Marine-Derived Alternariol Suppresses Inflammation by Regulating T Cell Activation and Migration
Abstract
1. Introduction
2. Results
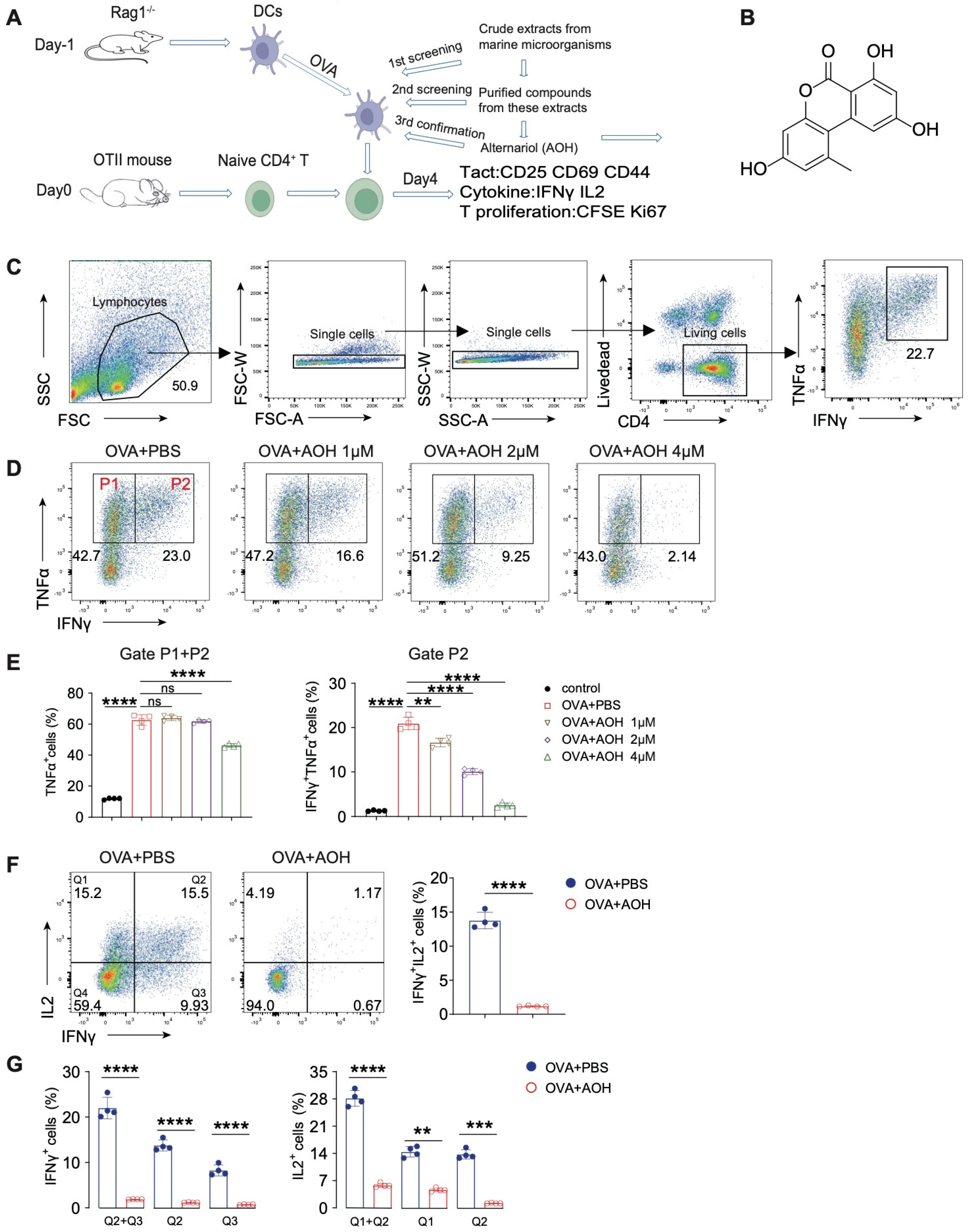
2.1. AOH Inhibits Proinflammatory Cytokine Production of T Cell In Vitro
2.2. AOH Affects T Cell Apoptosis and Inhibits T Cell Proliferation
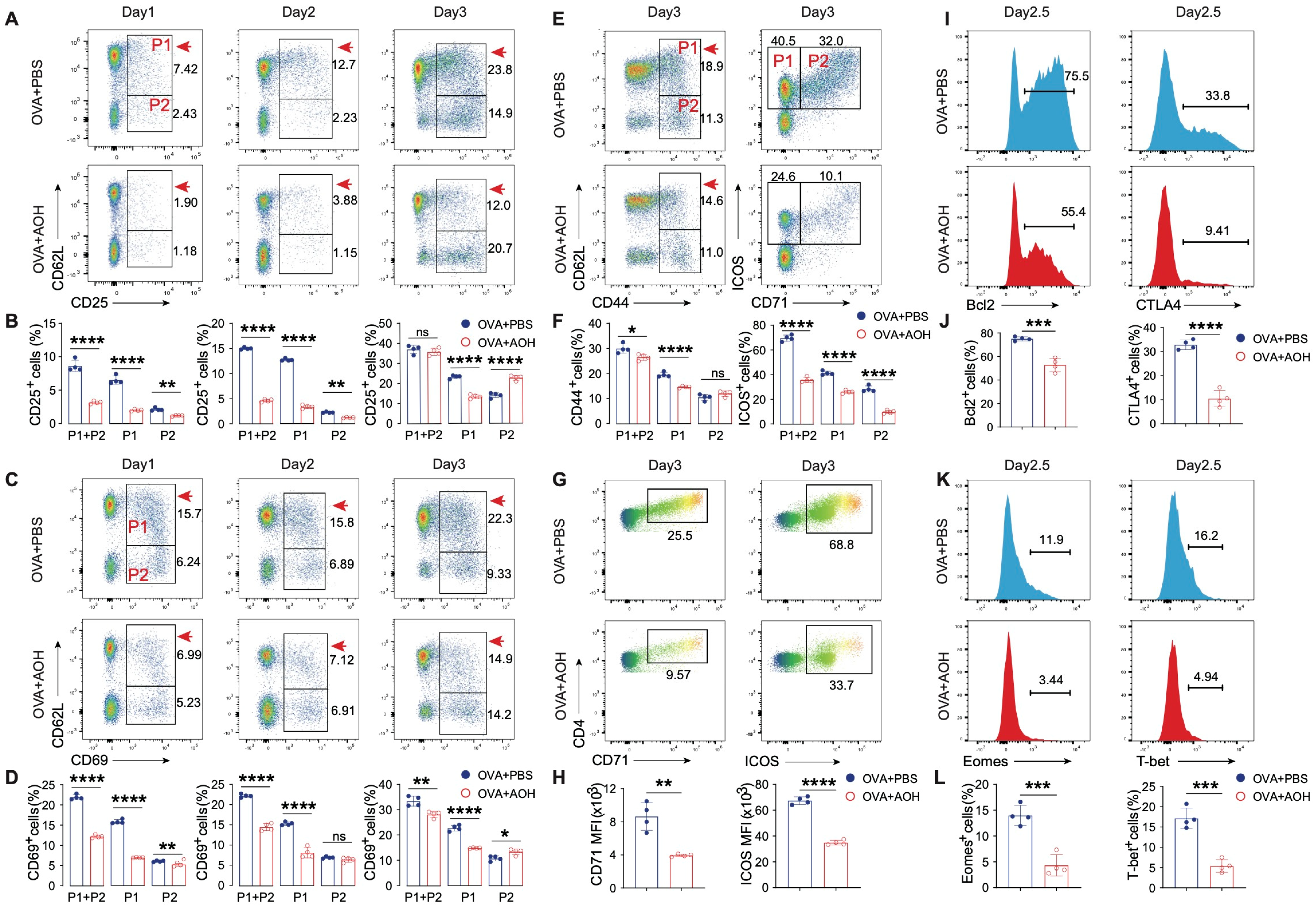
2.3. AOH Modulates Early T Cell Activation and Transcriptional Programming
2.4. AOH Targets Early T Cell Activation for Affecting T Cell Function Without Affecting Antigen Presentation
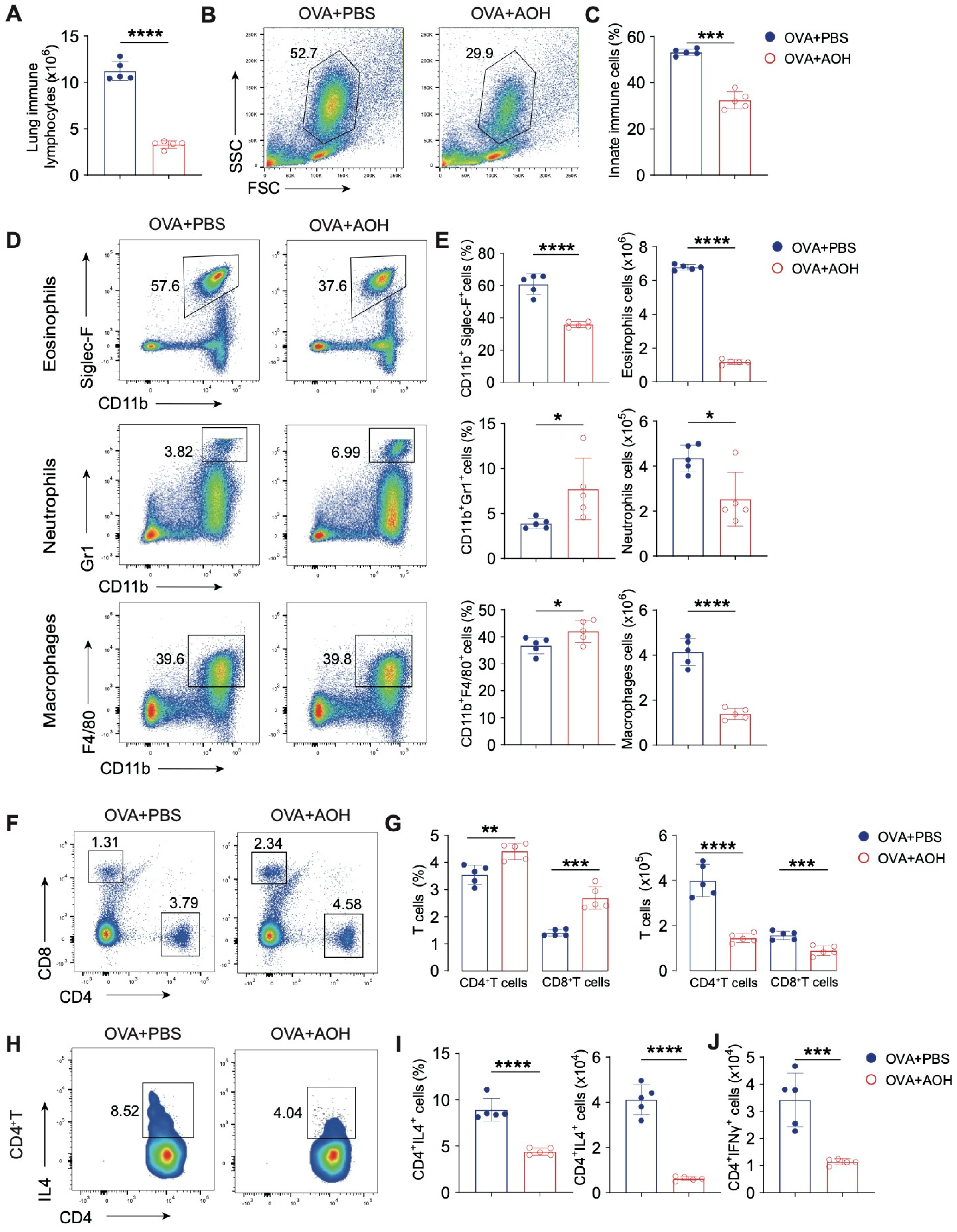
2.5. AOH Alleviates OVA-Induced Pulmonary Inflammation in Mice
2.6. AOH Suppresses T Cell Activation and Cytokine Production to Alleviate OVA- Induced Pulmonary Inflammation
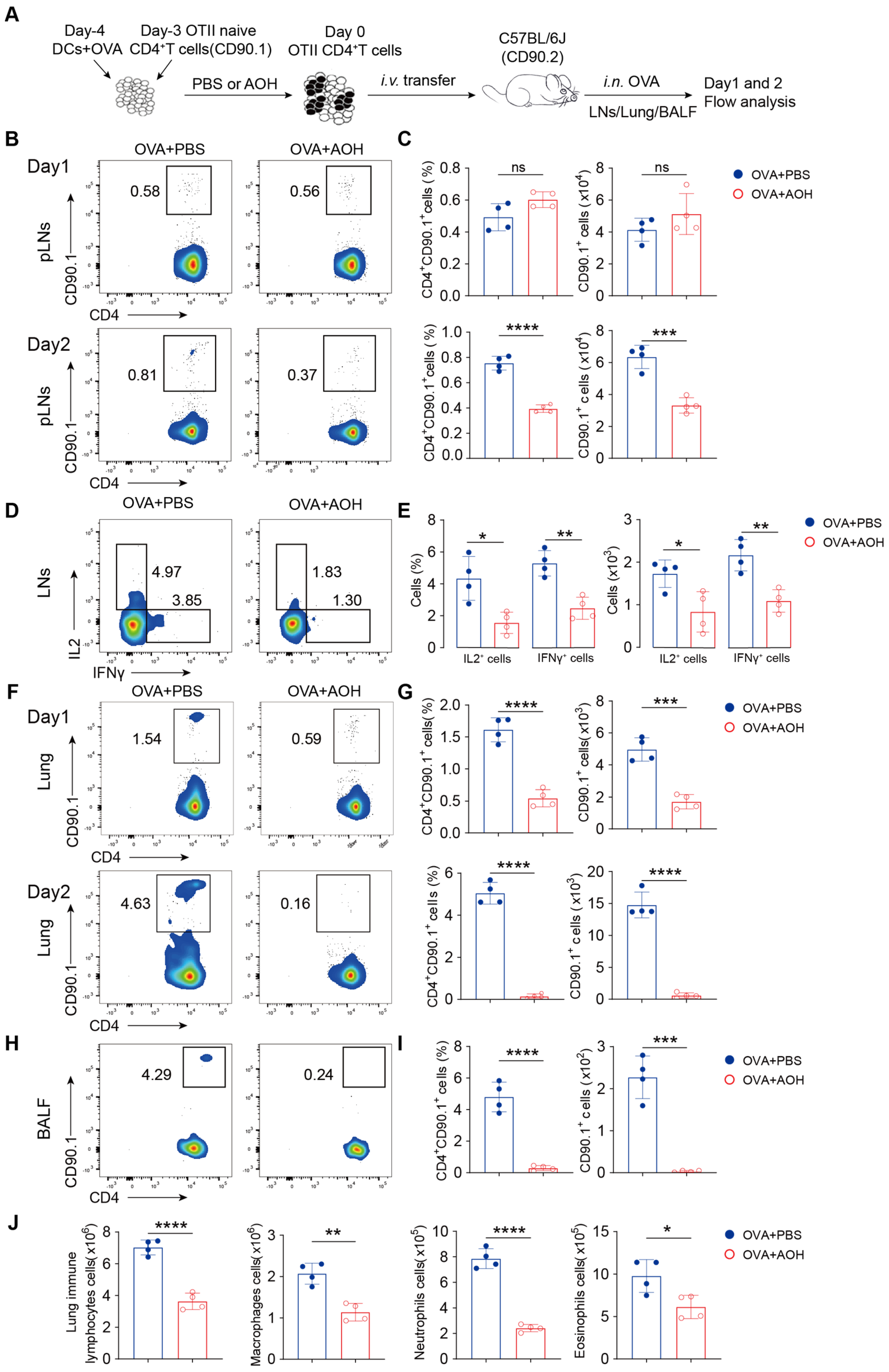
2.7. AOH Inhibits T Cell Migration to the Lung
3. Discussion
4. Materials and Methods
4.1. Alternariol Extraction, Isolation, and Purification
4.2. In Vitro Cell Models
4.3. Antibodies
4.4. Mouse Model
4.5. In Vivo Cell Migration Assay
4.6. Cell Acquisition
4.7. Flow Cytometry
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Giovanni, M.; Alessandro, M.; Alice, C.; Irene, M.; Silvia, S. Immune-mediated inflammatory diseases: Common and different pathogenic and clinical features. Autoimmun. Rev. 2023, 22, 103410. [Google Scholar]
- Søren, R.P.; Thomas, P.; Seth, L.M.; Trine, H.M. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat. Rev. Immunol. 2020, 21, 137–150. [Google Scholar]
- Ruslan, M.J.N. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar]
- Alessandro, L.; Domenico, F.; Marina, F.; Alessandra, P.; Sergio, O.; Mauro, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar]
- Li, W.S.; Zhang, Q.Q.; Li, Q.; Liu, S.Y.; Yuan, G.Q.; Pan, Y.W. Innate immune response restarts adaptive immune response in tumors. Front. Immunol. 2023, 14, 1260705. [Google Scholar] [CrossRef]
- Katherine, A.D.; Russell, E.V. Inflammasomes and adaptive immune responses. Nat. Immunol. 2021, 22, 412–422. [Google Scholar]
- Watanabe, S.; Alexander, M.; Misharin, A.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal. Transduct. Target. Ther. 2023, 8, 235. [Google Scholar]
- Bastian, K.; Anthony, C.B.; Naveen, S.; Andreas, D.B.; Susan, G.; Kristin, K.; Joanna, P.; Johannes, P.; Paulina, D.; Miriam, M.; et al. Cd4(+) t cell-induced inflammatory cell death controls immune-evasive tumours. Nature 2023, 618, 1033–1040. [Google Scholar]
- Asuka, T.; Kentaro, I.; Yuka, T.; Yu, S.; Marlen, D.; Hiroyuki, T.; Masahiro, O.; Hideki, O. Evaluation of T-cell immune status of reduced-dose cyclosporine and everolimus combination therapy in kidney transplant patients. Transplant Proc. 2023, 55, 797–802. [Google Scholar]
- Tobias, R.; Sumanta, B.; Andreas, S.M.; Steffen, P.; Falk, S.; Christopher, N.; Christina, B.S.; Alice, W.; Michael, H.; Thomas, M.; et al. Alemtuzumab-induced immune phenotype and repertoire changes: Implications for secondary autoimmunity. Brain 2022, 145, 1711–1725. [Google Scholar]
- Gou, Q.; Dong, C.; Xu, H.; Khan, B.; Jin, J.; Liu, Q.; Shi, J.; Hou, Y. PD-L1 degradation pathway and immunotherapy for cancer. Cell. Death Dis. 2020, 11, 955. [Google Scholar] [CrossRef]
- Renée, B.; Tom, V.M.; Edwin, B. CD47-sirpα blocking-based immunotherapy: Current and prospective therapeutic strategies. Clin. Transl. Med. 2022, 12, e943. [Google Scholar]
- Jiri, P.; Eugenie, N.; Kamil, K.; Wenda, W. Cyclosporine a: Chemistry and toxicity—A review. Curr. Med. Chem. 2020, 28, 3925–3934. [Google Scholar]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs. 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Montuori, E.; De Pascale, D.; Lauritano, C. Recent discoveries on marine organism immunomodulatory activities. Mar. Drugs. 2022, 20, 422. [Google Scholar] [CrossRef]
- Shih-Chao, L.; Caitlin, W.L.; Allison, K.S.; Lauren, P.; Nicole, B.; Jeffrey, L.C.W.; Mikell, P.; Wendy, K.S.; Kylene, K.H. Homoseongomycin, a compound isolated from marine actinomycete bacteria k3-1, is a potent inhibitor of encephalitic alphaviruses. Antiviral. Res. 2021, 191, 105087. [Google Scholar]
- Kim, E.N.; Gao, M.; Choi, H.; Jeong, G.S. Marine microorganism-derived macrolactins inhibit inflammatory mediator effects in LPS-induced macrophage and microglial cells by regulating bach1 and HO-1/Nrf2 signals through inhibition of tlr4 activation. Molecules 2020, 25, 656. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, Y.S.; Shih, S.P.; Lee, S.B.; El-Shazly, M.; Chang, K.M.; Yang, Y.S.H.; Lee, Y.L.; Lu, M.C. The anti-proliferative activity of secondary metabolite from the marine Streptomyces sp. against prostate cancer cells. Life 2021, 11, 1414. [Google Scholar] [CrossRef]
- Grover, S.; Lawrence, C.B. The alternaria alternata mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation. Int. J. Mol. Sci. 2017, 18, 1577. [Google Scholar] [CrossRef]
- Karolina, K.; Dominika Ewa, H.-G.; Marta Justyna, K.; Kinga Anna, U.; Kamila, D.; Agnieszka Wanda, P.-C. Mycotoxin alternariol (AOH) affects viability and motility of mammary breast epithelial cells. Int. J. Mol. Sci. 2021, 22, 696. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Z.B.; Gu, F.D.; Lin, Y.F.; Li, Y.; Chen, H.Y.; Liu, H.; Yang, X.W.; Liu, G.M.; Liu, Q.M. Alternariol Monomethyl Ether Alleviates Ovalbumin- Induced Food Allergy by Suppressing MAPK and NF-κB Signaling Pathways of Mast Cells. J. Agric. Food Chem. 2024, 72, 5463–5476. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Ma, L.; Gu, F.; Liao, K.; Liu, Y.; Zhang, Y.; Liu, H.; Hong, Y.; Cao, M.; et al. Sulfate oligosaccharide of gracilaria lemaneiformis modulates type 1 immunity by restraining t cell activation. Carbohydr. Polym. 2022, 288, 119377. [Google Scholar] [CrossRef]
- Heikrujam Thoihen, M.; Girdhari, L. T cell receptor signaling in the differentiation and plasticity of CD4(+) t cells. Cytokine Growth Factor Rev. 2022, 69, 14–27. [Google Scholar]
- Daniel, Y.W.; Joe-Elie, S.; Justine, V.C.; Sunandana, C.; Christian, M.; Fei, Y.; Shilin, Z.; Satya, D.; Kathryn, E.B.; Lisa, H.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar]
- Scheinecker, C.; Göschl, L.; Bonelli, M. Treg cells in health and autoimmune diseases: New insights from single cell analysis. J. Autoimmun. 2020, 110, 102376. [Google Scholar] [CrossRef]
- Rui, Z.; Ying, C.; Jing, L.; Min, C.; Hongling, W.; Meifang, H.; Shi, L.; Xiaobing, W.; Qiu, Z. JNK pathway-associated phosphatase/dusp22 suppresses CD4(+) T-cell activation and TH1/TH17-cell differentiation and negatively correlates with clinical activity in inflammatory bowel disease. Front. Immunol. 2017, 8, 781. [Google Scholar]
- Hyun-Su, L.; Gil-Saeng, J. Salinosporamide a, a marine-derived proteasome inhibitor, inhibits t cell activation through regulating proliferation and the cell cycle. Molecules 2020, 25, 5031. [Google Scholar] [CrossRef]
- Raquel, G.-B.; Angela, S.; Beatriz, H.F.; Cristina, R.; Hector, S.M.; Jose, M.G.G. CD4 T-cell subsets and the pathophysiology of inflammatory bowel disease. Int. J. Mol. Sci. 2023, 24, 2696. [Google Scholar] [CrossRef]
- Gregory, P.W.; Aubrey, M.S.; Asta, J.; Nicole, J.G.; David, G.S.; Ashley, S.H. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of parkinson’s disease. Brain 2021, 144, 2047–2059. [Google Scholar]
- Tingxia, L.; Wei, C.; Taisheng, L. Hiv-related immune activation and inflammation: Current understanding and strategies. Immunol Res. 2021, 2021, 7316456. [Google Scholar]
- Noor, B.; Alexander, I.M.; Tiago, Z.; Anastasia, N.R.; Evan, M.C.; Scott, A.L.; Deborah, J.F. T cell activation niches-optimizing t cell effector function in inflamed and infected tissues. Immunol. Rev. 2021, 306, 164–180. [Google Scholar]
- Keiko, N.; Gregory, S.W.; Miranda, R.L.C.; Sara, A.G.; Christina, L.W.; Gentaro, I.; Alessandra, L.-B.; Donald, N.C.; Hideki, N. Chemokine ccl19 promotes type 2 t-cell differentiation and allergic airway inflammation. J. Allergy Clin. Immunol. 2023, 153, 487–502. [Google Scholar]
- Yi-Fu, Y.; Takao, M.; Ping, G.; Nobuya, Y.; Shiro, O.; Hiroshi, I.; Satoshi, O.; Takeshi, I.; Takahiro, T.; Toshiyuki, H.; et al. A non-peptide ccr5 antagonist inhibits collagen-induced arthritis by modulating t cell migration without affecting anti-collagen t cell responses. Eur. J. Immunol. 2002, 32, 2124–2132. [Google Scholar]
- Solhaug, A.; Wisbech, C.; Christoffersen, T.E.; Hult, L.O.; Lea, T.; Eriksen, G.S. Holme JAThe mycotoxin alternariol induces DNA damage and modify macrophage phenotype and inflammatory responses. Toxicol. Lett. 2015, 239, 9–21. [Google Scholar] [CrossRef]
- Jessica, K.; Ebru, C.; Cornelia, S.; Doris, M. The mycotoxin alternariol suppresses lipopolysaccharide-induced inflammation in thp-1 derived macrophages targeting the nf-κb signalling pathway. Arch. Toxicol. 2018, 92, 3347–3358. [Google Scholar]
- Sun, S.; Zhao, G.; Liu, C.; Fan, W.; Zhou, X.; Zeng, L.; Guo, Y.; Kou, Z.; Yu, H.; Li, J.; et al. Treatment with anti-c5a antibody improves the outcome of h7n9 virus infection in african green monkeys. Clin. Infect Dis. 2014, 60, 856–895. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic t-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Gu, F.; Zou, Z.; Wang, F.; Li, D.; Song, J.; Hong, Y.; Wu, X.; Yang, X.; Liu, W.-H.; et al. Marine-Derived Alternariol Suppresses Inflammation by Regulating T Cell Activation and Migration. Mar. Drugs 2025, 23, 133. https://doi.org/10.3390/md23030133
Liu C, Gu F, Zou Z, Wang F, Li D, Song J, Hong Y, Wu X, Yang X, Liu W-H, et al. Marine-Derived Alternariol Suppresses Inflammation by Regulating T Cell Activation and Migration. Marine Drugs. 2025; 23(3):133. https://doi.org/10.3390/md23030133
Chicago/Turabian StyleLiu, Chenfeng, Fudie Gu, Zhengbiao Zou, Fengli Wang, Dashuai Li, Jing Song, Yazhen Hong, Xuhui Wu, Xianwen Yang, Wen-Hsien Liu, and et al. 2025. "Marine-Derived Alternariol Suppresses Inflammation by Regulating T Cell Activation and Migration" Marine Drugs 23, no. 3: 133. https://doi.org/10.3390/md23030133
APA StyleLiu, C., Gu, F., Zou, Z., Wang, F., Li, D., Song, J., Hong, Y., Wu, X., Yang, X., Liu, W.-H., Liu, G., Zhou, Y., & Liu, Q. (2025). Marine-Derived Alternariol Suppresses Inflammation by Regulating T Cell Activation and Migration. Marine Drugs, 23(3), 133. https://doi.org/10.3390/md23030133








