G.P.; Brooks, P.R.; Kurtböke, D.I. The occurrence of bioactive micromonosporae in aquatic habitats of the Sunshine Coast in Australia. Mar. Drugs 2008, 6, 243–261. [Google Scholar] [CrossRef]
- Huang, H.; Lv, J.; Hu, Y.; Fang, Z.; Zhang, K.; Bao, S. Micromonospora rifamycinica sp. nov., a novel actinomycete from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2008, 58, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.T.; Liang, Z.Y.; Wen, Z.C.; Guo, Z.K.; Xu, J. Research Progress on Bioactive Products from Mangrove-Derived Endophytic Fungi. J. Hainan Norm. Univ. (Nat. Sci. Ed.) 2022, 39, 373–385. (In Chinese) [Google Scholar] [CrossRef]
- Heinig, U.; Scholz, S.; Jennewein, S. Getting to the bottom of Taxol biosynthesis by fungi. Fungal Divers. 2013, 60, 161–170. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Kim, S.; Yu, N.H.; Park, A.R.; Yoon, H.; Bae, C.; Yeo, J.H.; Kim, I.S.; Kim, J. Antimicrobial activities of an oxygenated cyclohexanone derivative isolated from Amphirosellinia nigrospora JS-1675 against various plant pathogenic bacteria and fungi. J. Appl. Microbiol. 2019, 126, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, B.; Chen, D.; Deng, X.; Yuan, J.; Zhang, S.; Xiong, Z.; Xu, J. New Isocoumarin and Pyrone Derivatives from the Chinese Mangrove Plant Rhizophora mangle-Associated Fungus Phomopsis sp. DHS-11. Molecules 2023, 28, 3756. [Google Scholar] [CrossRef]
- Chen, B.T.; Wu, W.C.; Zhou, D.D.; Deng, X.L.; Zhang, S.Q.; Yuan, J.Z.; Xu, J.; Guo, Z. Bioactive components of endophytic fungi from two Hainan mangrove plants. J. Shenzhen Univ. Sci. Eng. 2022, 39, 245–252. (In Chinese) [Google Scholar] [CrossRef]
- Zou, G.; Li, T.; Yang, W.; Sun, B.; Chen, Y.; Wang, B.; Ou, Y.; Yu, H.; She, Z. Antioxidative indenone and benzophenone derivatives from the mangrove-derived fungus Cytospora heveae NSHSJ-2. Mar. Drugs 2023, 21, 181. [Google Scholar] [CrossRef]
- Li, K.L.; Dai, Y.; She, J.L.; Zeng, Y.B.; Dai, H.F.; Ou, S.L.; Zhou, X.F.; Liu, Y.H. Bisabolanoic acid A, a new polychiral sesquiterpene with AChE inhibitory activity from a mangrove-derived fungus Colletotrichum sp. J. Asian Nat. Prod. Res. 2022, 24, 88–95. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, X.; Wu, J.; Wei, C.; Feng, T.; Zhou, D.; Wen, Z.; Xu, J. Immunosuppressive cytochalasins from the mangrove endophytic fungus Phomopsis asparagi DHS-48. Mar. Drugs 2022, 20, 526. [Google Scholar] [CrossRef]
- Feng, T.; Wei, C.; Deng, X.; Chen, D.; Wen, Z.; Xu, J. Epigenetic manipulation induced production of immunosuppressive chromones and cytochalasins from the mangrove endophytic fungus Phomopsis asparagi DHS-48. Mar. Drugs 2022, 20, 616. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, D.Y.; Xu, J. Two new polyketides from endophytic fungus Pestalotiopsis sp. HQD-6 isolated from the Chinese mangrove plant Rhizophora mucronata. J. Asian Nat. Prod. Res. 2022, 24, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhu, X.C.; Zeng, W.N.; Wang, B.; Luo, Y.P.; Liu, J.; Chen, M.-J.; Li, G.-Y.; Huang, G.-L.; Chen, G.-Y.; et al. Talaromarins A–F: Six new isocoumarins from mangrove-derived fungus Talaromyces flavus TGGP35. Mar. Drugs 2022, 20, 361. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, X.; Wang, B.; Zhang, X.; Luo, M.; Huang, L.; Wang, R.; Chen, Y.; Li, X.; Luo, Y.; et al. Bioactive polyketides and meroterpenoids from the mangrove-derived fungus Talaromyces flavus TGGP35. Front. Microbiol. 2024, 15, 1342843. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.; Yuan, Y.; Zou, G.; Yang, W.; Tan, Q.; Kang, W.; She, Z. Metabolites with cytotoxic activities from the mangrove endophytic fungus Fusarium sp. 2ST2. Front. Chem. 2022, 10, 842405. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Wei, C.; Zhang, X.; Zhou, D.; Xu, J. Alkaloids from endophytic fungus Aspergillus fumigatus HQD24 isolated from the Chinese mangrove plant Rhizophora mucronata. Nat. Prod. Res. 2022, 36, 5069–5073. [Google Scholar] [CrossRef]
- Huang, D.Y.; Nong, X.H.; Zhang, Y.Q.; Xu, W.; Sun, L.Y.; Zhang, T.; Chen, G.-Y.; Han, C.-R. Two new 2, 5-diketopiperazine derivatives from mangrove-derived endophytic fungus Nigrospora camelliae-sinensis S30. Nat. Prod. Res. 2022, 36, 3651–3656. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wu, L.; Liu, R.; Li, J.; Liu, L.; Chen, C.; Li, J.; Zhang, K.; Liao, J.; Long, Y. Penifuranone A: A Novel Alkaloid from the Mangrove Endophytic Fungus Penicillium crustosum SCNU-F0006. Int. J. Mol. Sci. 2024, 25, 5032. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, W.; Chen, T.; Tan, Q.; Zou, G.; Zang, Z.; Li, J.; Wang, B.; She, Z. Cytosporones W and X: Two mutually converting epimers from a mangrove endophytic fungus Diaporthe sp. ZJHJYZ-1. ACS Omega 2023, 8, 26628–26634. [Google Scholar] [CrossRef]
- Jiang, H.; Cai, R.; Zang, Z.; Yang, W.; Wang, B.; Zhu, G.; Yuan, J.; She, Z. Azaphilone derivatives with anti-inflammatory activity from the mangrove endophytic fungus Penicillium sclerotiorum ZJHJJ-18. Bioorg. Chem. 2022, 122, 105721. [Google Scholar] [CrossRef]
- Long, K.Y.; Dai, D.C.; Zheng, C.J.; Wang, Y.T.; Song, X.M.; Chen, X.; Zhou, X.-M.; Chen, G.-Y. Three new long-chain polyenes from the mangrove-derived fungus Penicillium herquei JX4. J. Asian Nat. Prod. Res. 2023, 25, 422–428. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Zou, G.; Wang, G.; Kang, W.; Yuan, J.; She, Z. Cytotoxic bromine-and iodine-containing cytochalasins produced by the mangrove endophytic fungus Phomopsis sp. QYM-13 using the OSMAC approach. J. Nat. Prod. 2022, 85, 1229–1238. [Google Scholar] [CrossRef]
- Wang, G.; Yin, Z.; Wang, S.; Yuan, Y.; Chen, Y.; Kang, W. Diversified polyketides with anti-inflammatory activities from mangrove endophytic fungus Daldinia eschscholtzii KBJYZ-1. Front. Microbiol. 2022, 13, 900227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, Z.; Zhao, F.; Zheng, J.; Zheng, X.; Zhang, K.; Huang, H. Two new pyrone derivatives from the mangrove-derived endophytic fungus Aspergillus sydowii# 2B. Nat. Prod. Res. 2022, 36, 3872–3878. [Google Scholar]
- Guo, S.; Zhou, H.; Huang, X.; Peng, S.; Li, J.; Ding, B.; Tao, Y.; Huang, H. New glucosidated indole-quinazoline alkaloids from mangrove endophytic fungus Aspergillus fumigatus SAl12. Nat. Prod. Res. 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hao, L.; Qin, X.; Huang, J.; Yang, R.; Li, J.; Huang, X. A new lactone from mangrove endophytic fungus Aspergillus sp. GXNU-A9. Nat. Prod. Res. 2023, 37, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Luo, L.; Liu, Y.C.; Bo, X.L.; Wu, F.R.; Wang, F.F.; Tan, M.J.; Wei, Y.Q.; Dou, X.B.; Wang, C.Y.; et al. Diisoprenyl-cyclohexene-type meroterpenoids from a mangrove endophytic fungus Aspergillus sp. GXNU-Y65 and their anti-nonalcoholic steatohepatitis activity in AML12 cells. Phytochemistry 2024, 218, 113955. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.E.; Huang, X.S.; Wei, W.X.; Lu, W.L.; Tan, Z.; Liu, Y.H. Anti-inflammatory secondary metabolites from a Beihai mangrove endophytic fungus Aspergillus sp. GXIMD00016. Acta Sci. Nat. Univ. Sunyatseni/Zhongshan Daxue Xuebao 2022, 61, 69–73. (In Chinese) [Google Scholar] [CrossRef]
- Bo, X.; Zhao, Y.; Qin, F.; Wu, F.; Tan, M.; Ju, S.; Song, Z.; Li, W.; He, F.; Wei, Y.; et al. Cytotoxic metabolites from the mangrove endophytic fungus Aspergillus sp. GXNU QG1a. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Tan, M.; Xu, X.; Zhang, W.; Wu, F.; Bo, X.; Qin, F.; Ju, S.; Song, Z.; Yang, T.; Li, J.; et al. Isolation and insecticidal activities of new cyclic peptides from mangrove endophytic fungus Aspergillus sp. GXNU-4QQY1a. Fitoterapia 2023, 171, 105693. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, G.; She, Z.; Chen, Y.; Kang, W. Metabolites isolated from the mangrove endophytic fungus Didymella sp. CYSK-4 and their cytotoxic activities. Fitoterapia 2023, 171, 105692. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, W.; Chen, T.; Ouyang, H.; Liu, Y.; Wang, B.; Yu, H.; She, Z. Five secondary metabolites from mangrove endophytic fungus Fusarium. proliferatum NSD-1. J. Mol. Struct. 2024, 1302, 137434. [Google Scholar] [CrossRef]
- Wang, B.; Nong, X.H.; Zeng, W.N.; Li, S.S.; Li, G.Y.; Liu, J.; Huang, G.L.; Zheng, C.J. Study on bioactive secondary metabolites from the mangrove-derived fungus Penicillium verruculosum TGM14. Chem. Nat. Compd. 2022, 58, 812–815. [Google Scholar] [CrossRef]
- Gan, Y.M.; Xia, J.L.; Zhao, L.Y.; Liu, K.; Tang, Z.Z.; Huang, B.Y.; Liu, Y.-H.; Gao, C.-H.; Bai, M. Two new isocoumarins isolated from a mangrove-derived Penicillium sp. Phytochem. Lett. 2022, 50, 21–24. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Ye, Y.; Xu, Z.; Zhao, Q.; Luo, X.; Liu, Y.; Guo, P. Anti-inflammatory compounds from the mangrove endophytic fungus Amorosia sp. SCSIO 41026. Front. Microbiol. 2022, 13, 976399. [Google Scholar] [CrossRef]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef]
- Peng, B.; Cai, J.; Xiao, Z.; Liu, M.; Li, X.; Yang, B.; Fang, W.; Huang, Y.-Y.; Chen, C.; Zhou, X.; et al. Bioactive Polyketides and Benzene Derivatives from Two Mangrove Sediment-Derived Fungi in the Beibu Gulf. Mar. Drugs 2023, 21, 327. [Google Scholar] [CrossRef]
- Wu, M.D.; Chen, J.J.; Cheng, M.J. Secondary metabolites with antifungal activities from mangrove derived fungus Monascus purpureus WMD2424. Mar. Drugs 2023, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, M.; Qiu, Y.; Liu, X.; Wang, C.; Chen, J.; Zhang, H.; Wei, B.; Yu, Y.; Ying, Y.; et al. α-Glucosidase inhibitors from two mangrove-derived actinomycetes. Molecules 2023, 28, 3822. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Zhang, J.; Niu, S.; Liu, L. New prenylated indole diketopiperazine alkaloids and polyketides from the mangrove-derived fungus Penicillium sp. Front. Mar. Sci. 2022, 9, 1097594. [Google Scholar] [CrossRef]
- Cai, J.; Wang, X.; Yang, Z.; Tan, Y.; Peng, B.; Liu, Y.; Zhou, X. Thiodiketopiperazines and alkane derivatives produced by the mangrove sediment–derived fungus Penicillium ludwigii SCSIO 41408. Front. Microbiol. 2022, 13, 857041. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, M.; Wang, X.B.; Li, Y.Q.; Ding, J.L.; Lan, M.X.; Gao, X.; Zhao, D.L.; Zhang, C.S.; Wu, G.X. Phytotoxic azaphilones from the mangrove-derived fungus Penicillium sclerotiorum HY5. Front. Microbiol. 2022, 13, 880874. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Ying, C.H.E.N.; Yanchun, H.E.; Bin, Y.A.N.G. Secondary metabolites from the mangrove soil derived fungus Xylariaceae sp. SCSIO41212. J. Holist. Integr. Pharm. 2022, 3, 230–235. [Google Scholar] [CrossRef]
- Newaz, A.W.; Yong, K.; Yi, W.; Wu, B.; Zhang, Z. Antimicrobial metabolites from the Indonesian mangrove sediment-derived fungus Penicillium chrysogenum sp. ZZ1151. Nat. Prod. Res. 2023, 37, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Li, Z.; Pan, X.L.; Huang, Y.L.; Hu, W.J.; Li, F.; Wang, Q.Z.; Huang, S.S.; Zhou, X.Y.; Wen, W.S. Screening of Mangrove Soil Bacteria Producing in Vitro Anti-na sopharyngeal Carcinoma Substance and Analysis of Its Active Component. Guangxi Sci. 2022, 29, 846–853. (In Chinese) [Google Scholar] [CrossRef]
- Naito, T. Development of new synthetic reactions for nitrogen-containing compounds and their application. Chem. Pharm. Bull. 2008, 56, 1367–1383. [Google Scholar] [CrossRef]
- Cai, J.; Li, M.; Chen, C.; Yang, B.; Gao, C.; Liu, Y.; Luo, X.; Tan, Y.; Zhou, X. Peniditerpenoids A and B: Oxidized Indole Diterpenoids with Osteoclast Differentiation Inhibitory Activity from a Mangrove-Sediment-Derived Penicillium sp. J. Nat. Prod. 2024, 87, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; He, J.; Huang, S.; Wu, S.; Zeng, L.; Wang, J.; Hong, B.; Chen, Z. Phaeosphamides A and B, cytotoxic cyclodecadepsipeptides from the mangrove-derived fungus Phaeosphaeriopsis sp. S296. Mar. Drugs 2022, 20, 591. [Google Scholar] [CrossRef]
- Lu, P.; Shi, Y.; Zhang, J.; Hong, K.; Xue, Y.; Liu, L. New prenylated indole-benzodiazepine-2, 5-diones with α-glucosidase inhibitory activities from the mangrove-derived Aspergillus spinosus. Int. J. Biol. Macromol. 2024, 257, 128808. [Google Scholar] [CrossRef]
- Wu, Q.; Chang, Y.; Che, Q.; Li, D.; Zhang, G.; Zhu, T. Citreobenzofuran D–F and phomenone A–B: Five novel sesquiterpenoids from the mangrove-derived fungus Penicillium sp. HDN13-494. Mar. Drugs 2022, 20, 137. [Google Scholar] [CrossRef]
- Butler, A.; Sandy, M. Mechanistic considerations of halogenating enzymes. Nature 2009, 460, 848–854. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Cai, J.; Wang, X.; Gan, X.; Zhou, Q.; Luo, X.; Yang, B.; Liu, Y.; Ratnasekera, D.; Zhou, X. New chlorinated metabolites and antiproliferative polyketone from the mangrove sediments-derived fungus Mollisia sp. SCSIO41409. Mar. Drugs 2022, 21, 32. [Google Scholar] [CrossRef]
- Conrado, R.; Gomes, T.C.; Roque, G.S.C.; De Souza, A.O. Overview of bioactive fungal secondary metabolites: Cytotoxic and antimicrobial compounds. Antibiotics 2022, 11, 1604. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Somarelli, J.A.; Boddy, A.M.; Gardner, H.L.; DeWitt, S.B.; Tuohy, J.; Megquier, K.; Sheth, M.U.; Hsu, S.D.; Thorne, J.L.; London, C.A.; et al. Improving cancer drug discovery by studying cancer across the tree of life. Mol. Biol. Evol. 2020, 37, 11–17. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar]
- Ziemert, N.; Alanjary, M.; Weber, T. The evolution of genome mining in microbes—A review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, S.; Hahn, F. Opportunities for enzyme catalysis in natural product chemistry. Tetrahedron 2015, 71, 1473–1508. [Google Scholar] [CrossRef]
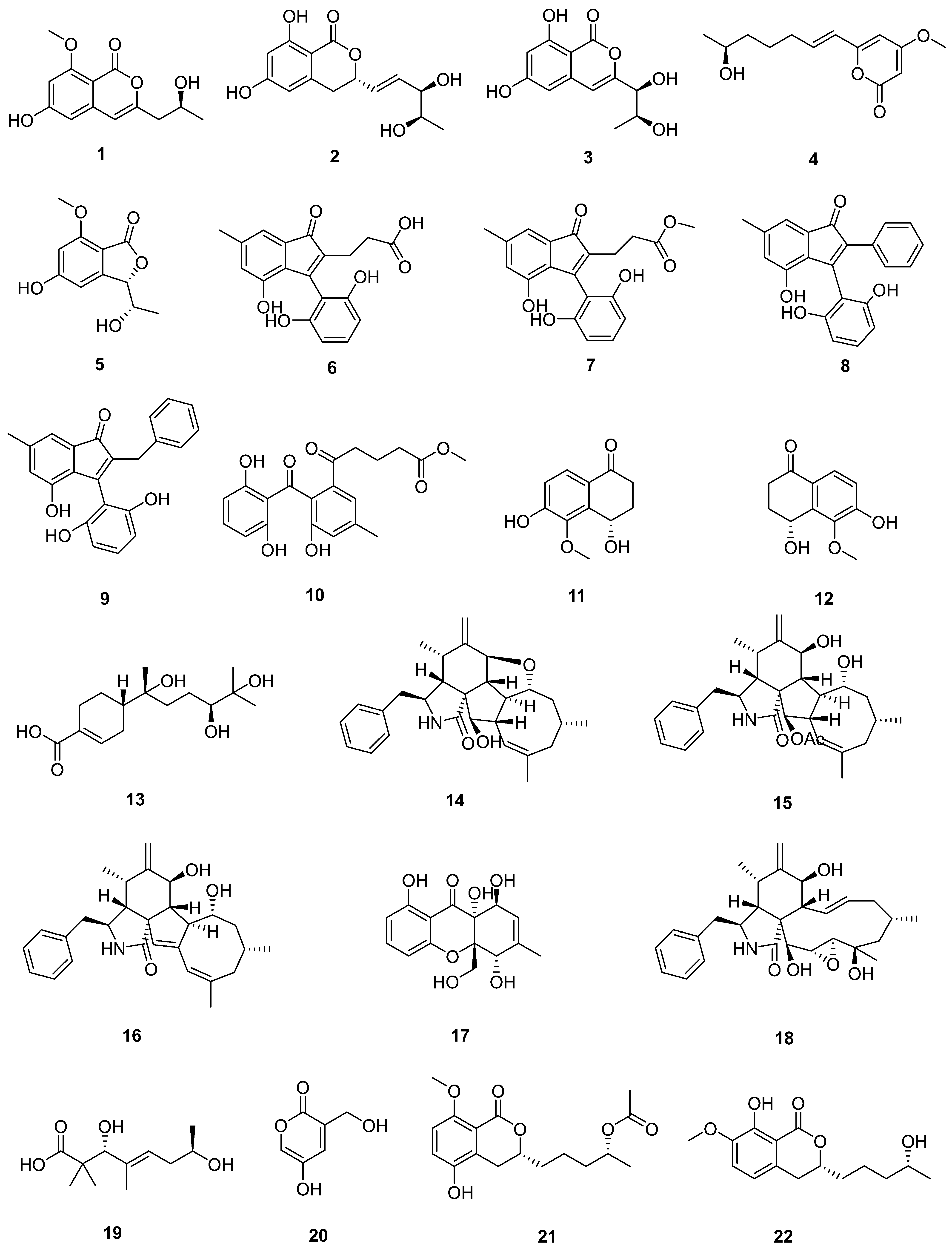
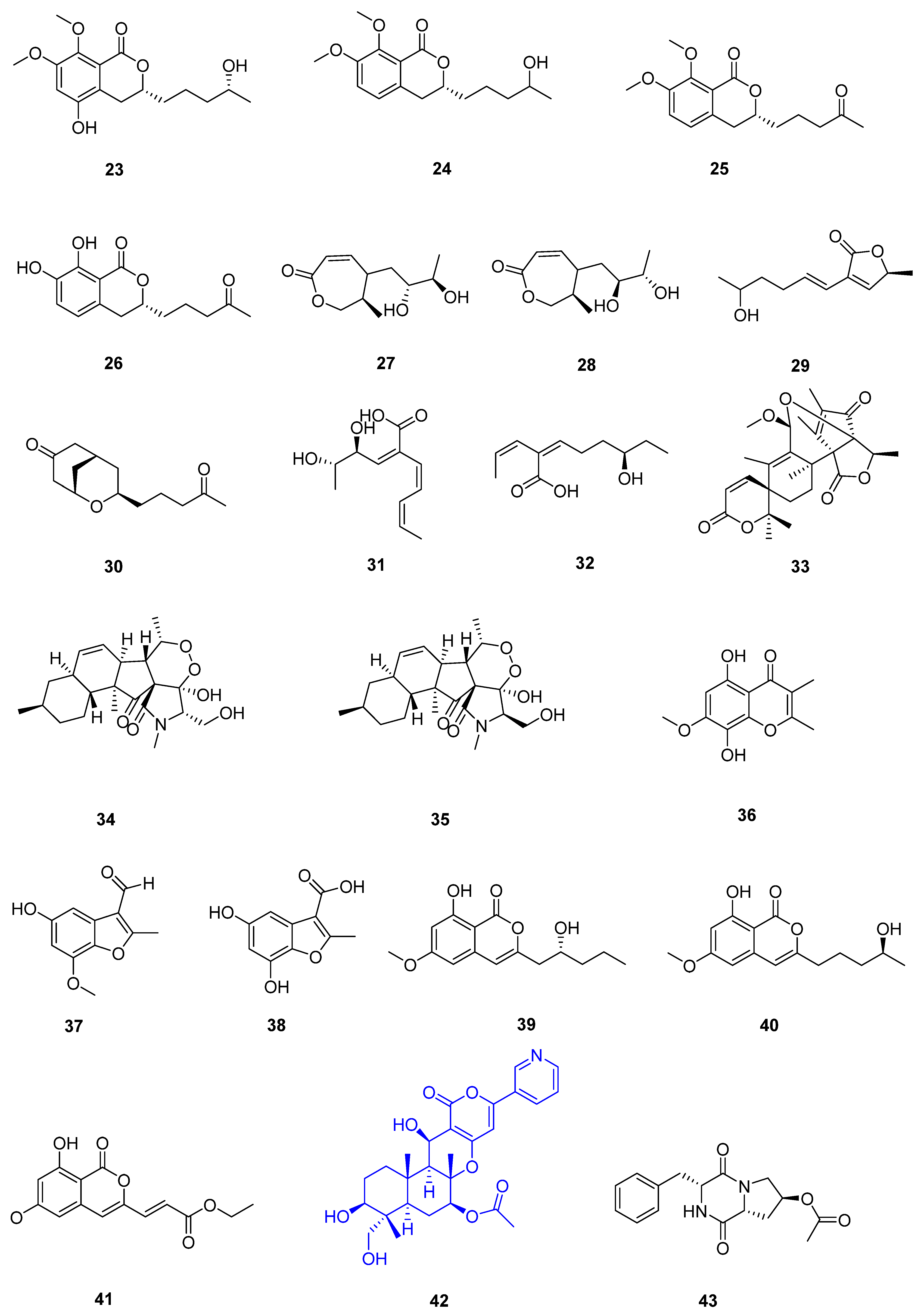
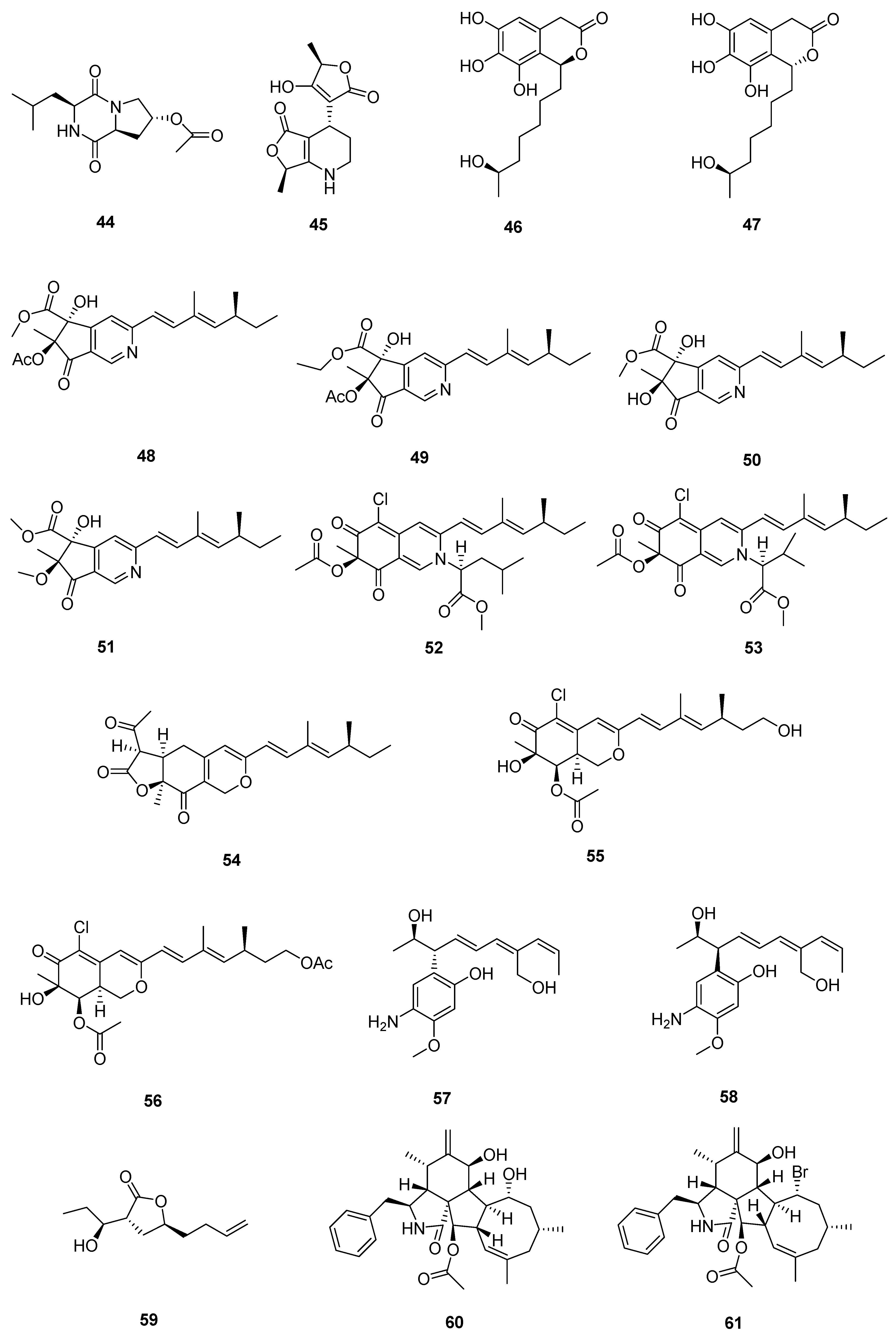


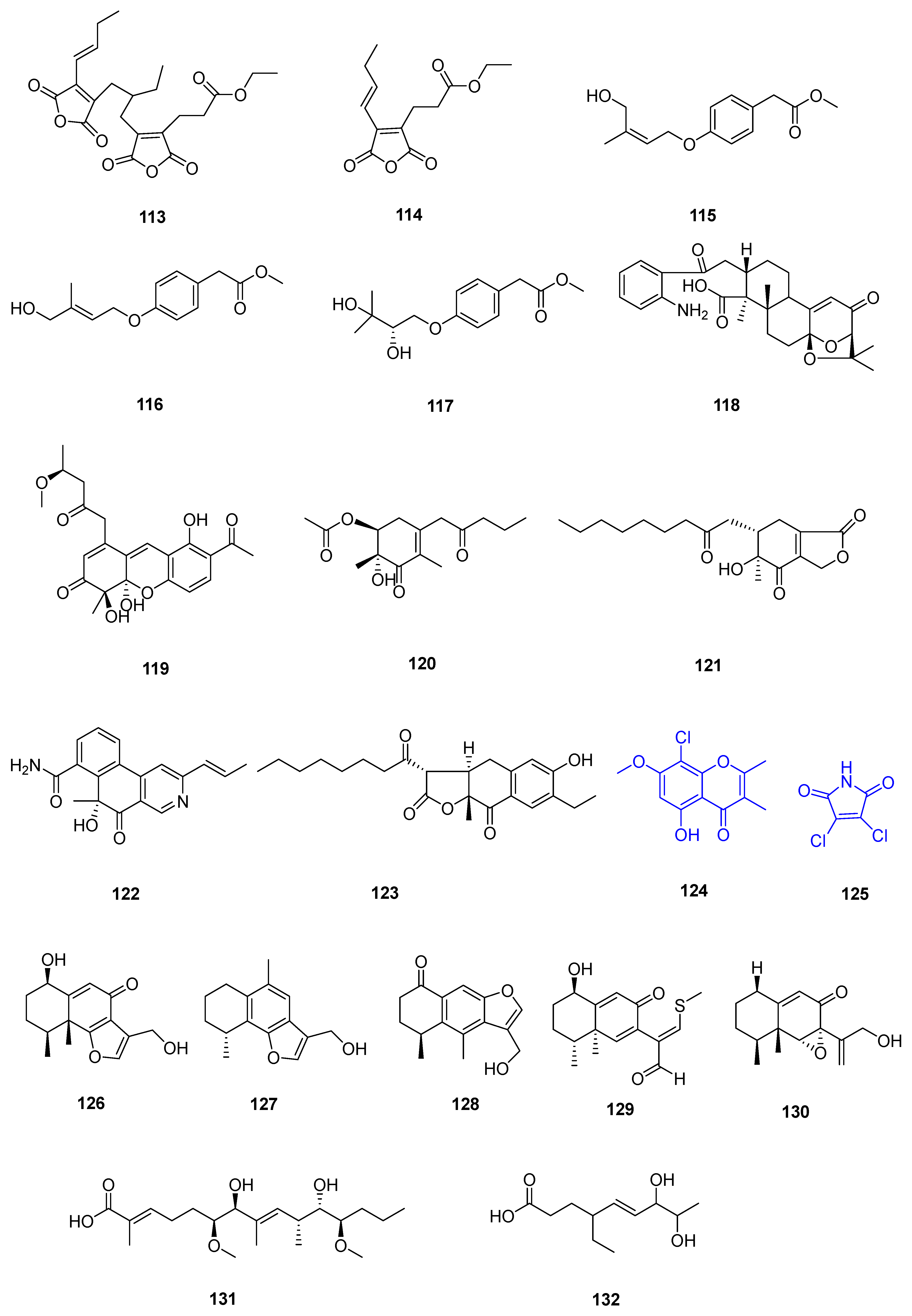
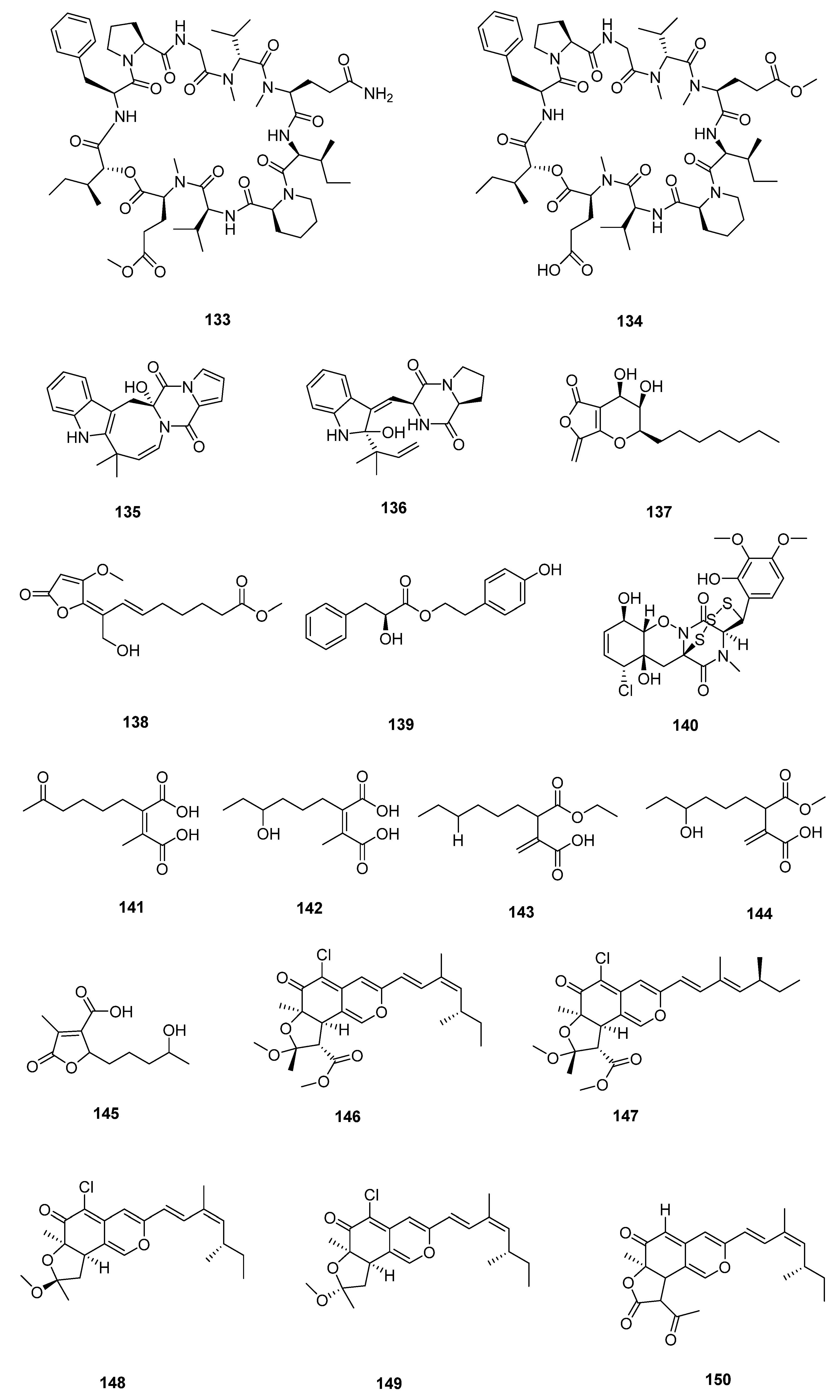
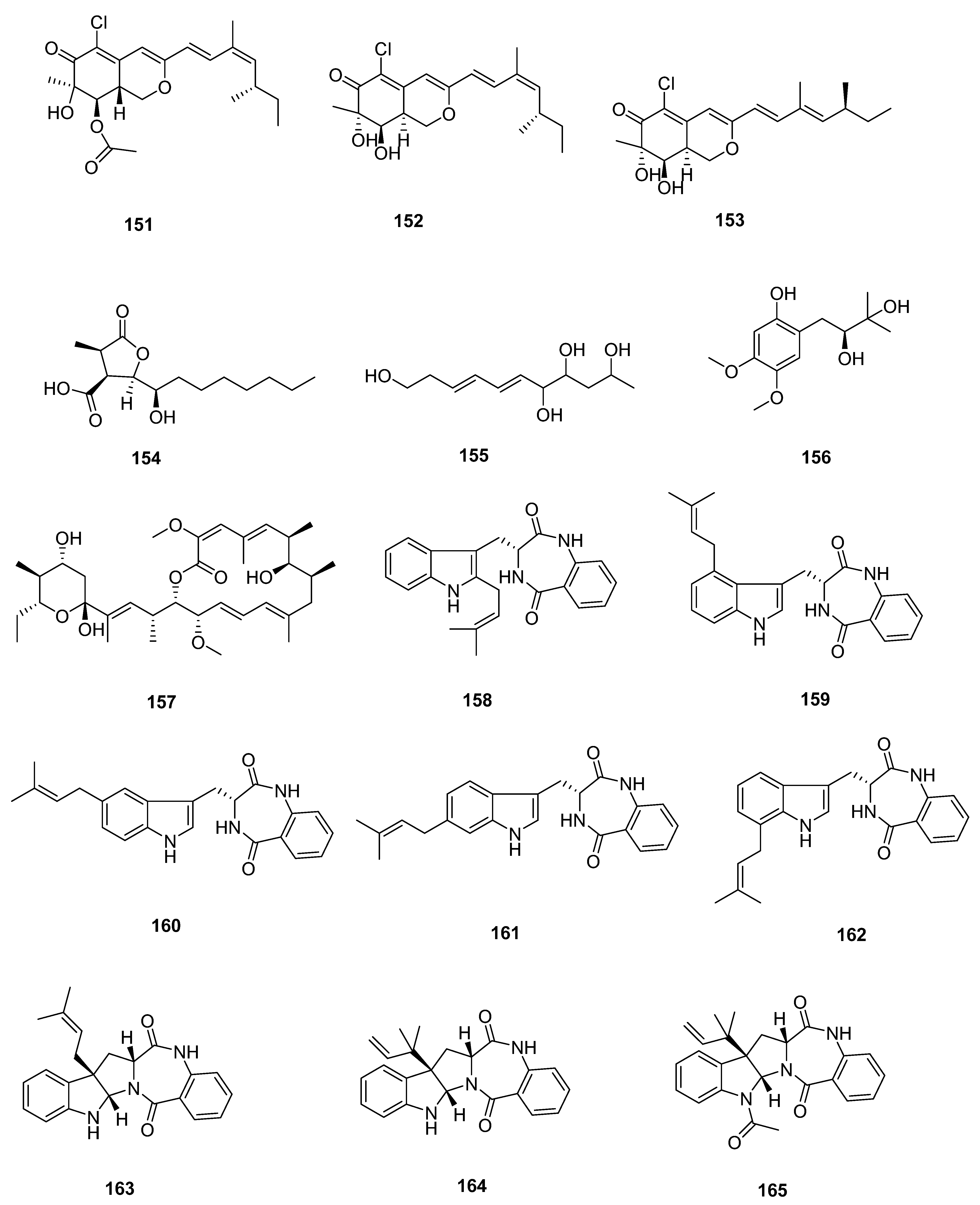

| Sr. No. | Endophytic Fungus | Host Plant(s) | Sources | Compound Isolated | Bioactivity and IC50/EC50/Inhibition | Reference |
|---|---|---|---|---|---|---|
| 1 | Phomopsis sp. DHS-11 | the living root of the mangrove plant Rhizophora mangle | Dong Zhai Gang mangrove garden on Hainan Island, China | 1 | HeLa cells (11.49 ± 1.64 μmol L−1) | [28] |
| 2 | ||||||
| 3 | HeLa cells (8.70 ± 0.94 μmol L−1) | |||||
| 4 | HepG 2 (34.10 ± 2.92 μmol L−1) | |||||
| 5 | [29] | |||||
| 2 | Cytospora heveae NSHSJ-2 | the fresh stem of mangrove plant Sonneratia caseolaris | the Nansha Mangrove National Nature Reserve in Guangdong Province, China | 6 | DPPH radical scavenging activity (11.5 ± 0.1 μmol L−1) | [30] |
| 7 | DPPH radical scavenging activity (21.5 ± 1.0 μmol L−1) | |||||
| 8 | DPPH radical scavenging activity (19.7 ± 1.8 μmol L−1) | |||||
| 9 | DPPH radical scavenging activity (16.6 ± 0.4 μmol L−1) | |||||
| 10 | DPPH radical scavenging activity (9.5 ± 0.1 μmol L−1) | |||||
| 11 | ||||||
| 12 | ||||||
| 3 | Colletotrichum sp. SCSIO KcB3-2 | a mangrove plant, Kandelia candel | Dayawan, Shenzhen, Guangdong Province, China | 13 | AChE inhibitory activity (2.2 ± 0.18 μmol L−1) | [31] |
| 4 | Phomopsis asparagi DHS-48 | a healthy tree root of the mangrove plant Rhizophora mangle | Dong Zhai Gang-Mangrove Garden in Hainan Province | 14 | [32] | |
| 15 | normal splenocytes (111.7 ± 1.1 μmol L−1) ConA-Induced T-Cell Proliferation (21.6 ± 1.7 μmol L−1) LPS-Induced B-Cell Proliferation (78.5 ± 1.3 μmol L−1) | |||||
| 16 | ||||||
| 17 | ConA-Induced T-Cell Proliferation (42.35 ± 2.49 μmol L−1) LPS-Induced B-Cell Proliferation (88.19 ± 2.59 μmol L−1) | [33] | ||||
| 18 | HepG2 (59.14 ± 15.79 μmol L−1) Hela (5.82 ± 0.82 μmol L−1) | |||||
| 5 | Pestalotiopsis sp. HQD-6 | fresh, healthy leaf of Chinese mangrove plant Rhizophora mucronata | Dong Zhai Gang-Mangrove Garden on Hainan Island, China | 19 | [34] | |
| 20 | Hela (50.42 ± 0.07 μmol L−1) | |||||
| 6 | Talaromyces flavus TGGP35 | the stem of the mangrove plant Acanthus ilicifolius | Dongzhai Port, Haikou, Hainan Province | 21 | [35] | |
| 22 | ||||||
| 23 | ||||||
| 24 | ||||||
| 25 | ||||||
| 26 | antioxidant activity (0.14 mmol L−1) | |||||
| 27 | [36] | |||||
| 28 | ||||||
| 29 | Hela (62.23 ± 0.23 μmol L−1) | |||||
| 30 | ||||||
| 31 | antioxidant activity (0.40 mmol L−1) | |||||
| 32 | Hela (57.14 ± 0.15 μmol L−1) | |||||
| 33 | anti-insect activity against newly hatched larvae of Helicoverpa armigera Hubner (50–200 µgmL−1) | |||||
| 7 | Fusarium sp. 2ST2 | healthy leaves of Kandelia candel | the South China Sea, Dong Zhai Harbor Mangrove Nature Reserve Area, Hainan Province, China | 34 | A549 (8.7 μmol L−1) | [37] |
| 35 | A550 (4.38 μmol L−1) | |||||
| 36 | ||||||
| 37 | ||||||
| 38 | ||||||
| 39 | ||||||
| 40 | ||||||
| 41 | ||||||
| 8 | Aspergillus fumigatus HQD24 | the flower of the Chinese mangrove plant Rhizophora mucronata | 42 | decreased ACAT2 inhibitory activity (12.0 mmol L−1) | [38] | |
| 9 | Nigrospora camelliae-sinensis S30 | mangrove Lumnitzera littorea | 43 | [39] | ||
| 44 | ||||||
| 10 | Penicillium crustosum SCNU-F0006 | Acanthus ilicifolius L. mangrove plant | the Yangjiang Mangrove Nature Reserve in Guangdong Province | 45 | RAW 264.7 cells (above 50 μmol L−1) anti-inflammatory activity (42.22 ± 2.26 μmol L−1) DPPH radical scavenging activity (180.2 μmol L−1) antimicrobial activities against Bacillus subtilis, Penicillium italicum, and Pseudomonas aeruginosa | [40] |
| 11 | Diaporthe sp. ZJHJYZ-1 | a fresh leaf of the semi-mangrove plant Hibiscus tiliaceus L. | Zhanjiang Mangrove National Nature Reserve in Guangdong Province, China | 46 | [41] | |
| 47 | ||||||
| 12 | Penicillium sclerotiorum ZJHJJ-18 | the stems of the mangrove plant Hibiscus tiliaceus | the shore of the Zhanjiang Mangrove Nature Reserve in Guangdong Province, China | 48 | inhibit LPS-induced NO production in RAW264.7 macrophage cells | [42] |
| 49 | inhibit LPS-induced NO production in RAW264.8 macrophage cells | |||||
| 50 | inhibit LPS-induced NO production in RAW264.9 macrophage cells | |||||
| 51 | inhibit LPS-induced NO production in RAW264.10 macrophage cells | |||||
| 52 | inhibit LPS-induced NO production in RAW264.11 macrophage cells | |||||
| 53 | inhibit LPS-induced NO production in RAW264.12 macrophage cells | |||||
| 54 | ||||||
| 55 | ||||||
| 56 | ||||||
| 13 | Penicillium herquei JX4 | the mangrove Ceriops tagal | the South China Sea | 57 | anti-inflammatory activities | [43] |
| 58 | anti-inflammatory activities | |||||
| 59 | anti-inflammatory activities | |||||
| 14 | Phomopsis sp. QYM-13 | healthy leaves of Kandelia candel | the South China Sea, Dongzhai Harbor Mangrove Nature Reserve Area, Hainan Province, China | 60 | [44] | |
| 61 | MDA-MB-435 (4.9–8.2 μmol L−1) | |||||
| 62 | ||||||
| 63 | ||||||
| 64 | ||||||
| 65 | ||||||
| 66 | ||||||
| 67 | ||||||
| 68 | ||||||
| 69 | ||||||
| 70 | ||||||
| 71 | ||||||
| 15 | Daldinia eschscholtzii KBJYZ-1 | the root of Pluchea indica Less | Zhanjiang Mangrove National Nature Reserve in Guangdong Province, China | 72 | anti-inflammatory activities (19.3 μmol L−1) | [45] |
| 73 | ||||||
| 74 | ||||||
| 75 | ||||||
| 76 | anti-inflammatory activities (12.9 μmol L−1) | |||||
| 16 | Aspergillus sydowii #2B | the leaves of the mangrove plant Aricennia marina | 77 | inhibit the production of nitric oxide (NO) in lipopolysaccharide (LPS)-induced RAW 246.7 cells (40.15 μmol L−1) | [46] | |
| 78 | VCaP (20.06 ± 2.01 μmol L−1) | |||||
| 17 | Aspergillus fumigatus SAI12 | leaves of mangrove plant Sonneratia apetala Buch.-Ham. | Dongzhaigang National Nature Reserve in south China’s Hainan Province | 79 | [47] | |
| 80 | ||||||
| 18 | Aspergillus sp. GXNU-A9 | a leaf of mangrove Acanthus ilicifolius L. | Qinzhou City, China | 81 | moderate inhibitory activity against nitric oxide (NO) production anti-inflammatory activity | [48] |
| 19 | Aspergillus sp. GXNU-Y65 | fresh fruit of the mangrove plant Kandelia candel | Beihai, China | 82 | [49] | |
| 83 | ||||||
| 84 | anti-nonalcoholic steatohepatitis activity | |||||
| 85 | ||||||
| 86 | ||||||
| 87 | ||||||
| 88 | ||||||
| 89 | ||||||
| 90 | ||||||
| 20 | Aspergillus sp. GXIMD00016 | Fresh leaves of Kandelia candel | Beihai Golden Bay Mangrove Reserve | 91 | [50] | |
| 21 | Aspergillus QG1a | the mangrove Kandelia candel | the seaside of Qinzhou, Guangxi Province, China | 92 | A549 (59.283 μmol L−1) A2780 (46.197 μmol L−1) MIA PACA-2 (42.664 μmol L−1) | [51] |
| 93 | A2780 (6.808 μmol L−1) MIA PACA-2 (15.400 μmol L−1) | |||||
| 22 | Aspergillus sp. GXNU-4QQY1a | healthy leaves of Acanthus ilicifolius L. | 94 | insecticidal activity against citrus psyllids (lethality values of 92.31 ± 6.20%) | [52] | |
| 95 | exhibited good insecticidal activity against citrus psyllids (lethality values of 87.80 ± 9.32%) | |||||
| 23 | Didymella sp. CYSK-4 | a fresh branch of the mangrove plant Pluchea indica | Shankou Mangrove Na ture Reserve in Guangxi Province, China | 96 | A549 (11.0 μmol L−1) | [53] |
| 97 | A549 (2.8 μmol L−1) KYSE 150 (5.9 μmol L−1) | |||||
| 98 | ||||||
| 99 | ||||||
| 24 | Fusarium. proliferatum NSD-1 | a fresh twig from mangrove plant Kandelia candel | a Mangrove of Nansha District of Guangzhou in Guangdong Province, China | 100 | A549 (75.9 ± 10.4 μmol L−1) SW480 (37.5 ± 8.0 μmol L−1) | [54] |
| 101 | ||||||
| 102 | ||||||
| 103 | moderate IL-1β inhibitory activity | |||||
| 104 | A549 (24.9 ± 10.1 μmol L−1) SW480 (77.7 ± 3.6 μmol L−1) | |||||
| 25 | Penicillium verruculosum TGM14 | mangrove Xylocarpus granatum Koenig | the South China Sea | 105 | [55] | |
| 26 | Penicillium sp. GXIMD 03001 | the rhizophoraceous mangrove Kandelia candel | the Beibu Gulf | 106 | [56] | |
| 107 | ||||||
| 27 | Amorosia sp. SCSIO 41026 | the leaf of Avicennia marina (Forsk.) Vierh. | the mangrove wetland in Zhanjiang, Guangdong province, China | 108 | inhibit LPS-induced NO production in RAW264.7 macrophage cells | [57] |
| 109 | ||||||
| 110 | ||||||
| 111 | inhibit LPS-induced NO production in RAW264.7 macrophage cells | |||||
| 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, Z.; Xiong, D.; Zhou, L.; Kong, F.; Wang, Q. New Secondary Metabolites of Mangrove-Associated Strains. Mar. Drugs 2024, 22, 372. https://doi.org/10.3390/md22080372
Yu Y, Wang Z, Xiong D, Zhou L, Kong F, Wang Q. New Secondary Metabolites of Mangrove-Associated Strains. Marine Drugs. 2024; 22(8):372. https://doi.org/10.3390/md22080372
Chicago/Turabian StyleYu, Yunxia, Zimin Wang, Dingmi Xiong, Liman Zhou, Fandong Kong, and Qi Wang. 2024. "New Secondary Metabolites of Mangrove-Associated Strains" Marine Drugs 22, no. 8: 372. https://doi.org/10.3390/md22080372





