Influence of Supercritical Fluid Extraction Process on Techno-Functionality of Enzymatically Derived Peptides from Filter-Pressed Shrimp Waste
Abstract
1. Introduction
2. Results and Discussion
2.1. Biochemical Properties of SPHs
2.2. FPLC/SEC-MS
2.3. Intrinsic Fluorescence
2.4. Surface Hydrophobicity (H0)
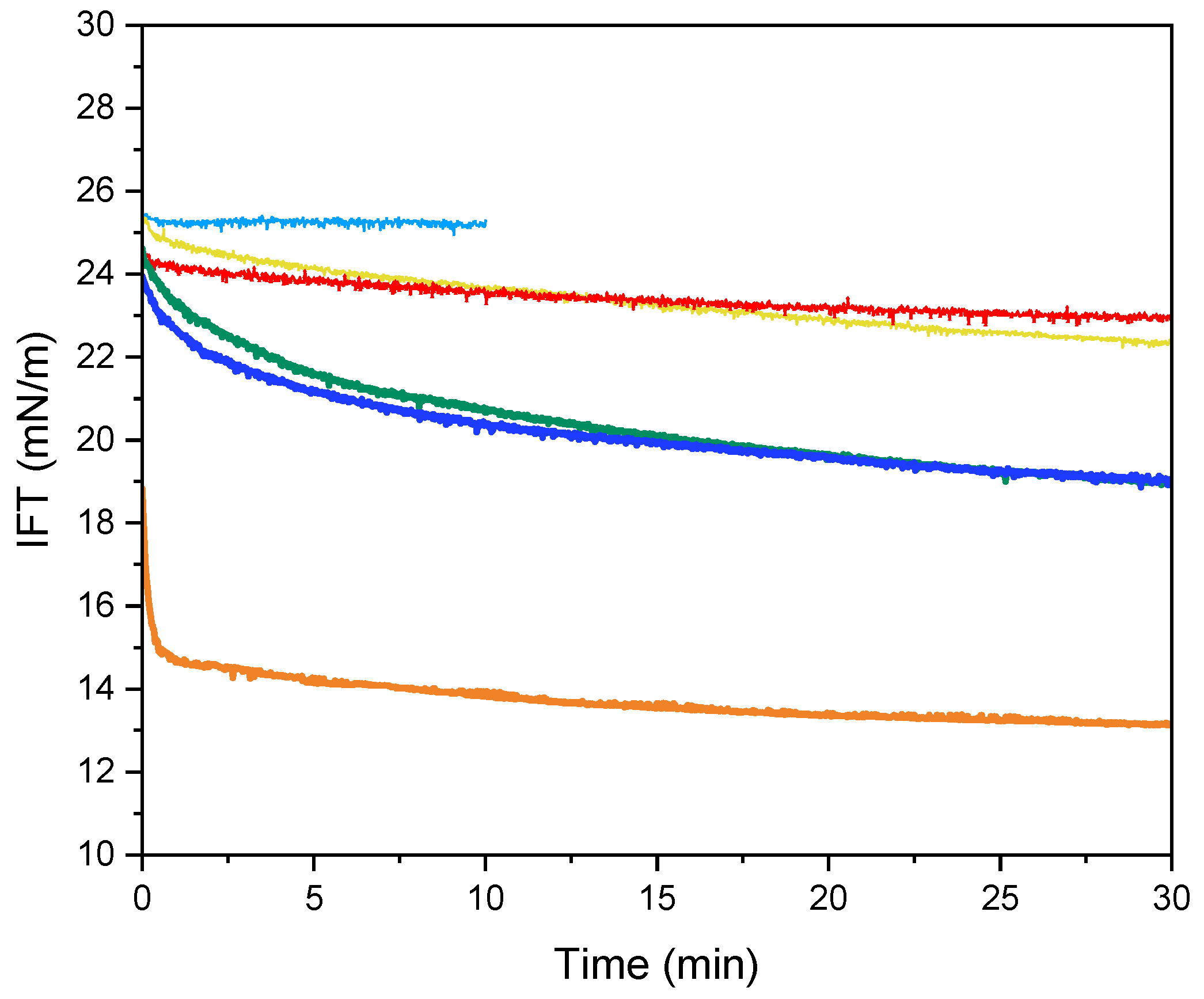
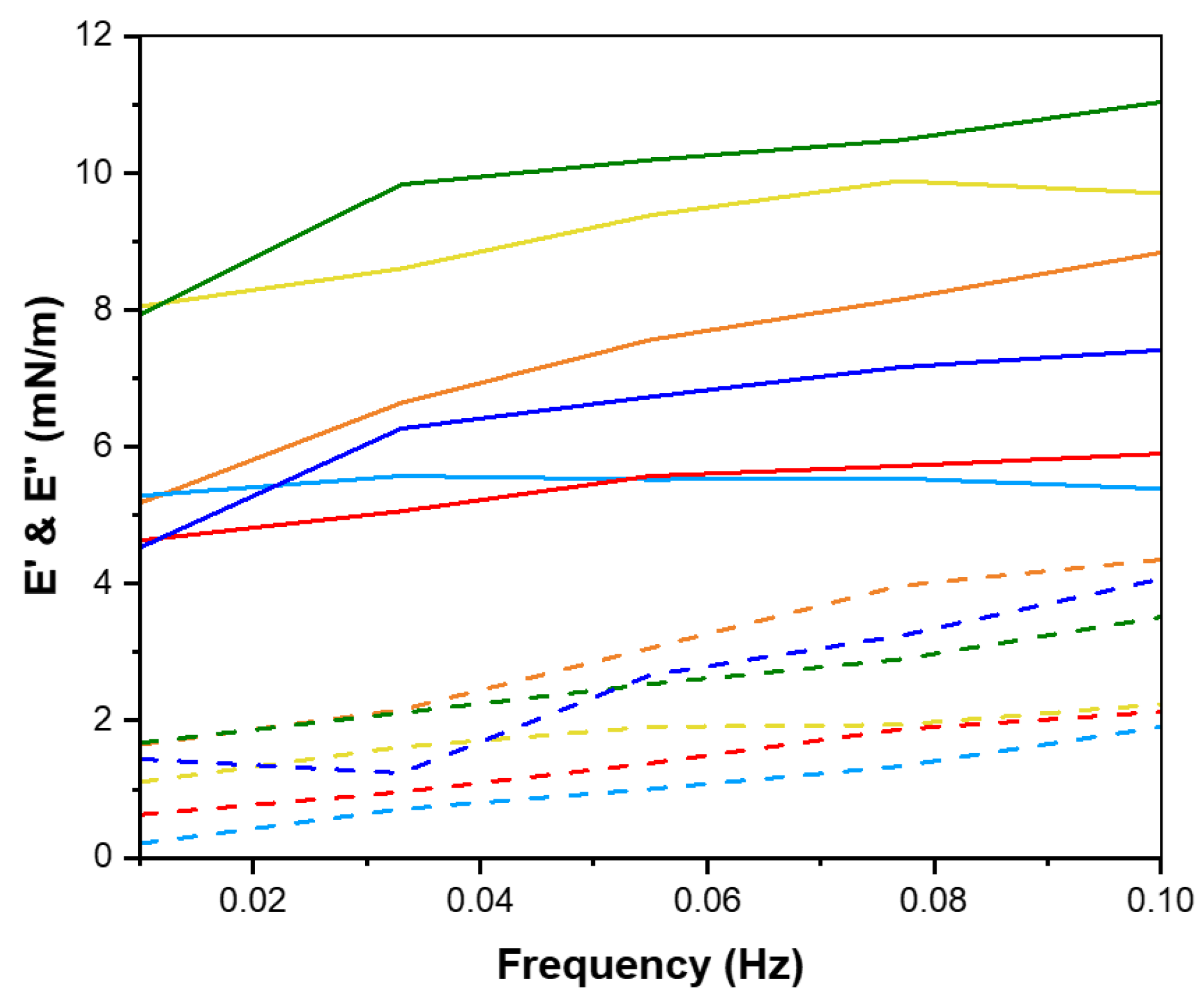
2.5. Interfacial Tension and Dilatational Rheology
2.6. Lissajous Plots
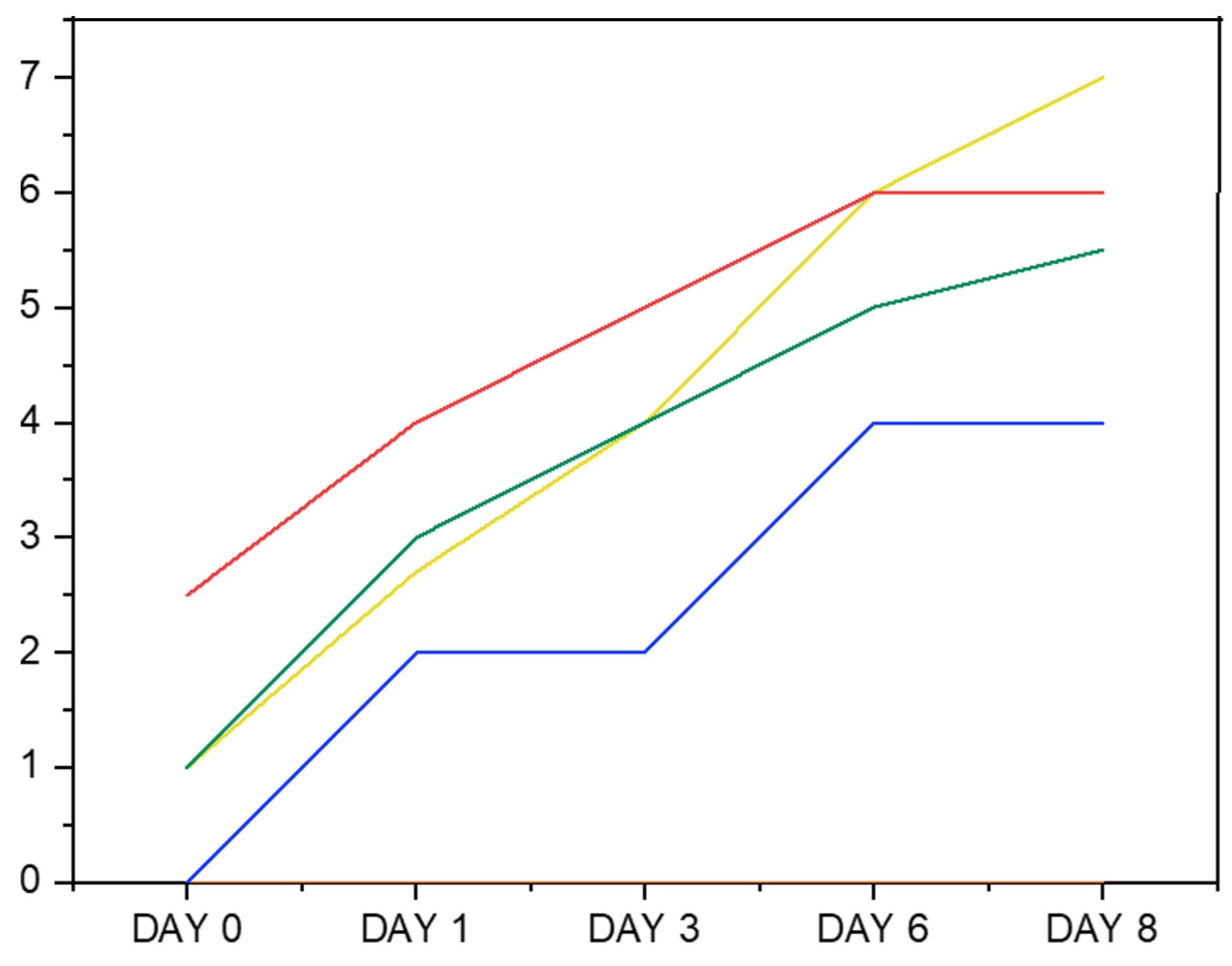
2.7. ζ-Potential, Droplet Size, and Creaming Index of Oil-in-Water Emulsions
3. Materials and Methods
3.1. Supercritical CO2 Extraction
3.2. Enzymatic Hydrolysis and Degree of Hydrolysis
3.3. Protein Content (Based on Total Nitrogen Content), Total Amino Acid Composition, and Protein Recovery % (PRP)
3.4. Fast Protein Liquid Chromatography (FPLC)
3.5. Intrinsic Fluorescence Emission Spectroscopy
3.6. Surface Hydrophobicity (H0)
3.7. Interfacial Tension and Dilatational Rheology
3.8. Emulsion Preparation and Storage Study
3.9. Determination of the Ζ-Potential, Particle Size, and Creaming Index (%)
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, C.E.; Davis, R.P.; McNevin, A.A. Perspectives on the Mangrove Conundrum, Land Use, and Benefits of Yield Intensification in Farmed Shrimp Production: A Review. J. World Aquac. Soc. 2022, 53, 8–46. [Google Scholar] [CrossRef]
- FAO. World Fisheries and Aquaculture the State of Sustainability in Action; FAO: Rome, Italy, 2018. [Google Scholar] [CrossRef]
- IMARC. Shrimp Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2020–2025. In Proceedings of the International Mining and Resources Conference (IMARC), Sydney, Australia, 21–23 October 2020. [Google Scholar]
- Dayakar, B.; Xavier, M.; Ngasotter, S.; Dhanabalan, V.; Porayil, L.; Balange, A.K.; Nayak, B.B. Extraction, Optimization, and Functional Quality Evaluation of Carotenoproteins from Shrimp Processing Side Streams through Enzymatic Process. Environ. Sci. Pollut. Res. 2023, 31, 62315–62328. [Google Scholar] [CrossRef] [PubMed]
- Rossi, N.; Grosso, C.; Delerue-Matos, C. Shrimp Waste Upcycling: Unveiling the Potential of Polysaccharides, Proteins, Carotenoids, and Fatty Acids with Emphasis on Extraction Techniques and Bioactive Properties. Mar. Drugs 2024, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Kandra, P.; Challa, M.M.; Kalangi Padma Jyothi, H. Efficient Use of Shrimp Waste: Present and Future Trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Jafarpour, A.; Sales Queiroz, L.; Casanova, F.; Badfar, N.; Jacobsen, C.; Jessen, F.; Sloth, J.J.; Petersen, H.O.; Knudsen, M.; Hansen, P.B.; et al. Biochemical and Physicochemical Properties of Shrimp (Pandalus borealis) Compounds after Compact Filter Press Process. JSFA Rep. 2024, 4, 135–147. [Google Scholar] [CrossRef]
- Jafarpour, A.; Gomes, R.M.; Gregersen, S.; Sloth, J.J.; Jacobsen, C.; Moltke Sørensen, A.D. Characterization of Cod (Gadus Morhua) Frame Composition and Its Valorization by Enzymatic Hydrolysis. J. Food Compos. Anal. 2020, 89, 103469. [Google Scholar] [CrossRef]
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering Food Matrix and Gastrointestinal Effects in Enhancing Bioactive Peptide Absorption and Bioavailability. J. Funct. Foods 2020, 64, 103680. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The Effects of Limited Enzymatic Hydrolysis on the Physicochemical and Emulsifying Properties of a Lentil Protein Isolate. Food Res. Int. 2013, 51, 162–169. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the Production of Bioactive Peptides: A Review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [PubMed]
- Yesiltas, B.; García-Moreno, P.J.; Gregersen, S.; Olsen, T.H.; Jones, N.C.; Hoffmann, S.V.; Marcatili, P.; Overgaard, M.T.; Hansen, E.B.; Jacobsen, C. Antioxidant Peptides Derived from Potato, Seaweed, Microbial and Spinach Proteins: Oxidative Stability of 5% Fish Oil-in-Water Emulsions. Food Chem. 2022, 385, 132699. [Google Scholar] [CrossRef] [PubMed]
- Fadimu, G.J.; Le, T.T.; Gill, H.; Farahnaky, A.; Olatunde, O.O.; Truong, T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods 2022, 11, 1823. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, Q.; Zhou, X.; Tang, C.; Chen, Y.; Cai, Z. Changes in Protein Structure and Physicochemical Properties of Egg White by Super Critical Carbon Dioxide Treatment. J. Food Eng. 2020, 284, 110076. [Google Scholar] [CrossRef]
- Xu, Y.; Bajaj, M.; Schneider, R.; Grage, S.L.; Ulrich, A.S.; Winter, J.; Gallert, C. Transformation of the Matrix Structure of Shrimp Shells during Bacterial Deproteination and Demineralization. Microb. Cell Fact. 2013, 12, 90. [Google Scholar] [CrossRef]
- Díaz-Rojas, E.I.; Argüelles-Monal, W.M.; Higuera-Ciapara, I.; Hernández, J.; Lizardi-Mendoza, J.; Goycoolea, F.M. Determination of Chitin and Protein Contents During the Isolation of Chitin from Shrimp Waste. Macromol. Biosci. 2006, 6, 340–347. [Google Scholar] [CrossRef]
- Williams, A.P. Enzymic Hydrolysis of Food Proteins. Food Chem. 1987, 26, 81–82. [Google Scholar] [CrossRef]
- Gregersen Echers, S.; Jafarpour, A.; Yesiltas, B.; García-Moreno, P.J.; Greve-Poulsen, M.; Hansen, D.K.; Jacobsen, C.; Overgaard, M.T.; Hansen, E.B. Targeted Hydrolysis of Native Potato Protein: A Novel Workflow for Obtaining Hydrolysates with Improved Interfacial Properties. Food Hydrocoll. 2023, 137, 108299. [Google Scholar] [CrossRef]
- Kishimura, H.; Tokuda, Y.; Yabe, M.; Klomklao, S.; Benjakul, S.; Ando, S. Trypsins from the Pyloric Ceca of Jacopever (Sebastes Schlegelii) and Elkhorn Sculpin (Alcichthys alcicornis): Isolation and Characterization. Food Chem. 2007, 100, 1490–1495. [Google Scholar] [CrossRef][Green Version]
- Ghorbel-Bellaaj, O.; Maalej, H.; Nasri, M.; Jellouli, K. Fermented Shrimp Waste Hydrolysates: Promising Source of Functional Molecules with Antioxidant Properties. J. Culin. Sci. Technol. 2018, 16, 357–377. [Google Scholar] [CrossRef]
- Mao, Y.; Krischke, M.; Hengst, C.; Kulozik, U. Comparison of the Influence of PH on the Selectivity of Free and Immobilized Trypsin for β-Lactoglobulin Hydrolysis. Food Chem. 2018, 253, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Opheim, M.; Šližytė, R.; Sterten, H.; Provan, F.; Larssen, E.; Kjos, N.P. Hydrolysis of Atlantic Salmon (Salmo Salar) Rest Raw Materials—Effect of Raw Material and Processing on Composition, Nutritional Value, and Potential Bioactive Peptides in the Hydrolysates. Process Biochem. 2015, 50, 1247–1257. [Google Scholar] [CrossRef]
- Park, J.-Y.; Back, S.-S.; Chun, B.-S. Fatty Acids and Protein Recovery of Squid Viscera with Supercritical Carbon Dioxide. J. Mar. Biosci. Biotechnol. 2006, 1, 206–212. [Google Scholar]
- Pattanaik, S.S.; Sawant, P.B.; Xavier, K.A.M.; Dube, K.; Srivastava, P.P.; Dhanabalan, V.; Chadha, N.K. Characterization of Carotenoprotein from Different Shrimp Shell Waste for Possible Use as Supplementary Nutritive Feed Ingredient in Animal Diets. Aquaculture 2020, 515, 734594. [Google Scholar] [CrossRef]
- Ghidalia, W. Integument, Pigments, and Hormonal Processes: Volume 9: Integument, Pigments—Google Books. Available online: https://books.google.dk/books?hl=en&lr=&id=JG1lh--v1sYC&oi=fnd&pg=PA301&dq=Structural+and+Biological+Aspects+of+Pigments+walter+Ghidalia&ots=aLQlXjXlsn&sig=OZXO9FcdF0RKAK2Tua9AvID8ueY&redir_esc=y#v=onepage&q=Structural%20and%20Biological%20Aspects%20of%20Pigments%20walter%20Ghidalia&f=false (accessed on 7 March 2024).
- Sheikh, M.A.; Saini, C.S.; Sharma, H.K. Supercritical Carbon Dioxide Treatment of Plum (Prunus domestica L.) Kernel Protein Isolate: Impact on Structural, Thermal, Physical, and Functional Properties. Sustain. Chem. Pharm. 2023, 32, 100979. [Google Scholar] [CrossRef]
- Khiari, Z.; Ndagijimana, M.; Betti, M. Low Molecular Weight Bioactive Peptides Derived from the Enzymatic Hydrolysis of Collagen after Isoelectric Solubilization/Precipitation Process of Turkey by-Products. Poult. Sci. 2014, 93, 2347–2362. [Google Scholar] [CrossRef]
- Batista, S.; Pintado, M.; Marques, A.; Abreu, H.; Silva, J.L.; Jessen, F.; Tulli, F.; Valente, L.M.P. Use of Technological Processing of Seaweed and Microalgae as Strategy to Improve Their Apparent Digestibility Coefficients in European Seabass (Dicentrarchus labrax) Juveniles. J. Appl. Phycol. 2020, 32, 3429–3446. [Google Scholar] [CrossRef]
- Vorob’ev, M.M.; Vogel, V.; Güler, G.; Mäntele, W. Monitoring of Demasking of Peptide Bonds During Proteolysis by Analysis of the Apparent Spectral Shift of Intrinsic Protein Fluorescence. Food Biophys. 2011, 6, 519–526. [Google Scholar] [CrossRef]
- Peyrano, F.; Speroni, F.; Avanza, M.V. Physicochemical and Functional Properties of Cowpea Protein Isolates Treated with Temperature or High Hydrostatic Pressure. Innov. Food Sci. Emerg. Technol. 2016, 33, 38–46. [Google Scholar] [CrossRef]
- Cui, Q.; Sun, Y.; Zhou, Z.; Cheng, J.; Guo, M. Effects of Enzymatic Hydrolysis on Physicochemical Properties and Solubility and Bitterness of Milk Protein Hydrolysates. Foods 2021, 10, 2462. [Google Scholar] [CrossRef]
- Chao, D.; He, R.; Jung, S.; Aluko, R.E. Effect of Pressure or Temperature Pretreatment of Isolated Pea Protein on Properties of the Enzymatic Hydrolysates. Food Res. Int. 2013, 54, 1528–1534. [Google Scholar] [CrossRef]
- Hui, Y.; Xue, X.; Xuesong, Z.; Yan, W. Intrinsic Fluorescence Spectra of Tryptophan, Tyrosine and Phenyloalanine. In Proceedings of the 5th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2015), Shenzhen, China, 19–20 September 2015; Atlantis Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Murtaza, A.; Iqbal, A.; Linhu, Z.; Liu, Y.; Xu, X.; Pan, S.; Hu, W. Effect of High-Pressure Carbon Dioxide on the Aggregation and Conformational Changes of Polyphenol Oxidase from Apple (Malus Domestica) Juice. Innov. Food Sci. Emerg. Technol. 2019, 54, 43–50. [Google Scholar] [CrossRef]
- Moro, A.; Gatti, C.; Delorenzi, N. Hydrophobicity of Whey Protein Concentrates Measured by Fluorescence Quenching and Its Relation with Surface Functional Properties. J. Agric. Food Chem. 2001, 49, 4784–4789. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, E.; Nakai, S. The Relationship between Hydrophobicity and Interfacial Tension of Proteins. Biochimica Biophysica Acta (BBA)-Protein Structure 1979, 576, 269–279. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of Ultrasound on Structural and Physical Properties of Soy Protein Isolate (SPI) Dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Zang, X.; Yue, C.; Wang, Y.; Shao, M.; Yu, G. Effect of Limited Enzymatic Hydrolysis on the Structure and Emulsifying Properties of Rice Bran Protein. J. Cereal Sci. 2019, 85, 168–174. [Google Scholar] [CrossRef]
- Lima, J.C.; Seixas, F.A.V.; Coimbra, J.S.R.; Pimentel, T.C.; Barão, C.E.; Cardozo-Filho, L. Continuous Fractionation of Whey Protein Isolates by Using Supercritical Carbon Dioxide. J. CO2 Util. 2019, 30, 112–122. [Google Scholar] [CrossRef]
- Ding, L.; Lu, L.; Sheng, L.; Tang, C.; Chen, Y.; Cai, Z. Mechanism of Enhancing Foaming Properties of Egg White by Super Critical Carbon Dioxide Treatment. Food Chem. 2020, 317, 126349. [Google Scholar] [CrossRef]
- Gultekin Subasi, B.; Yildirim-Elikoğlu, S.; Altay, İ.; Jafarpour, A.; Casanova, F.; Mohammadifar, M.A.; Capanoglu, E. Influence of Non-Thermal Microwave Radiation on Emulsifying Properties of Sunflower Protein. Food Chem. 2022, 372, 131275. [Google Scholar] [CrossRef]
- Yesiltas, B.; Gregersen, S.; Lægsgaard, L.; Brinch, M.L.; Olsen, T.H.; Marcatili, P.; Overgaard, M.T.; Hansen, E.B.; Jacobsen, C.; García-Moreno, P.J. Emulsifier Peptides Derived from Seaweed, Methanotrophic Bacteria, and Potato Proteins Identified by Quantitative Proteomics and Bioinformatics. Food Chem. 2021, 362, 130217. [Google Scholar] [CrossRef]
- Amine, C.; Dreher, J.; Helgason, T.; Tadros, T. Investigation of Emulsifying Properties and Emulsion Stability of Plant and Milk Proteins Using Interfacial Tension and Interfacial Elasticity. Food Hydrocoll. 2014, 39, 180–186. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Jacobsen, C.; Marcatili, P.; Gregersen, S.; Overgaard, M.T.; Andersen, M.L.; Sørensen, A.-D.M.; Hansen, E.B. Emulsifying Peptides from Potato Protein Predicted by Bioinformatics: Stabilization of Fish Oil-in-Water Emulsions. Food Hydrocoll. 2020, 101, 105529. [Google Scholar] [CrossRef]
- Schröder, A.; Berton-Carabin, C.; Venema, P.; Cornacchia, L. Interfacial Properties of Whey Protein and Whey Protein Hydrolysates and Their Influence on O/W Emulsion Stability. Food Hydrocoll. 2017, 73, 129–140. [Google Scholar] [CrossRef]
- Yesiltas, B.; García-Moreno, P.J.; Mikkelsen, R.K.; Echers, S.G.; Hansen, D.K.; Greve-Poulsen, M.; Hyldig, G.; Hansen, E.B.; Jacobsen, C. Physical and Oxidative Stability of Emulsions Stabilized with Fractionated Potato Protein Hydrolysates Obtained from Starch Production Side Stream. Antioxidants 2023, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Sagis, L.; Schroën, K. Formation, Structure, and Functionality of Interfacial Layers in Food Emulsions. Annu. Rev. Food Sci. Technol. 2018, 9, 551–587. [Google Scholar] [CrossRef]
- Lei, J.; Gao, Y.; Ma, Y.; Zhao, K.; Du, F. Improving the Emulsion Stability by Regulation of Dilational Rheology Properties. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123906. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Yang, J.; Gregersen, S.; Jones, N.C.; Berton-Carabin, C.C.; Sagis, L.M.C.; Hoffmann, S.V.; Marcatili, P.; Overgaard, M.T.; Hansen, E.B.; et al. The Structure, Viscoelasticity and Charge of Potato Peptides Adsorbed at the Oil-Water Interface Determine the Physicochemical Stability of Fish Oil-in-Water Emulsions. Food Hydrocoll. 2021, 115, 106605. [Google Scholar] [CrossRef]
- Yang, J.; Thielen, I.; Berton-Carabin, C.C.; van der Linden, E.; Sagis, L.M.C. Nonlinear Interfacial Rheology and Atomic Force Microscopy of Air-Water Interfaces Stabilized by Whey Protein Beads and Their Constituents. Food Hydrocoll. 2020, 101, 105466. [Google Scholar] [CrossRef]
- Badfar, N.; Abdollahi, M.; Stubbe, P.R.; Jafarpour, A. Texture and Viscoelastic Characteristics of Silver Carp (Hypophthalmichthys Molitrix) Surimi Affected by Combination of Washing Regimes and Hydrogen Peroxide. J. Texture Stud. 2022, 53, 490–502. [Google Scholar] [CrossRef]
- Dickinson, E. Mixed Biopolymers at Interfaces: Competitive Adsorption and Multilayer Structures. Food Hydrocoll. 2011, 25, 1966–1983. [Google Scholar] [CrossRef]
- Tamm, F.; Drusch, S. Impact of Enzymatic Hydrolysis on the Interfacial Rheology of Whey Protein/Pectin Interfacial Layers at the Oil/Water-Interface. Food Hydrocoll. 2017, 63, 8–18. [Google Scholar] [CrossRef]
- Leser, M.E.; Acquistapace, S.; Cagna, A.; Makievski, A.V.; Miller, R. Limits of Oscillation Frequencies in Drop and Bubble Shape Tensiometry. Colloids Surf. A Physicochem. Eng. Asp. 2005, 261, 25–28. [Google Scholar] [CrossRef]
- Cascão Pereira, L.G.; Théodoly, O.; Blanch, H.W.; Radke, C.J. Dilatational Rheology of BSA Conformers at the Air/Water Interface. Langmuir 2003, 19, 2349–2356. [Google Scholar] [CrossRef]
- Böttcher, S.; Keppler, J.K.; Drusch, S. Mixtures of Quillaja Saponin and Beta-Lactoglobulin at the Oil/Water-Interface: Adsorption, Interfacial Rheology and Emulsion Properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 46–56. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, X.; Gan, K.; Chen, Y.; Xu, J.; Yang, J. Effect of Zeta Potential on Properties of Foamed Colloidal Suspension. J. Eur. Ceram. Soc. 2019, 39, 574–583. [Google Scholar] [CrossRef]
- Bhatt, N.; Prasad, R.K.; Singh, K.; Panpalia, G.M. Stability Study of O/W Emulsions Using Zeta Potential. J. Chem. Pharm. Res. 2010, 2, 512–527. [Google Scholar]
- Queiroz, L.S.; Casanova, F.; Feyissa, A.H.; Jessen, F.; Ajalloueian, F.; Perrone, I.T.; de Carvalho, A.F.; Mohammadifar, M.A.; Jacobsen, C.; Yesiltas, B. Physical and Oxidative Stability of Low-Fat Fish Oil-in-Water Emulsions Stabilized with Black Soldier Fly (Hermetia Illucens) Larvae Protein Concentrate. Foods 2021, 10, 2977. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, C.Y.; Wang, S.T.; Li, Y.Q.; Mo, H.Z.; He, J.X. Physicochemical Properties and Antioxidant Activities of Tree Peony (Paeonia Suffruticosa Andr.) Seed Protein Hydrolysates Obtained with Different Proteases. Food Chem. 2021, 345, 128765. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. Comparison of Batch and Continuous Ultrasonic Emulsification Processes. J. Food Eng. 2015, 167, 114–121. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Dependence of Creaming and Rheology of Monodisperse Oil-in-Water Emulsions on Droplet Size and Concentration. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 79–86. [Google Scholar] [CrossRef]
- Tadros, T.F. Applied Surfactants: Principles and Applications; Wiley: Hoboken, NJ, USA, 2005; p. 634. [Google Scholar]
- Bjørlie, M.; Hartmann, J.C.; Rasmussen, L.H.; Yesiltas, B.; Sørensen, A.-D.M.; Gregersen Echers, S.; Jacobsen, C. Screening for Metal-Chelating Activity in Potato Protein Hydrolysates Using Surface Plasmon Resonance and Peptidomics. Antioxidants 2024, 13, 346. [Google Scholar] [CrossRef] [PubMed]





| Sample | DH (%) | Protein Content (%) (Based on Total Nitrogen Content (%)) | Protein Recovery (%) | Yield (%) |
|---|---|---|---|---|
| PC-ALC | 18.10 ± 0.14 a | 85.69 ± 0.62 b | 63.49 ± 0.41 a | 24.73 ± 0.70 a |
| PC-TRYP | 14.50 ± 1.27 a,b | 88.60 ± 0.23 a | 42.99 ± 0.19 d | 19.41 ± 0.25 c |
| SC-ALC | 17.05 ± 1.20 a | 80.89 ± 0.92 d | 52.30 ± 0.76 b | 21.00 ± 0.20 b |
| SC-TRYP | 11.85 ± 0.64 b | 83.92 ± 0.38 c | 50.26 ± 0.21 c | 18.78 ± 0.14 d |
| AAs * | PC-300 | SC-300 | SC-ALC | SC-TRYP | PC-ALC | PC-TRYP | |
|---|---|---|---|---|---|---|---|
| Hydrophobic Amino Acids | TRP | 16.29 ± 1.55 | 16.20 ± 2.01 | 8.71 ± 0.25 | 18.79 ± 0.43 | 41.74 ± 3.81 | 33.74 ± 1.48 |
| PHE | 12.43 ± 0.28 | 10.38 ± 0.54 | 48.25 ± 17.43 | 58.78 ± 0.79 | 41.73 ± 3.72 | 32.45 ± 1.00 | |
| LEU | 12.22 ± 1.68 | 8.14 ± 0.79 | 47.04 ± 7.35 | 56.65 ± 0.67 | 70.00 ± 5.64 | 58.63 ± 1.11 | |
| ILE | 17.84 ± 1.20 | 16.32 ± 1.12 | 33.29 ± 8.26 | 44.85 ± 7.69 | 50.10 ± 5.00 | 40.71 ± 0.72 | |
| MET | 7.24 ± 0.91 | 6.28 ± 0.55 | 14.21 ± 0.03 | 22.12 ± 1.09 | 23.35 ± 2.46 | 18.21 ± 0.40 | |
| VAL | 18.05 ± 1.01 | 18.76 ± 1.82 | 44.70 ± 8.04 | 53.61 ± 0.69 | 53.80 ± 5.64 | 44.86 ± 1.60 | |
| TYR | 16.20 ± 0.26 | 16.29 ± 1.55 | 32.53 ± 1.61 | 38.69 ± 0.21 | 41.74 ± 3.81 | 33.74 ± 1.48 | |
| ALA | 27.02 ± 1.73 | 26.12 ± 1.81 | 89.93 ± 0.95 | 52.90 ± 2.65 | 65.25 ± 4.28 | 55.84 ± 1.17 | |
| PRO | 16.84 ± 1.49 | 16.82 ± 1.28 | 39.23 ± 0.18 | 36.12 ± 4.02 | 42.38 ± 2.02 | 38.15 ± 0.40 | |
| Hydrophilic Amino Acids | THR | 28.50 ± 2.36 | 28.16 ± 1.56 | 58.24 ± 1.24 | 68.60 ± 1.99 | 77.06 ± 5.68 | 66.14 ± 1.02 |
| HIS | 23.75 ± 0.99 | 26.46 ± 0.43 | 73.57 ± 4.43 | 55.71 ± 0.53 | 68.69 ± 4.43 | 68.17 ± 4.38 | |
| LYS | 20.60 ± 1.30 | 19.52 ± 2.37 | 68.98 ± 1.70 | 60.97 ± 1.01 | 69.46 ± 12.35 | 63.38 ± 1.55 | |
| ARG | 31.21 ± 3.81 | 35.77 ± 3.88 | 75.22 ± 2.50 | 74.06 ± 0.13 | 72.81 ± 6.13 | 68.26 ± 10.13 | |
| SER | 21.62 ± 0.54 | 20.63 ± 4.72 | 47.66 ± 0.96 | 58.56 ± 4.38 | 52.69 ± 5.04 | 46.75 ± 0.09 | |
| HYP | 0.29 ± 0.06 | 0.20 ± 0.01 | 1.97 ± 0.07 | 3.43 ± 0.19 | 0.97 ± 0.07 | 1.39 ± 0.17 | |
| GLY | 9.87 ± 0.88 | 9.59 ± 0.58 | 49.90 ± 0.07 | 40.54 ± 0.70 | 26.91 ± 1.48 | 22.67 ± 0.01 | |
| GLU | 79.08 ± 9.21 | 91.94 ± 16.45 | 166.63 ± 1.84 | 143.23 ± 3.63 | 215.99 ± 3.24 | 192.34 ± 4.64 | |
| ASP | 2.08 ± 0.90 | 7.29 ± 1.52 | 79.98 ± 1.78 | 77.16 ± 0.38 | 48.51 ± 36.30 | 39.32 ± 2.36 | |
| C-C | 0.91 ± 0.55 | 1.74 ± 0.73 | 2.07 ± 0.01 | 3.67 ± 0.70 | 5.33 ± 0.88 | 2.10 ± 0.87 | |
| SUM | 966.80 ± 32.72 | 833.90 ± 5.06 | 984.10 ± 13.67 | 968.44 ± 32.33 | 362.04 ± 30.71 | 376.61 ± 43.72 |
| Emulsion | Size | ζ-Potential (mV) 0.2% Protein | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.2% Peptide | 0.4% Peptide | ||||||||
| D (4,3) (μm) | D (3,2) (μm) | D (4,3) (μm) | D (3,2) (μm) | ||||||
| Day 1 | Day 8 | Day 1 | Day 8 | Day 1 | Day 8 | Day 1 | Day 8 | Day 1 | |
| PC-ALC | 55.0 ± 2.5 b | 71.0 ± 0.3 a | 13.0 ± 1.3 b | 18.0 ± 1.8 c | 28.7±5.1 b | 43.8 ± 4.4 a | 7.0 ± 0.1 a | 18.0 ± 3.0 a | −25.1±7.2 a |
| PC-TRYP | 23.0 ± 2.8 c | 28.0 ± 6.6 c | 5.0 ± 1.3 c | 42.0 ± 3.3 a | 18.1 ± 2.3 c | 45.1 ± 3.4 a | 1.6 ± 0.1 b | 7.1 ± 3.9 b | −38.6 ± 0.0 b |
| SC-ALC | 67.0 ± 0.1 a | 69.0 ± 3.9 a | 21.0 ± 3.4 a | 27.0 ± 1.8b | 35.8 ± 3.2 a | 50.8 ± 3.2a | 1.5 ± 0.3 b,c | 24.7 ± 2.4 a | −47.2 ± 4.6 c |
| SC-TRYP | 23.0 ± 0.7 c | 54.0 ± 1.1 b | 0.7 ± 0.0c d | 8.5 ± 2.6 d | 10.3 ± 0.1 d | 32.8 ± 2.1b | 1.2 ± 0.0c | 8.9 ± 6 b | −49.9 ± 3.5 c |
| Na-Cas | 0.37 ± 0.0 d | 2.18 ± 0.0 d | 0.18 ± 0.0 d | 0.20 ± 0.0 e | −43.7 ± 1.1 b,c | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badfar, N.; Jafarpour, A.; Casanova, F.; Sales Queiroz, L.; Tilahun Getachew, A.; Jacobsen, C.; Jessen, F.; Gringer, N. Influence of Supercritical Fluid Extraction Process on Techno-Functionality of Enzymatically Derived Peptides from Filter-Pressed Shrimp Waste. Mar. Drugs 2025, 23, 122. https://doi.org/10.3390/md23030122
Badfar N, Jafarpour A, Casanova F, Sales Queiroz L, Tilahun Getachew A, Jacobsen C, Jessen F, Gringer N. Influence of Supercritical Fluid Extraction Process on Techno-Functionality of Enzymatically Derived Peptides from Filter-Pressed Shrimp Waste. Marine Drugs. 2025; 23(3):122. https://doi.org/10.3390/md23030122
Chicago/Turabian StyleBadfar, Narjes, Ali Jafarpour, Federico Casanova, Lucas Sales Queiroz, Adane Tilahun Getachew, Charlotte Jacobsen, Flemming Jessen, and Nina Gringer. 2025. "Influence of Supercritical Fluid Extraction Process on Techno-Functionality of Enzymatically Derived Peptides from Filter-Pressed Shrimp Waste" Marine Drugs 23, no. 3: 122. https://doi.org/10.3390/md23030122
APA StyleBadfar, N., Jafarpour, A., Casanova, F., Sales Queiroz, L., Tilahun Getachew, A., Jacobsen, C., Jessen, F., & Gringer, N. (2025). Influence of Supercritical Fluid Extraction Process on Techno-Functionality of Enzymatically Derived Peptides from Filter-Pressed Shrimp Waste. Marine Drugs, 23(3), 122. https://doi.org/10.3390/md23030122








