Light Spectra, a Promising Tool to Modulate Ulva lacinulata Productivity and Composition
Abstract
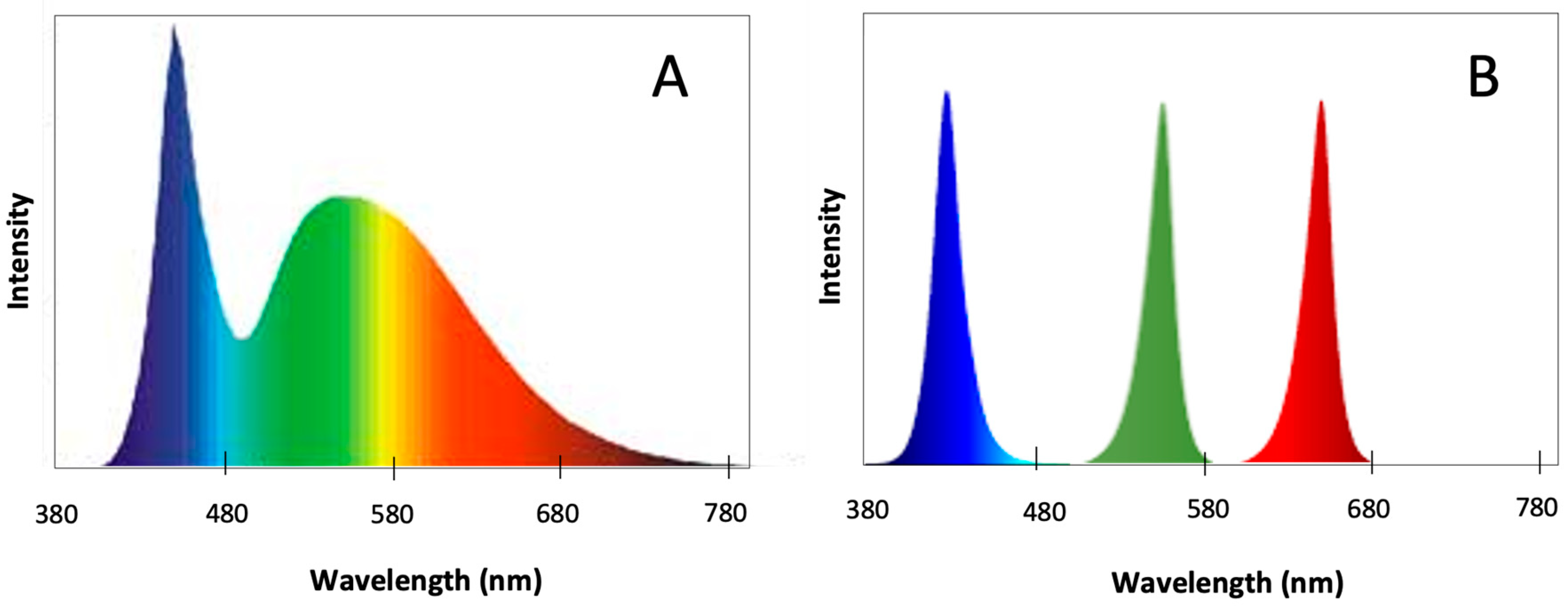
:1. Introduction
2. Results
2.1. Effects of Light Quality on U. lacinulata Growth and Composition
2.1.1. Effects of Light Quality on U. lacinulata Growth
2.1.2. Effects of Light Quality on Photosynthetic Activity
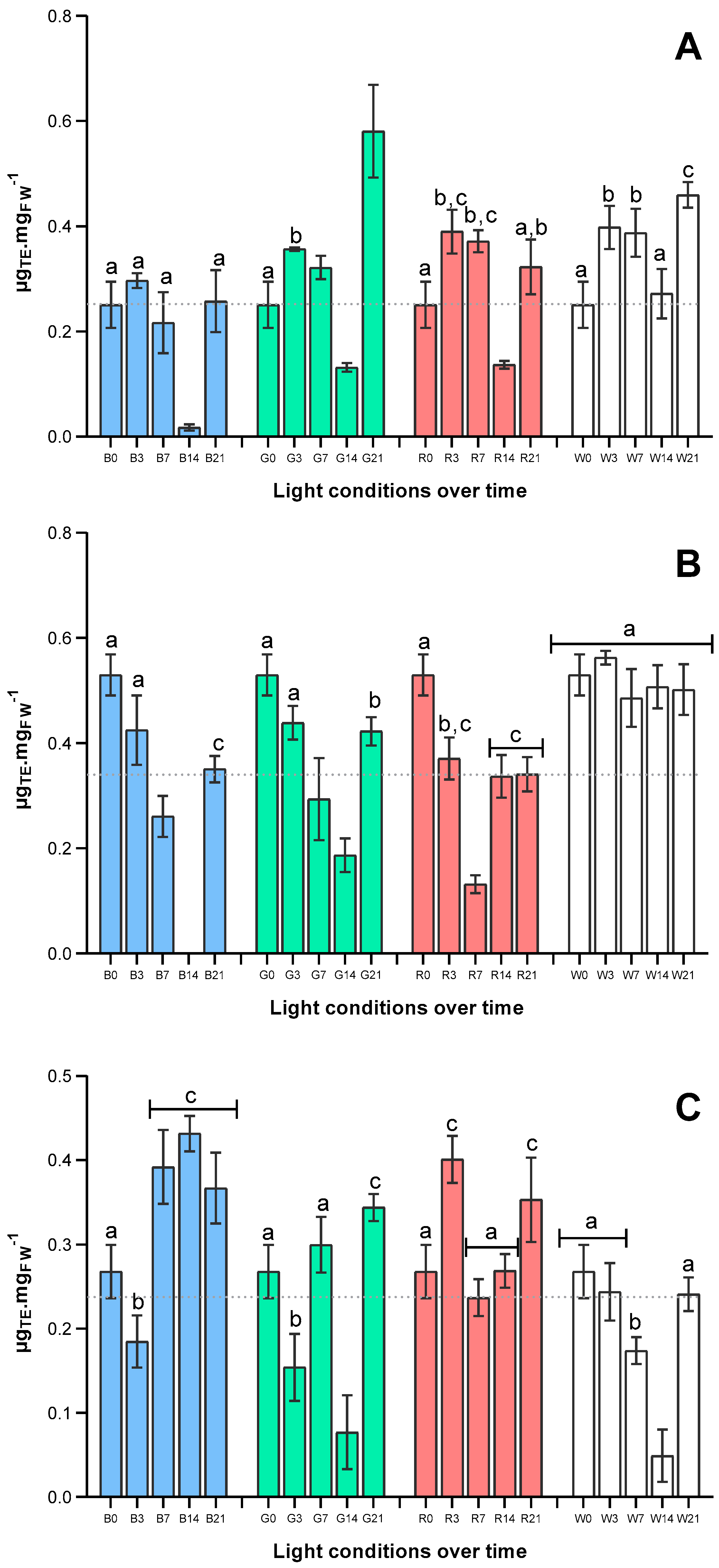
2.2. Effect on Pigment Composition over Time
2.3. Effects of Light Quality on Antioxidant Capacity of U. lacinulata
2.4. Pearson Correlation of U. lacinulata Performance Parameters under Each Light Quality
3. Discussion
3.1. Effects of Light Quality on U. lacinulata Growth
3.2. Effects of Light Quality on Photosynthetic Activity and Events
3.3. Pigment Content and Antioxidant Capacity
4. Materials and Methods
4.1. Sampling and Ulva Maintenance
4.2. Species Identification
4.3. Light Quality Experiments
4.4. Growth Evaluation
4.5. Photosynthetic Activity Measurement
4.6. Pigment Composition and Antioxidant Capacity
4.6.1. Pigment Quantification
4.6.2. U. lacinulata Antioxidant Capacity Assessment
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
 0.7–0.9;
0.7–0.9;  0.8–0.9;
0.8–0.9;  ≥0.9), if is positive (+) or negative (−), and the thickness edge line indicates the significance levels for each comparison, none- p < 0.05,
≥0.9), if is positive (+) or negative (−), and the thickness edge line indicates the significance levels for each comparison, none- p < 0.05,  p < 0.01, and
p < 0.01, and  p < 0.001.
p < 0.001.Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elizondo-González, R.; Quiroz-Guzmán, E.; Escobedo-Fregoso, C.; Magallón-Servín, P.; Peña-Rodríguez, A. Use of seaweed Ulva lactuca for water bioremediation and as feed additive for white shrimp Litopenaeus vannamei. PeerJ 2018, 6, e4459. [Google Scholar] [CrossRef] [PubMed]
- Favot, G.; Cunha, M.E.; Quental-Ferreira, H.; Álvares Serrão, M.E. Production of Ulva sp. In multitrophic aquaculture in earth ponds. Aquac. Fish. Stud. 2019, 1, 1–8. [Google Scholar]
- van den Burg, S.W.K.; Dagevos, H.; Helmes, R.J.K. Towards sustainable european seaweed value chains: A triple p perspective. ICES J. Mar. Sci. 2019, 78, 443–450. [Google Scholar] [CrossRef]
- Macchiavello, J.; Bulboa, C. Nutrient uptake efficiency of Gracilaria chilensis and Ulva lactuca in an imta system with the red abalone haliotis rufescens. Lat. Am. J. Aquat. Res. 2014, 42, 523–533. [Google Scholar] [CrossRef]
- Israel, A.; Friedlander, M.; Neori, A. Biomass yield, photosynthesis and morphological expression of Ulva lactuca. Bot. Mar. 1995, 38, 297–302. [Google Scholar] [CrossRef]
- Fort, A.; Lebrault, M.; Allaire, M.; Esteves-Ferreira, A.A.; McHale, M.; Lopez, F.; Fariñas-Franco, J.M.; Alseekh, S.; Fernie, A.R.; Sulpice, R. Extensive variations in diurnal growth patterns and metabolism among Ulva spp. Strains. Plant Physiol. 2019, 180, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Gadberry, B.A.; John, C.; Desmond, M.; Diane, C.B.; Ken, W.; Ronald, B.J.; Gary, W.S.; Richard, H.B. Intensive land-based production of red and green macroalgae for human consumption in the pacific northwest: An evaluation of seasonal growth, yield, nutritional composition, and contaminant levels. ALGAE 2018, 33, 109–125. [Google Scholar] [CrossRef]
- Muthuvelan, B.; Noro, T.; Nakamura, K. Effect of light quality on the cell integrity in marine alga Ulva pertusa (chlorophyceae). Indian J. Mar. Sci. 2002, 31, 21–25. [Google Scholar]
- Rochaix, J.-D. Regulation of photosynthetic electron transport. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 375–383. [Google Scholar] [CrossRef]
- Ouzounis, T.; Frette, X.; Ottosen, C.O.; Rosenqvist, E. Spectral effects of leds on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘vivien’ and ‘purple star’. Physiol. Plant. 2015, 154, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Xiaolei, F.; Guangce, W.; Demao, L.; Pu, X.; Songdong, S. Study on early-stage development of conchospore in Porphyra yezoensis ueda. Aquaculture 2008, 278, 143–149. [Google Scholar] [CrossRef]
- Barufi, J.B.; Figueroa, F.L.; Plastino, E.M. Effects of light quality on reproduction, growth and pigment content of Gracilaria birdiae (rhodophyta: Gracilariales). Sci. Mar. 2015, 79, 15–24. [Google Scholar] [CrossRef]
- Dring, M. Pigment composition and photosynthetic action spectra of sporophytes of laminaria (phaeophyta) grown in different light qualities and irradiances. Br. Phycol. J. 1986, 21, 199–207. [Google Scholar] [CrossRef]
- Marques, R.; Cruz, S.; Calado, R.; Lillebø, A.; Abreu, H.; Pereira, R.; Pitarma, B.; da Silva, J.M.; Cartaxana, P. Effects of photoperiod and light spectra on growth and pigment composition of the green macroalga Codium tomentosum. J. Appl. Phycol. 2021, 33, 471–480. [Google Scholar] [CrossRef]
- Le, B.; Shin, J.-A.; Kang, M.-G.; Sun, S.; Yang, S.h.; Chung, G. Enhanced growth rate and ulvan yield of Ulva pertusa using light-emitting diodes (leds). Aquac. Int. 2018, 26, 937–946. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Z.; Zou, D. Growth and photosynthetic characteristics of Gracilaria lemaneiformis (rhodophyta) and Ulva lactuca (chlorophyta) cultured under fluorescent light and different led light. J. Appl. Phycol. 2020, 32, 3265–3272. [Google Scholar] [CrossRef]
- Miki, O.; Okumura, C.; Marzuki, M.; Tujimura, Y.; Fujii, T.; Kosugi, C.; Kato, T. Contrasting effects of blue and red led irradiations on the growth of sargassum horneri during the germling and immature stages. J. Appl. Phycol. 2017, 29, 1461–1469. [Google Scholar] [CrossRef]
- Kang, L.-K.; Huang, Y.-J.; Lim, W.-T.; Hsu, P.-H.; Hwang, P.-A. Growth, pigment content, antioxidant activity, and phytoene desaturase gene expression in Caulerpa lentillifera grown under different combinations of blue and red light-emitting diodes. J. Appl. Phycol. 2020, 32, 1971–1982. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Kumaran, R.S.; Jeon, H.J.; Song, H.-J.; Yang, Y.-H.; Lee, S.H.; Song, K.-G.; Kim, K.J.; Singh, V.; Kim, H.J. Led light stress induced biomass and fatty acid production in microalgal biosystem, Acutodesmus obliquus. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 245–253. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Conde-Álvarez, R.; Gómez, I. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth. Res. 2003, 75, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue led light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Wang, H.; Lin, A.; Gu, W.; Huan, L.; Gao, S.; Wang, G. The sporulation of the green alga Ulva prolifera is controlled by changes in photosynthetic electron transport chain. Sci. Rep. 2016, 6, 24923. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bogaert, K.; Engelen, A.H.; Leliaert, F.; Roleda, M.Y.; Clerck, O.D. Seaweed reproductive biology: Environmental and genetic controls. Bot. Mar. 2017, 60, 89–108. [Google Scholar] [CrossRef]
- Mine, I.; Okuda, K.; Tatewaki, M. Gamete discharge by Bryopsis plumosa (codiales, chlorophyta) induced by blue and uv-a light. Phycol. Res. 1996, 44, 185–191. [Google Scholar] [CrossRef]
- Vu, A.L. Role of Light, Humidity, and Nutrition in Sporulation of Phytophthora Species. Ph.D. Thesis, University of California, Riverside, CA, USA, 2020. [Google Scholar]
- Chen, M.; Chory, J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 2011, 21, 664–671. [Google Scholar] [CrossRef]
- Duanmu, D.; Bachy, C.; Sudek, S.; Wong, C.-H.; Jiménez, V.; Rockwell, N.C.; Martin, S.S.; Ngan, C.Y.; Reistetter, E.N.; van Baren, M.J.; et al. Marine algae and land plants share conserved phytochrome signaling systems. Proc. Natl. Acad. Sci. USA 2014, 111, 15827–15832. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates Inc. Publishers: Sunderland, MA, USA, 2002. [Google Scholar]
- Senger, H. Blue Light Control of Pigment Synthesis-Chlorophyll Biosynthesis; Senger, H., Ed.; CRC Press: Boca Raton, FL, USA, 1987; Volume 1, ISBN 0-8493-5235-5. [Google Scholar]
- Lopez-Figueroa, F.; Niell, F.X. Red-light and blue-light photoreceptors controlling chlorophyll a synthesis in the red alga porphyra umbilicalis and in the green alga ulva rigida. Physiol. Plant. 1989, 76, 391–397. [Google Scholar] [CrossRef]
- Wu, H. Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (bangiales, rhodophyta). BioMed Res. Int. 2016, 2016, 7383918. [Google Scholar] [CrossRef]
- Bohne, F.; Linden, H. Regulation of carotenoid biosynthesis genes in response to light in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 2002, 1579, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Fort, A.; McHale, M.; Cascella, K.; Potin, P.; Perrineau, M.-M.; Kerrison, P.D.; da Costa, E.; Calado, R.; Domingues, M.D.R.; Costa Azevedo, I.; et al. Exhaustive reanalysis of barcode sequences from public repositories highlights ongoing misidentifications and impacts taxa diversity and distribution. Mol. Ecol. Resour. 2022, 22, 86–101. [Google Scholar] [CrossRef]
- Fort, A.; Linderhof, C.; Coca-Tagarro, I.; Inaba, M.; McHale, M.; Cascella, K.; Potin, P.; Guiry, M.D.; Sulpice, R. A sequencing-free assay for foliose ulva species identification, hybrid detection and bulk biomass characterisation. Algal Res. 2021, 55, 102280. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Hall-Spencer, J.M.; Horta, P.A.; Milazzo, M.; Korbee, N.; Cornwall, C.E.; Figueroa, F.L. Macroalgal responses to ocean acidification depend on nutrient and light levels. Front. Mar. Sci. 2015, 2, 26. [Google Scholar] [CrossRef]
- Schreiber, U.; Endo, T.; Mi, H.; Asada, K. Quenching analysis of chlorophyll fluorescence by the saturation pulse method: Particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol. 1995, 36, 873–882. [Google Scholar] [CrossRef]
- Platt, T.; Harrison, W.G.; Irwin, B.; Horne, E.P.; Gallegos, C.L. Photosynthesis and photoadaptation of marine phytoplankton in the arctic. Deep Sea Res. Part A Oceanogr. Res. Pap. 1982, 29, 1159–1170. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of temperature and ph on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef]
- Amaro, H.M.; Fernandes, F.; Valentão, P.; Andrade, P.B.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Effect of solvent system on extractability of lipidic components of Scenedesmus obliquus (m2-1) and Gloeothece sp. On antioxidant scavenging capacity thereof. Mar. Drugs 2015, 13, 6453–6471. [Google Scholar] [CrossRef]



| Light Quality | Time | Fv/Fm | ETRmax | Ek | NPQmax |
|---|---|---|---|---|---|
| B | 0 | 0.793 ± 0.009 a | 150.6 ± 2.6 a | 601.9 ± 18.6 a | 0.680 ± 0.038 a |
| 3 | 0.704 ± 0.004 b | 107.3 ± 1.8 b | 448.5 ± 8.8 b | 0.675 ± 0.028 a | |
| 7 | 0.738 ± 0.006 c | 116.8 ± 2.4 b | 496.8 ± 3.6 b | 0.622 ± 0.016 a | |
| 14 | 0.696 ± 0.005 b | 64.8 ± 0.2 c | 143.5 ± 0.8 c | 0.343 ± 0.047 b | |
| 21 | 0.671 ± 0.013 b | 67.2 ± 9.8 c,d | 300.7 ± 11.8 d | 0.781 ± 0.022 c | |
| G | 0 | 0.782 ± 0.007 a | 164.9 ± 2.9 a | 624.3 ± 7.1 a | 0.689 ± 0.015 a |
| 3 | 0.868 ± 0.008 d | 118.4 ± 1.2 b | 392.8 ± 3.6 e | 0.698 ± 0.029 a | |
| 7 | 0.808 ± 0.005 a | 93.6 ± 1.2 e | 245.9 ± 3.3 d | 0.518 ± 0.053 d | |
| 14 | 0.758 ± 0.006 c | 75.9 ± 2.2 d | 193.9 ± 8.9 f | 0.510 ± 0.030 d | |
| 21 | 0.730 ± 0.005 c | 76.1 ± 3.2 d | 266.5 ± 8.3 d | 0.415 ± 0.027 b | |
| R | 0 | 0.794 ± 0.002 a | 156.7 ± 3.2 a | 614.5 ± 5.5 a | 0.672 ± 0.017 a |
| 3 | 0.694 ± 0.005 b | 117.8 ± 1.3 b | 456.3 ± 5.6 b | 0.701 ± 0.036 a | |
| 7 | 0.744 ± 0.003 c | 152.1 ± 2.9 a | 495.7 ± 3.8 b | 0.916 ± 0.043 d | |
| 14 | 0.752 ± 0.009 c | 126.5 ± 2.4 f | 488.5 ± 6.3 b | 0.866 ± 0.084 c,d | |
| 21 | 0.729 ± 0.011 c | 76.7 ± 3.3 d | 266.7 ± 12.3 d | 0.672 ± 0.088 a | |
| W | 0 | 0.793 ± 0.005 a | 155.1 ± 1.9 a | 611.6 ± 1.1 a | 0.699 ± 0.023 a |
| 3 | 0.815 ± 0.005 a | 111.6 ± 1.1 b | 339.6 ± 2.9 g | 0.696 ± 0.037 a | |
| 7 | 0.763 ± 0.004 c | 116.2 ± 3.6 b | 366.5 ± 2.9 g | 0.692 ± 0.081 a | |
| 14 | 0.702 ± 0.003 b | 73.1 ± 0.9 d | 206.4 ± 4.1 f | 0.571 ± 0.061 a,d | |
| 21 | 0.703 ± 0.008 b | 76.8 ± 4.1 d | 280.8 ± 3.7 d | 0.664 ± 0.012 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, H.M.; Pagels, F.; Melo, R.; Fort, A.; Sulpice, R.; Lopes, G.; Costa, I.; Sousa-Pinto, I. Light Spectra, a Promising Tool to Modulate Ulva lacinulata Productivity and Composition. Mar. Drugs 2024, 22, 404. https://doi.org/10.3390/md22090404
Amaro HM, Pagels F, Melo R, Fort A, Sulpice R, Lopes G, Costa I, Sousa-Pinto I. Light Spectra, a Promising Tool to Modulate Ulva lacinulata Productivity and Composition. Marine Drugs. 2024; 22(9):404. https://doi.org/10.3390/md22090404
Chicago/Turabian StyleAmaro, Helena M., Fernando Pagels, Rosa Melo, Antoine Fort, Ronan Sulpice, Graciliana Lopes, Isabel Costa, and Isabel Sousa-Pinto. 2024. "Light Spectra, a Promising Tool to Modulate Ulva lacinulata Productivity and Composition" Marine Drugs 22, no. 9: 404. https://doi.org/10.3390/md22090404
APA StyleAmaro, H. M., Pagels, F., Melo, R., Fort, A., Sulpice, R., Lopes, G., Costa, I., & Sousa-Pinto, I. (2024). Light Spectra, a Promising Tool to Modulate Ulva lacinulata Productivity and Composition. Marine Drugs, 22(9), 404. https://doi.org/10.3390/md22090404









