The Origin, Properties, Structure, Catalytic Mechanism, and Applications of Fucoidan-Degrading Enzymes
Abstract
1. Introduction
2. Sources and Classification of Fucoidanase
| Sources | Family | pH | OpT | Km | Vmax | M.W. (kDa) | Substrate Source | Specific Activity | Products | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Muricauda eckloniae (Mef1) | GH107 | 8 | 37 °C | ND | ND | 45 | Fucus evanescens | ND | [62] | |
| Muricauda eckloniae (Mef2) | GH107 | 8 | 35 °C | ND | ND | 105 | Fucus evanescens | 1.2 × 10−3 Uf/μM | ND | [63] |
| Formosa algae strain KMM 3553 (FFA) | GH107 | 6.5–9.1 | 45 °C | ND | ND | 96 | ND | ND | ND | [64] |
| Formosa algae strain KMM 3553 T (FFA2) | GH107 | 6.5–9 | 25–37 °C | ND | ND | 101.2 | Fucus evanescens | ND | DP4, DP6 | [65] |
| Flavobacterium algicola 12,076 (OUC-FaFcn1) | GH107 | 9.0 | 40 °C | ND | ND | 110 | Fucales | 4.11 U/mg | DP2 | [37] |
| Alteromonas sp. SN-1009 (tFda1B) | GH107 | 7.0 | 35 °C | 3.88 ± 0.81 mg/mL | ND | 100 | Kjellmaniella crassifolia | 0.0038 U/mg | ND | [45] |
| Formosa haliotis (Fhf1Δ470) | GH107 | 8 | 37–40 °C | ND | ND | 71 | Fucus evanescens Fucus vesiculosus | ND | DP4, DP8, DP10 | [43] |
| Formosa haliotis (Fhf2Δ484) | GH107 | 8 | 37 °C | ND | ND | 98 | Fucus evanescens (Fucus vesiculosus, Sargassum mccluei, and Sargassum polycystum) | 2.4 × 10−4 Uf/μM | DP8, DP10 | [44] |
| Wenyingzhuangia fucanilytica CZ1127 T (Fwf1) | GH107 | 6.4–7.2 | 24–35 °C | ND | ND | 83 | Fucus evanescen, Fucus vesiculosus, and Sargassum horneri | ND | DP4, DP6 | [66] |
| Wenyingzhuangia fucanilytica CZ1127 T (Fwf2) | GH107 | 6.0–6.8 | 24–40 °C | ND | ND | 95 | ND | DP4, DP6, DP8 | ||
| Psychromonas sp. SW19D | GH107 | ND | ND | ND | ND | ND | Laminaria hyperborea and Macrocystis pyifera | ND | ND | [67] |
| Wenyingzhuangia fucanilytica (FunA) | GH168 | 8.0 | 40 °C | 1.05 ± 0.10 mg/mL | 25.45 ± 0.97 U/mg | 48 | Isostichopus badionotus | 13.7 U/mg | DP4 | [40] |
| Wenyingzhuangia fucanilytica CZ1127 T (Fun168D) | GH168 | 7.5 | 35 °C | 2.28 mg/mL | 64.10 U/mg | 49.5 | Isostichopus badionotus | 24.5 ± 1.1 U/mg | ND | [68] |
| Holothuria tubeulosa | 69.3 ± 0.9 U/mg | |||||||||
| Wenyingzhuangia fucanilytica CZ1127 T (Fwf5) | GH168 | 6.0–6.4 | 25–40 °C | ND | ND | 44.3 ± 1 | Fucus evanescens | ND | DP2, DP4 | [69] |
| Wenyingzhuangia fucanilytica (Fun168E) | GH168 | 8.5 | 35 °C | 1.07 mg/mL | 5.07 U/mg | 46.2 | Isostichopus badionotus | ND | DP4 | [70] |
| 3.66 mg/mL | 4.46 U/mg | Holothuria tubeulosa | ||||||||
| Wenyingzhuangia aestuarii OF219 (Fun174A) | GH174 | 5.5 | 30 °C | 5.60 mg/mL | 11.04 U/mg | 80 | Isostichopus badionotus | 2.87 U/mg | ND | [41] |
| ND (Fun174Sb) | GH174 | 7.5 | 35–50 °C | 4.37 mg/mL | 45.05 U/mg | 54.3 | Isostichopus badionotus | 29.3 ± 2.1 U/mg | DP4 | [71] |
| ND (Fun174Rm) | GH174 | 8.5 | 50 °C | 2.84 mg/mL | 4.27 U/mg | 70.5 | 2.5 ± 0.1 U/mg | DP4 | ||
| ND (FunRi) | GH174 | 6.5 | 35 °C | 1.18 mg/mL | 11.05 U/mg | 56.8 | 5.2 ± 0.1 U/mg | DP4 | ||
| Wenyingzhuangia aestuarii OF219 (Fun187A) | GH187 | 7.5 | 30 °C | 3.51 mg/mL | 1.51 U/mg | 101 | Holothuria tubulosa | 1.4 U/mg | ND | [42] |
| Cobetia amphilecti | ND | 8 | 30 °C | 1.3 mg/mL | ND | 35 | ND | 0.43 U/mg | ND | [51] |
| Vasticardium flavum | ND | 3–4 | ND | ND | ND | ND | Stichopus variegatus, Holothuria spinifera | ND | ND | [72] |
| Sphingomonas paucimobilis PF-1 (FNase S) | ND | 6.0–7.0 | 40–50 °C | 1.7 mg/mL | 0.62 mg·min−1 | 130 | ND | ND | ND | [54] |
| Flavobacterium sp. RC2-3 (Fcn1) | ND | 8.0 | 50 °C | 1.17 mg/mL | 10.53 g/L·min | 46.8 | ND | 332 U/mg | ND | [73] |
| Fusarium sp. LD8 | ND | 6 | 60 °C | ND | ND | 64 | ND | 0.25 IU/mg | ND | [56] |
| Aspergillus flavus FS018 | ND | 5 | 55 °C | 1.9 mg/mL | ND | ND | Sargassum vulgare | ND | ND | [59] |
| Dendryphiella arenaria TM94 | ND | 6 | 50 °C | 6.56 mg/mL | ND | 180 | ND | 0.32 IU/mg | ND | [56] |
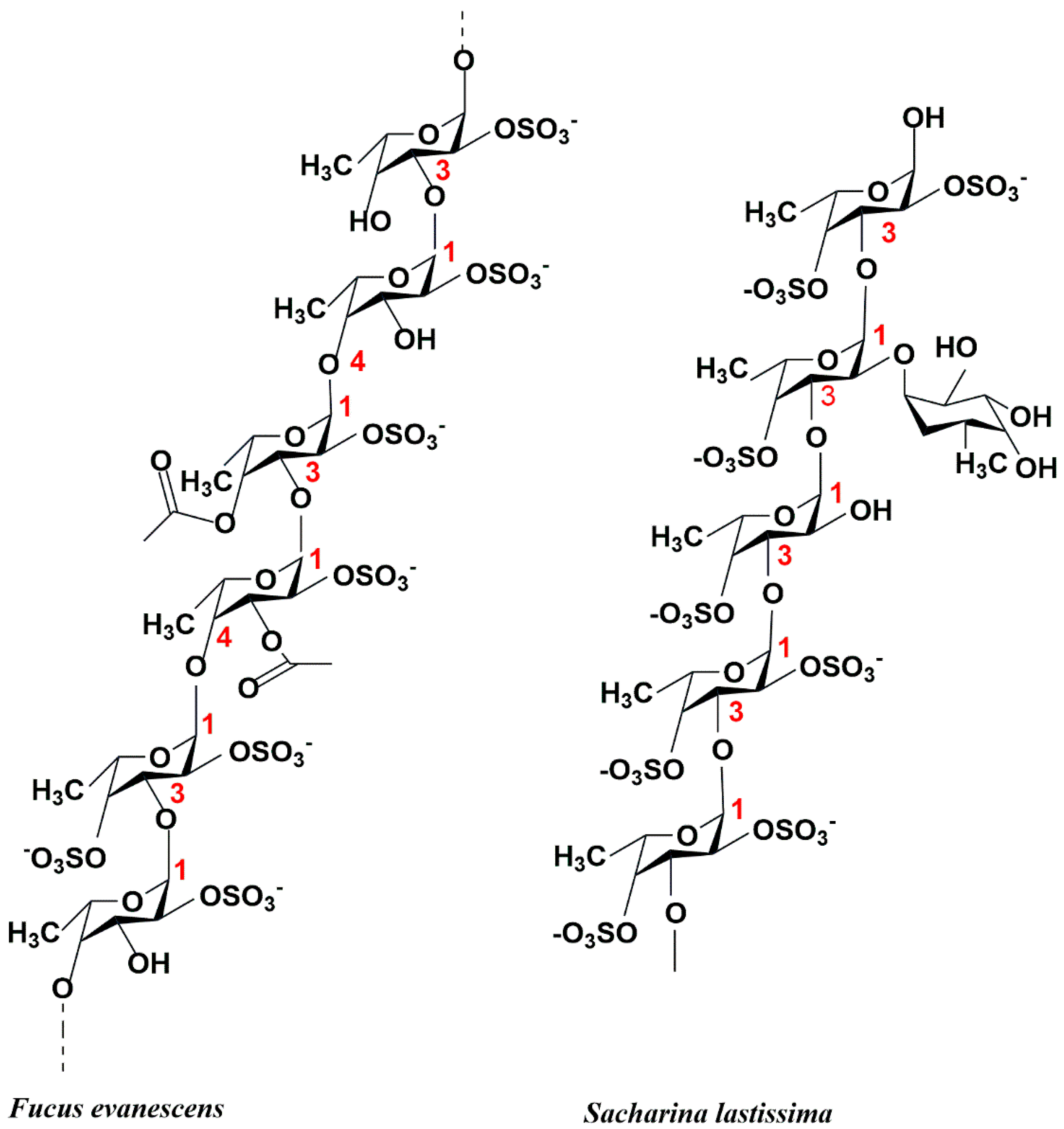
2.1. Structure of Fucoidanase
2.2. Three-Dimensional Structure of Fucoidanase
2.3. Carbohydrate-Binding Modules (CBMs)
2.4. Enzymatic Properties of Fucoidanase
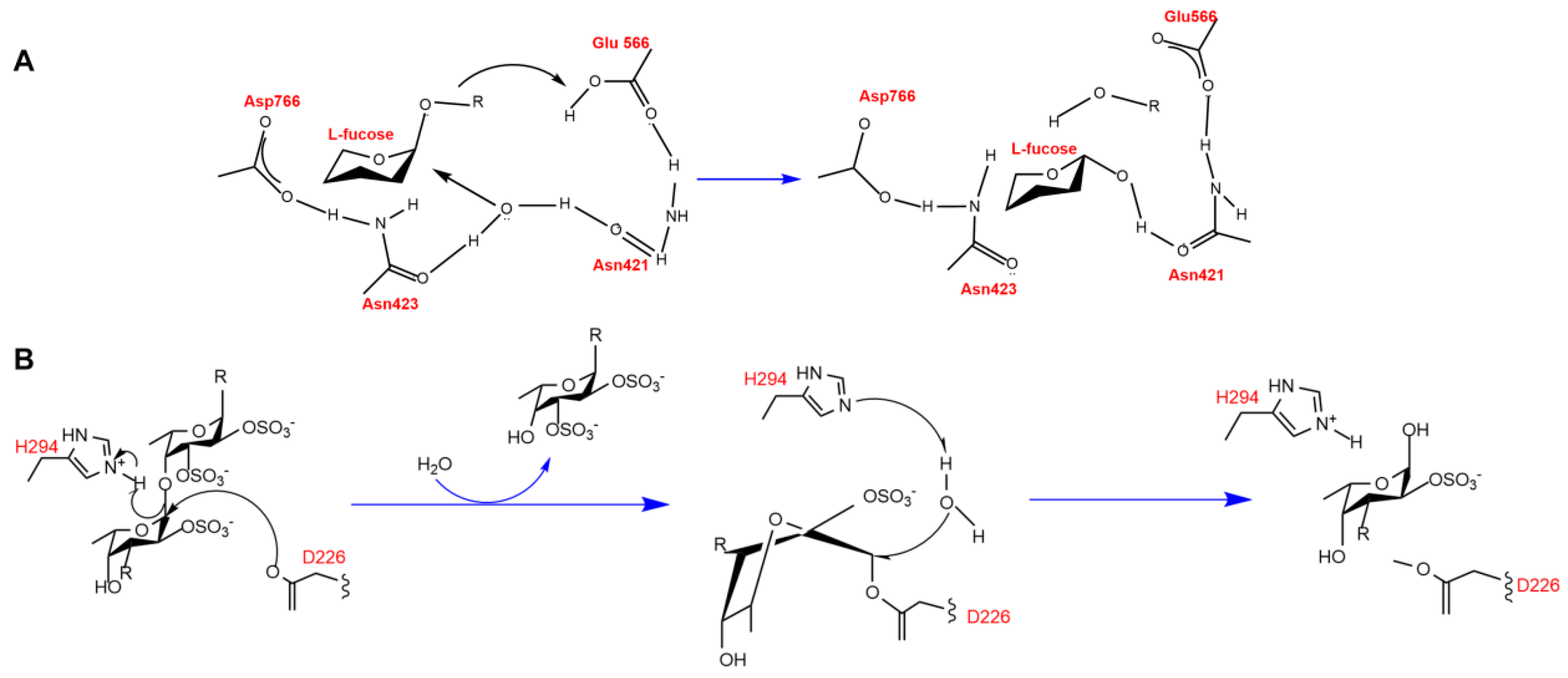
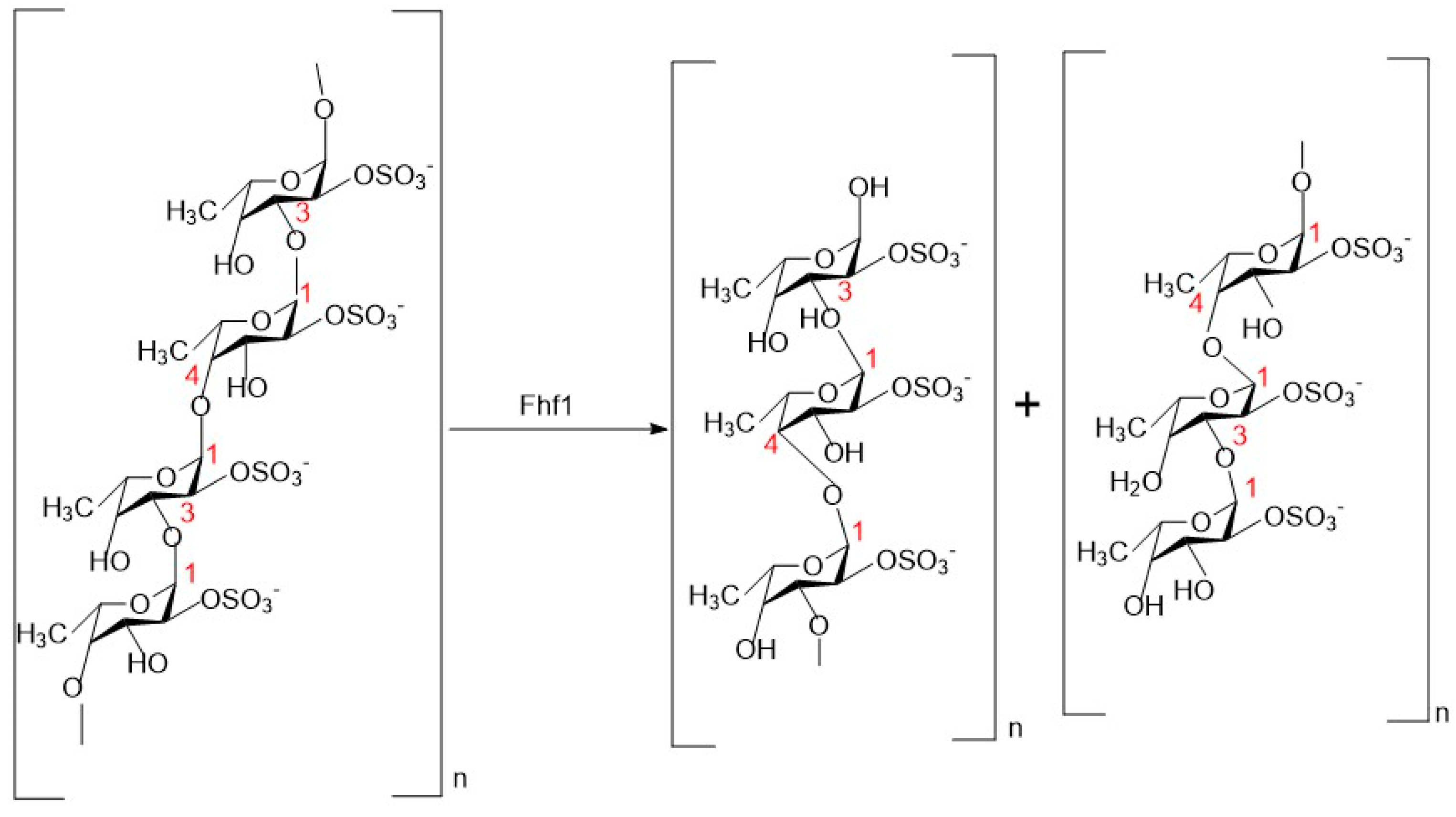
3. Catalytic Mechanism and Substrate Specificity
4. Application of Fucoidanase
4.1. Production of Low-Molecular-Weight Fucoidan
4.2. For Inferring the Structure of Fucoidan
4.3. In Biotechnology
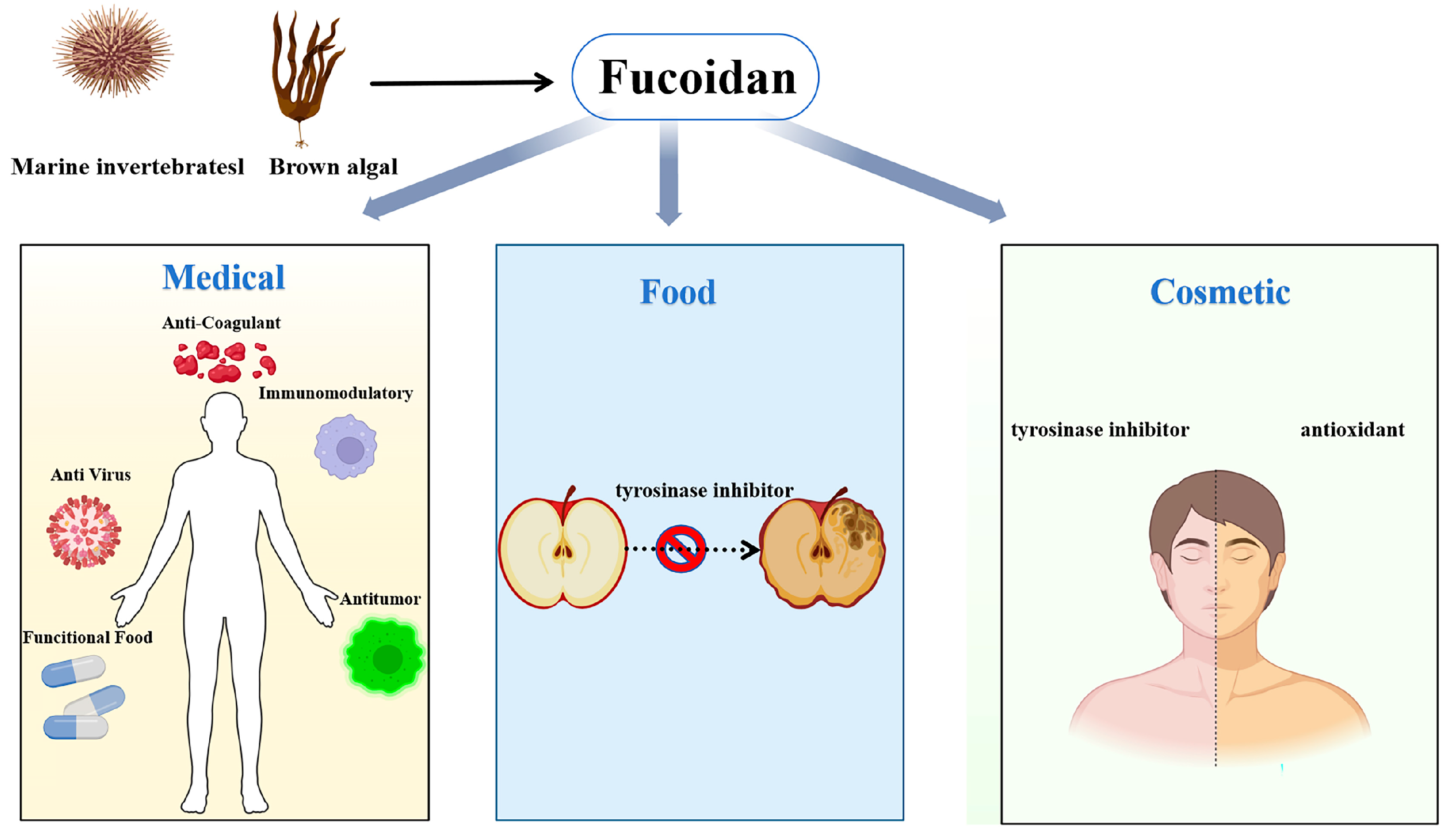
4.4. Medical and Cosmetic Applications
4.5. Food Industry
5. Prospects and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.J.; Ragan, M.A. Eleventh International Seaweed Symposium. In Proceedings of the Eleventh International Seaweed Symposium, Qingdao, China, 19–25 June 2012; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 22. [Google Scholar]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, L.; You, Y.; Sun, X.; Wen, C.; Fu, Y.; Song, S. Preparation of low-molecular-weight fucoidan with anticoagulant activity by photocatalytic degradation method. Foods 2022, 11, 822. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.-S.; Lee, D.-S.; Jeon, Y.-J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Men’shova, R.V.; Lepeshkin, F.D.; Ermakova, S.P.; Pokrovskii, O.I.; Zvyagintseva, T.N. Effect of pretreatment conditions of brown algae by supercritical fluids on yield and structural characteristics of fucoidans. Chem. Nat. Compd. 2013, 48, 923–926. [Google Scholar] [CrossRef]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Extraction of sulfated polysaccharides by autohydrolysis of brown seaweed Fucus vesiculosus. J. Appl. Phycol. 2013, 25, 31–39. [Google Scholar] [CrossRef]
- Ha, V.T.N. Kinetics and Technology Functionality of Microbial Fucoidanase. Ph.D. Thesis, DTU Bioengineering, Lyngby, Denmark, 2022. [Google Scholar]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Wan Aida, W.M.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mazita Mohd, D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Itoh, H.; Amano, H.; Kakinuma, M.; Noda, H. Antitumor activities and immunological studies with algal polysaccharides. Fish. Sci. 2002, 68, 1453–1456. [Google Scholar] [CrossRef][Green Version]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef]
- Shibata, H.; Kimura-Takagi, I.; Nagaoka, M.; Hashimoto, S.; Sawada, H.; Ueyama, S.; Yokokura, T. Inhibitory effect of Cladosiphon fucoidan on the adhesion of Helicobacter pylori to human gastric cells. J. Nutr. Sci. Vitaminol. 1999, 45, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lai, S.; Wu, D.; Liu, D.; Zou, X.; Ismail, A.; El-Seedi, H.; Arroo, R.R.; Xiao, J. miRNAs as regulators of antidiabetic effects of fucoidans. eFood 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gu, Q.; Yu, X. The preparation and anti-atherosclerotic effects of different low-molecular weights fucoidan. Food Biosci. 2024, 58, 103755. [Google Scholar] [CrossRef]
- Bruhn, A.; Janicek, T.; Manns, D.; Nielsen, M.M.; Balsby, T.J.S.; Meyer, A.S.; Rasmussen, M.B.; Hou, X.; Saake, B.; Göke, C.; et al. Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata—Seasonal variation and impact of environmental factors. J. Appl. Phycol. 2017, 29, 3121–3137. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K. Marine Nutraceuticals: Prospects and Perspectives; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Rasin, A.B.; Zueva, A.O.; Kusaykin, M.I.; Zvyagintseva, T.N.; Rubtsov, N.K.; Ermakova, S.P. Discovery of a fucoidan endo-4O-sulfatase: Regioselective 4O-desulfation of fucoidans and its effect on anticancer activity in vitro. Carbohydr. Polym. 2021, 271, 118449. [Google Scholar] [CrossRef] [PubMed]
- Kasai, A.; Arafuka, S.; Koshiba, N.; Takahashi, D.; Toshima, K. Systematic synthesis of low-molecular weight fucoidan derivatives and their effect on cancer cells. Org. Biomol. Chem. 2015, 13, 10556–10568. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Y.; Cao, M.-J.; Liu, G.-M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, L.; Wang, F.; Wang, Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohydr. Polym. 2019, 225, 115211. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Gao, L.; Zhou, H.; Ai, C.; Huang, X.; Wang, M.; Zhang, Y.; Zhao, C. Opportunities and challenges of algal fucoidan for diabetes management. Trends Food Sci. Technol. 2021, 111, 628–641. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Purification and molecular characterization of fucoidan isolated from Ascophyllum nodosum brown seaweed grown in Ireland. Mar. Drugs 2023, 21, 315. [Google Scholar] [CrossRef] [PubMed]
- Kusaykin, M.I.; Silchenko, A.S.; Zakharenko, A.M.; Zvyagintseva, T.N. Fucoidanases. Glycobiology 2016, 26, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, X.; Miao, Y.; Zhou, Y.; Shi, J.; Yan, M.; Chen, A. Studies on Antiviral and Immuno-Regulation Activity of Low Molecular Weight Fucoidan from Laminaria japonica. J. Ocean. Univ. China 2018, 17, 705–711. [Google Scholar] [CrossRef]
- Choi, J.-i.; Kim, H.-J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 2013, 97, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Choi, J.-i. Melanogenesis inhibitory effect of low molecular weight fucoidan from Undaria pinnatifida. J. Appl. Phycol. 2017, 29, 2213–2217. [Google Scholar]
- Tsai, H.-L.; Tai, C.-J.; Huang, C.-W.; Chang, F.-R.; Wang, J.-Y. Efficacy of low-molecular-weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients: A double-blind randomized controlled trial. Mar. Drugs 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, H.; Dong, Y.; Wang, Y.; Hamouda, H.I.; Balah, M.A.; Mao, X. Expression and biochemical characterization of a novel fucoidanase from Flavobacterium algicola with the principal product of fucoidan-derived disaccharide. Foods 2022, 11, 1025. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, D.; Que, F.; Li, Y.; Chen, Q. Low molecular weight fucoidan prepared by fucoidanase degradation—A promising browning inhibitor. LWT 2021, 148, 111739. [Google Scholar] [CrossRef]
- Colin, S.; Deniaud, E.; Jam, M.; Descamps, V.; Chevolot, Y.; Kervarec, N.; J.-Yvin, C.; Barbeyron, T.; Michel, G.; Kloareg, B. Cloning and biochemical characterization of the fucanase FcnA: Definition of a novel glycoside hydrolase family specific for sulfated fucans. Glycobiology 2006, 16, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chang, Y.; Zhang, Y.; Mei, X.; Xue, C. Discovery and characterization of an endo-1, 3-fucanase from marine bacterium Wenyingzhuangia fucanilytica: A novel glycoside hydrolase family. Front. Microbiology. 2020, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shen, J.; Chang, Y.; Mei, X.; Chen, G.; Zhang, Y.; Xue, C. Characterization of an endo-1,3-fucanase from marine bacterium Wenyingzhuangia aestuarii: The first member of a novel glycoside hydrolase family GH174. Carbohydr. Polym. 2023, 306, 120591. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zheng, L.; Zhang, Y.; Chen, G.; Mei, X.; Chang, Y.; Xue, C. Discovery of a catalytic domain defines a new glycoside hydrolase family containing endo-1,3-fucanase. Carbohydr. Polym. 2024, 323, 121442. [Google Scholar] [CrossRef] [PubMed]
- Vuillemin, M.; Silchenko, A.S.; Cao, H.T.T.; Kokoulin, M.S.; Trang, V.T.D.; Holck, J.; Ermakova, S.P.; Meyer, A.S.; Mikkelsen, M.D. Functional characterization of a new GH107 endo-α-(1,4)-fucoidanase from the marine bacterium Formosa haliotis. Mar. Drugs 2020, 18, 562. [Google Scholar] [CrossRef]
- Trang, V.T.D.; Mikkelsen, M.D.; Vuillemin, M.; Meier, S.; Cao, H.T.T.; Muschiol, J.; Perna, V.; Nguyen, T.T.; Tran, V.H.N.; Holck, J. The endo-α (1,4) specific fucoidanase Fhf2 from Formosa haliotis releases highly sulfated fucoidan oligosaccharides. Front. Plant Sci. 2022, 13, 823668. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, Z.; Ren, L.; Jiao, S.; Zhang, X.; Wang, Q.; Li, Z.; Du, Y.; Li, J.-J. Overexpression and biochemical characterization of a truncated endo-α (1 → 3)-fucoidanase from Alteromonas sp. SN-1009. Food Chem. 2021, 353, 129460. [Google Scholar] [PubMed]
- Manivasagan, P.; Oh, J. Production of a novel fucoidanase for the green synthesis of gold nanoparticles by Streptomyces sp. and its cytotoxic effect on HeLa cells. Mar. Drugs 2015, 13, 6818–6837. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Ponce, A.; Stortz, C.A.; Lozada, M.; Dionisi, H.M. Mining an Intertidal Sediment Metagenome for Fucanases for the Production of Oligosaccharides from Brown Algae Fucoidans. Red Argentina de Tecnología Enzimática 2021. Available online: https://ri.conicet.gov.ar/handle/11336/183000 (accessed on 21 February 2025).
- Kim, W.-J.; Kim, S.-M.; Lee, Y.-H.; Kim, H.-G.; Kim, H.-K.; Moon, S.-H.; Suh, H.-H.; Jang, K.-H.; Park, Y.-I. Isolation and characterization of marine bacterial strain degrading fucoidan from Korean Undaria pinnatifida sporophylls. J. Microbiol. Biotechnol. 2008, 18, 616–623. [Google Scholar] [PubMed]
- Setyawan, A.; Juliasih, N.L.G.R.; Darmawan, M.; Susanto, G.N.; Sarida, M. Screening, characterization, and identification of fucoidanase producing bacteria from Sargassum polycystum. AACL Bioflux 2023, 16, 1357–1371. [Google Scholar]
- Burtseva, Y.V.; Kusaikin, M.; Sova, V.; Shevchenko, N.; Skobun, A.; Zvyagintseva, T.N. Distribution of fucoidan hydrolases and some glycosidases among marine invertebrates. Russ. J. Mar. Biol. 2000, 26, 453–456. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Shao, Z.; Liu, Q.; He, Y.; Ren, D.; Yang, H.; Li, X. Purification and Characterization of the enzyme fucoidanase from Cobetia amphilecti utilizing fucoidan from Undaria pinnatifida. Foods 2023, 12, 1555. [Google Scholar] [CrossRef]
- Tran, V.H.N.; Perna, V.; Mikkelsen, M.D.; Nguyen, T.T.; Dieu Trang, V.T.; Baum, A.; Thuy Cao, H.T.; Thanh Van, T.T.; Meyer, A.S. A new FTIR assay for quantitative measurement of endo-fucoidanase activity. Enzym. Microb. Technol. 2022, 158, 110035. [Google Scholar] [CrossRef]
- Furukawa, S.-i.; Fujikawa, T.; Koga, D.; Ide, A. Purification and some properties of exo-type fucoidanases from Vibrio sp. N-5. Biosci. Biotechnol. Biochem. 1992, 56, 1829–1834. [Google Scholar] [CrossRef]
- Kim, W.J.; Park, J.W.; Park, J.K.; Choi, D.J.; Park, Y.I. Purification and characterization of a fucoidanase (FNase S) from a marine bacterium Sphingomonas paucimobilis PF-1. Mar. Drugs 2015, 13, 4398–4417. [Google Scholar] [CrossRef]
- Nagao, T.; Arai, Y.; Yamaoka, M.; Komatsu, F.; Yagi, H.; Suzuki, H.; Ohshiro, T. Identification and characterization of the fucoidanase gene from Luteolibacter algae H18. J. Biosci. Bioeng. 2018, 126, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Qianqian, W.; Shuang, M.; Hourong, X.; Min, Z.; Jingmin, C. Purification and the secondary structure of fucoidanase from Fusarium sp. LD8. Evid. Based Complement. Altern. Med. 2011, 2011, 196190. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, M.; Wu, K.; Liu, B.; Cai, J.; Pan, R. Purification and characteristics of fucoidanase obtained from Dendryphiella arenaria TM94. J. Appl. Phycol. 2011, 23, 197–203. [Google Scholar] [CrossRef]
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of enzymatic saccharification of fucoidan and alginate from brown seaweed using fucoidanase and alginate lyase from the marine fungus Dendryphiella arenaria. J. Appl. Phycol. 2019, 31, 1955–1965. [Google Scholar] [CrossRef]
- Garuba, E.O.; Adeleye, P.A.; Onilude, A.A. Purification and properties of thermostable fucoidanase produced by recently isolated terrestrial Aspergillus flavus FS018: Characteristics of fucoidanase extracted from Aspergillus flavus FS018. Trends Pept. Protein Sci. 2020, 5, 1–7(e3). [Google Scholar]
- Silchenko, A.S.; Kusaykin, M.I.; Zakharenko, A.M.; Menshova, R.V.; Khanh, H.H.N.; Dmitrenok, P.S.; Isakov, V.V.; Zvyagintseva, T.N. Endo-1,4-fucoidanase from Vietnamese marine mollusk Lambis sp. which producing sulphated fucooligosaccharides. J. Mol. Catal. B: Enzym. 2014, 102, 154–160. [Google Scholar] [CrossRef]
- Kitamura, K.; Matsuo, M.; Tsuneo, Y. Enzymic degradation of fucoidan by fucoidanase from the hepatopancreas of Patinopecten yessoensis. Biosci. Biotechnol. Biochem. 1992, 56, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.D.; Tran, V.H.N.; Meier, S.; Nguyen, T.T.; Holck, J.; Cao, H.T.T.; Van, T.T.T.; Thinh, P.D.; Meyer, A.S.; Morth, J.P. Structural and functional characterization of the novel endo-α (1,4)-fucoidanase Mef1 from the marine bacterium Muricauda eckloniae. Acta Crystallogr. Sect. D Struct. Biol. 2023, 79, 1026–1043. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.N.; Nguyen, T.T.; Meier, S.; Holck, J.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S.; Mikkelsen, M.D.J. The endo-α (1, 3)-fucoidanase Mef2 releases uniquely branched oligosaccharides from Saccharina latissima fucoidans. Mar. Drugs 2022, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kusaykin, M.I.; Kurilenko, V.V.; Zakharenko, A.M.; Isakov, V.V.; Zaporozhets, T.S.; Gazha, A.K.; Zvyagintseva, T.N.J. Hydrolysis of fucoidan by fucoidanase isolated from the marine bacterium, Formosa algae. Mar. Drugs 2013, 11, 2413–2430. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Ustyuzhanina, N.E.; Kusaykin, M.I.; Krylov, V.B.; Shashkov, A.S.; Dmitrenok, A.S.; Usoltseva, R.V.; Zueva, A.O.; Nifantiev, N.E.; Zvyagintseva, T.N. Expression and biochemical characterization and substrate specificity of the fucoidanase from Formosa algae. Glycobiology 2017, 27, 254–263. [Google Scholar] [PubMed]
- Zueva, A.O.; Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Usoltseva, R.V.; Kalinovsky, A.I.; Kurilenko, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Expression and biochemical characterization of two recombinant fucoidanases from the marine bacterium Wenyingzhuangia fucanilytica CZ1127T. Int. J. Biol. Macromol. 2020, 164, 3025–3037. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.; Liu, F.; Abe, K.; Salama-Alber, O.; Jenkins, M.; Springate, C.M.K.; Burke, J.E.; Withers, S.G.; Boraston, A.B. Endo-fucoidan hydrolases from glycoside hydrolase family 107 (GH107) display structural and mechanistic similarities to fucosidases from GH29. J. Biol. Chem. 2018, 293, 18296–18308. [Google Scholar] [CrossRef]
- Shen, J.; Chen, G.; Zhang, Y.; Mei, X.; Chang, Y.; Xue, C. Characterization of a novel endo-1,3-fucanase from marine bacterium Wenyingzhuangia fucanilytica reveals the presence of diversity within glycoside hydrolase family 168. Carbohydr. Polym. 2023, 318, 121104. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Zhang, Y.; Shen, J.; Liu, G.; Chen, F.; Li, X.; Xue, C.; Cui, Q.; Feng, Y.; et al. Structural investigation of Fun168A unraveling the recognition mechanism of endo-1,3-fucanase towards sulfated fucan. Int. J. Biol. Macromol. 2024, 271, 132622. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, G.; Zhang, Y.; Mei, X.; Zheng, L.; Xue, C.; Chang, Y. Characterization of a novel endo-1,3-fucanase from Wenyingzhuangia fucanilytica within glycoside hydrolase family 168. Int. J. Biol. Macromol. 2024, 281, 136447. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Chen, G.; Zhang, Y.; Mei, X.; Zheng, L.; Xue, C.; Chang, Y. Biochemical characterization and cleavage specificities analyses of three endo-1,3-fucanases within glycoside hydrolase family 174. Carbohydr. Polym. 2024, 335, 122083. [Google Scholar] [CrossRef] [PubMed]
- Khanh, H.H.N.; Trang, V.T.D.; Thinh, P.D.; San, P.T.J. Catalytic conditions of fucoidan degrading enzymes from Vasticardium flavum. Vietnam. J. Sci. Technol. 2019, 57, 28–37. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Wu, J.; Wang, Y.; Qin, M.; Chen, Q.; Chen, T. Heterologous Expression and Enzymatic Properties of Fucoidanase Fcn1 from Marine Flavobacterium sp. J. Food Sci. Technol. 2024, 42, 135–144. [Google Scholar]
- Mohammed, A.; Guda, C. Computational Approaches for Automated Classification of Enzyme Sequences. J. Proteom. Bioinform. 2011, 4, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kundrotas, P.J.; Lensink, M.F.; Alexov, E. Homology-based modeling of 3D structures of protein–protein complexes using alignments of modified sequence profiles. Int. J. Biol. Macromol. 2008, 43, 198–208. [Google Scholar] [CrossRef]
- Hospital, A.; Goñi, J.R.; Orozco, M.; Gelpí, J.L. Molecular dynamics simulations: Advances and applications. Adv. Appl. Bioinform. Chem. 2015, 8, 37–47. [Google Scholar]
- Chen, Q.; Zhang, W.; Mu, W. Molecular Dynamics Simulation for Food Enzyme Engineering: Why This Technique Should Be Encouraged To Learn. J. Agric. Food Chem. 2021, 69, 4–6. [Google Scholar] [CrossRef]
- Mikkelsen, M.D.; Cao, H.T.T.; Roret, T.; Rhein-Knudsen, N.; Holck, J.; Tran, V.T.T.; Nguyen, T.T.; Tran, V.H.N.; Lezyk, M.J.; Muschiol, J.; et al. A novel thermostable prokaryotic fucoidan active sulfatase PsFucS1 with an unusual quaternary hexameric structure. Sci. Rep. 2021, 11, 19523. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Taran, I.V.; Usoltseva, R.V.; Zvyagintsev, N.V.; Zueva, A.O.; Rubtsov, N.K.; Lembikova, D.E.; Nedashkovskaya, O.I.; Kusaykin, M.I.; Isaeva, M.P.J. The Discovery of the Fucoidan-Active Endo-1→ 4-α-L-Fucanase of the GH168 Family, Which Produces Fucoidan Derivatives with Regular Sulfation and Anticoagulant Activity. Int. J. Mol. Sci. 2023, 25, 218. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.A.; Ponce, N.M.; Lozada, M.; Daglio, Y.; Stortz, C.A.; Dionisi, H.M.J. Fucanases Related to the GH107 Family from Members of the PVC Superphylum. J. Mar. Sci. Eng. 2024, 12, 181. [Google Scholar] [CrossRef]
- Liu, G.; Chang, Y.; Mei, X.; Chen, G.; Zhang, Y.; Jiang, X.; Tao, W.; Xue, C. Identification and structural characterization of a novel chondroitin sulfate-specific carbohydrate-binding module: The first member of a new family, CBM100. Int. J. Biol. Macromol. 2024, 255, 127959. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Bolam, D.N.; Harry Gilbert, J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Chang, Y.; Shen, J.; Zhang, Y.; Chen, G.; Liu, Y.; Xue, C. Characterization of a sulfated fucan-specific carbohydrate-binding module: A promising tool for investigating sulfated fucans. Carbohydr. Polym. 2022, 277, 118748. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.; Sørensen, I.; Bernal, A.J.; Blaukopf, C.; Lee, K.; Øbro, J.; Pettolino, F.; Roberts, A.; Mikkelsen, J.D.; Knox, J.P.J. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Liu, G.; Shen, J.; Chen, G.; Zhang, Y.; Xue, C.; Chang, Y. Discovery of a sulfated fucan-specific carbohydrate-binding module: The first member of a new carbohydrate-binding module family. Int. J. Biol. Macromol. 2023, 238, 124037. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Bakunina, I.Y.; Nedashkovskaya, O.I.; Gorshkova, N.M.; Alexeeva, Y.V.; Zelepuga, E.A.; Zvaygintseva, T.N.; Nicolau, D.V.; Mikhailov, V.V. Ecophysiological Variabilities in Ectohydrolytic Enzyme Activities of Some Pseudoalteromonas Species, P. citrea, P. issachenkonii, and P. nigrifaciens. Curr. Microbiol. 2003, 46, 0006–0010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, C.; Yu, L.; Wang, Y.; Xu, X.; Chang, Y. Fucoidanase activity determination method on basis of pHBH method. J. Chin. Inst. Food Sci. Technol. 2013, 13, 200–206. [Google Scholar]
- Bilan, M.I.; Kusaykin, M.I.; Grachev, A.A.; Tsvetkova, E.A.; Zvyagintseva, T.N.; Nifantiev, N.E.; Usov, A.I. Effect of Enzyme Preparation from the Marine Mollusk Littorina kurila on Fucoidan from the Brown Alga Fucus distichus. Biochemistry 2005, 70, 1321–1326. [Google Scholar] [CrossRef]
- Sasaki, K.; Sakai, T.; Kojima, K.; Nakayama, S.; Nakanishi, Y.; Kato, I.J. Partial purification and characterization of an enzyme releasing 2-sulfo-α-l-fucopyranose from 2-sulfo-α-l-fucopyranosyl-(1→ 2) pyridylaminated fucose from a sea urchin, Strongylocentrotus nudus. Biosci. Biotechnol. Biochem. 1996, 60, 666–668. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Kalinovsky, A.I.; Miansong, Z.; Changheng, L.; Malyarenko, O.; Zueva, A.O.; Zvyagintseva, T.N.; Ermakova, S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017, 175, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Shingu, Y.; Yagi, H.; Suzuki, H.; Ohshiro, T. Occurrence of different fucoidanase genes in Flavobacterium sp. SW and enzyme characterization. J. Biosci. Bioeng. 2022, 134, 187–194. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Imbs, T.I.; Zvyagintseva, T.N.; Fedoreyev, S.A.; Ermakova, S.P. Brown Alga Metabolites—Inhibitors of Marine Organism Fucoidan Hydrolases. Chem. Nat. Compd. 2017, 53, 345–350. [Google Scholar] [CrossRef]
- Imbs, T.I.; Silchenko, A.S.; Fedoreev, S.A.; Isakov, V.V.; Ermakova, S.P.; Zvyagintseva, T.N. Fucoidanase inhibitory activity of phlorotannins from brown algae. Algal Res. 2018, 32, 54–59. [Google Scholar] [CrossRef]
- Nagae, M.; Tsuchiya, A.; Katayama, T.; Yamamoto, K.; Wakatsuki, S.; Kato, R. Structural Basis of the Catalytic Reaction Mechanism of Novel 1,2-L-Fucosidase from Bifidobacterium bifidum. J. Biol. Chem. 2007, 282, 18497–18509. [Google Scholar] [CrossRef]
- Davies, G.J.; Wilson, K.S.; Henrissat, B.J. Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochem. J. 1997, 321, 557. [Google Scholar] [CrossRef]
- Zueva, A.O.; Silchenko, A.S.; Rasin, A.B.; Malyarenko, O.S.; Kusaykin, M.I.; Kalinovsky, A.I.; Ermakova, S.P. Production of high- and low-molecular weight fucoidan fragments with defined sulfation patterns and heightened in vitro anticancer activity against TNBC cells using novel endo-fucanases of the GH107 family. Carbohydr. Polym. 2023, 318, 121128. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chang, Y.; Xue, C.; Wang, J.; Shen, J. Gastric Protective Activities of Sea Cucumber Fucoidans with Different Molecular Weight and Chain Conformations: A Structure–Activity Relationship Investigation. J. Agric. Food Chem. 2018, 66, 8615–8622. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Malyarenko, O.S.; Shevchenko, N.M.; Zueva, A.O.; Kalinovsky, A.I.; Zvyagintseva, T.N.; Ermakova, S.P. Modification of native fucoidan from Fucus evanescens by recombinant fucoidanase from marine bacteria Formosa algae. Carbohydr. Polym. 2018, 193, 189–195. [Google Scholar] [CrossRef]
- Ptak, S.H.; Hjuler, A.L.; Ditlevsen, S.I.; Fretté, X.; Errico, M.; Christensen, K.V. The effect of seasonality and geographic location on sulphated polysaccharides from brown algae. Aquac. Res. 2021, 52, 6235–6243. [Google Scholar] [CrossRef]
- Honya, M.; Mori, H.; Anzai, M.; Araki, Y.; Nisizawa, K. Monthly Changes in the Content of fucans, Their Constituent Sugars and Sulphate in Cultured Laminaria Japonica, Sixteenth Interna-tional Seaweed Symposium, Dordrecht, 1999; Kain, J.M., Brown, M.T., Lahaye, M., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 411–416. [Google Scholar]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Hifney, A.F.; Gomaa, M.; Fawzy, M.A.; Abdel-Gawad, K.M. Optimizing a Low-Cost Production Process of Crude Fucoidanase by Dendryphiella arenaria Utilizing Cystoseira trinodis (Phaeophyceae) and Enzymatic Hydrolysis of the Brown Algal Biomass. Waste Biomass Valorization 2019, 10, 2773–2781. [Google Scholar] [CrossRef]
- Ohmes, J.; Mikkelsen, M.D.; Nguyen, T.T.; Tran, V.H.N.; Meier, S.; Nielsen, M.S.; Ding, M.; Seekamp, A.; Meyer, A.S.; Fuchs, S. Depolymerization of fucoidan with endo-fucoidanase changes bioactivity in processes relevant for bone regeneration. Carbohydr. Polym. 2022, 286, 119286. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yu, L.; Zhang, Y.; Chang, Y.; Liu, Y.; Shen, J.; Xue, C. Utilizing heterologously overexpressed endo-1,3-fucanase to investigate the structure of sulfated fucan from sea cucumber (Holothuria hilla). Carbohydr. Polym. 2021, 272, 118480. [Google Scholar] [CrossRef] [PubMed]
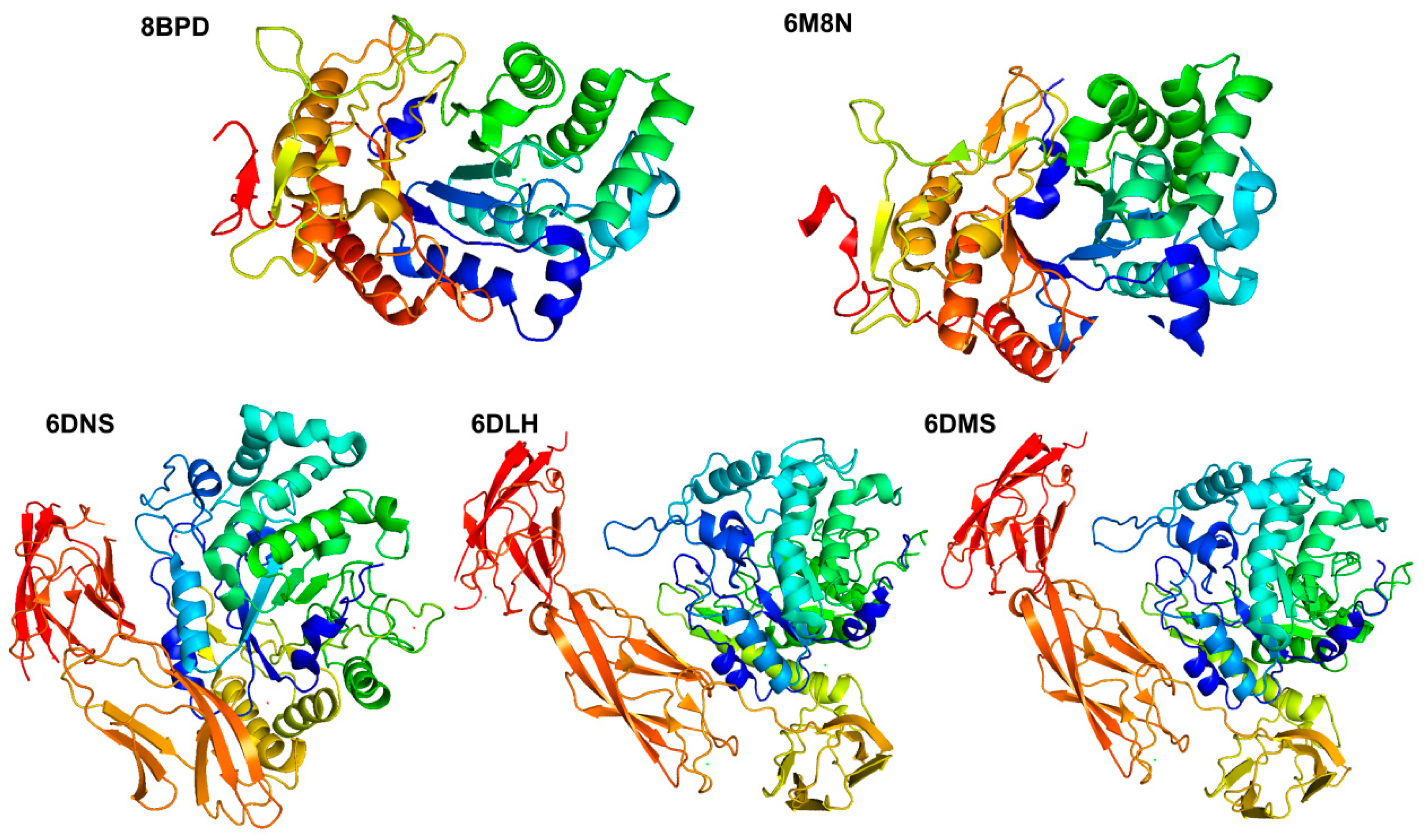

| Family | Enzyme | GeneBank | 3D Structure Status | Resolution (Å) | PDB | Ref. |
|---|---|---|---|---|---|---|
| GH107 | Mef1 | KQC28683.1 | (β/α) 8 barrel | 1.8 | 8BPD[A] | [62] |
| MfFcnA4 | CAI47003.1Q08I46 | (β/α) 8 barrel | 2.20 | 6DLH[A] | [67] | |
| MfFcnA4_H294Q | 2.85 | 6DMS[A] | ||||
| MfFcnA9 | 2.24 | 6DNS[A] | ||||
| P5AFcnA | AYF59291.1A0A452CSY7 | (β/α) 8 barrel | 1.55 | 6M8N[A] | ||
| GH168 | Fun168A | ANW96599.1 WP_068826898.1 | (β/α) 8 barrel | 1.92 | 8YA6[A] | [69] |
| FunA | 1.99 | 8YA7[A] | ||||
| Poly41_55130 | TWU32535.1 | ND | 1.7 | 9JOS | https://www.cazy.org/ | |
| 2.02 | 9JP2 | |||||
| 1.4 | 9JP3 | |||||
| FUN168E | ANW96379.1 | 2.04 | 9JOM | |||
| 1.97 | 9JOO | |||||
| FUN168D | ANW96381.1 | 1.29 | 9JOC | |||
| 1.5 | 9JOF | |||||
| 1.4 | 9JOG | |||||
| 1.36 | 9JOH |
| Enzyme | Family | Action | Catalytic Bases | Active Site | Ref. |
|---|---|---|---|---|---|
| Mef2 | GH107 | endo-α-1,3-L-fucanase | Asp182/His260 | ND | [63] |
| OUC-FaFcn1 | GH107 | endo-α-1,4-L-fucoidanase | Asp231 | ND | [37] |
| tFda1B | GH107 | endo-α(1,3)-fucoidanase | Asp202 | ND | [45] |
| FWf1 | GH107 | endo-α-1,4-L-fucoidanase | Asp226/His294 | ND | [66] |
| FWf2 | GH107 | endo-α-1,4-L-fucoidanase | Asp464/His537 | ND | |
| FWf3 | GH107 | endo-α-1,4-L-fucanase | Asp401/His469 | ND | |
| FWf4 | GH107 | endo-α-1,4-L-fucanase | Asp229/His297 | ND | |
| Fhf1 | GH107 | endo-α-(1,4)-fucoidanase | Asp225/His293 | ND | [43] |
| Mef1 | GH107 | endo-a(1,4)-fucoidanase | Asp187/His27 | ND | [62] |
| Fun168E | GH168 | endo-1,3-fucanase | ND | ND | [70] |
| FunA | GH168 | endo-1,3-fucanase | ND | D206, E264 | [40] |
| Fun174A | GH174 | endo-1,3-fucanase | ND | D119, E120, E218 | [41] |
| Fun187A | GH187 | endo-α-1,3-L-fucanase | ND | ND | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Ning, L.; Zhu, P.; Jiang, J.; Yao, Z.; Zhu, B. The Origin, Properties, Structure, Catalytic Mechanism, and Applications of Fucoidan-Degrading Enzymes. Mar. Drugs 2025, 23, 97. https://doi.org/10.3390/md23030097
Zhao Y, Ning L, Zhu P, Jiang J, Yao Z, Zhu B. The Origin, Properties, Structure, Catalytic Mechanism, and Applications of Fucoidan-Degrading Enzymes. Marine Drugs. 2025; 23(3):97. https://doi.org/10.3390/md23030097
Chicago/Turabian StyleZhao, Yi, Limin Ning, Penghui Zhu, Jinju Jiang, Zhong Yao, and Benwei Zhu. 2025. "The Origin, Properties, Structure, Catalytic Mechanism, and Applications of Fucoidan-Degrading Enzymes" Marine Drugs 23, no. 3: 97. https://doi.org/10.3390/md23030097
APA StyleZhao, Y., Ning, L., Zhu, P., Jiang, J., Yao, Z., & Zhu, B. (2025). The Origin, Properties, Structure, Catalytic Mechanism, and Applications of Fucoidan-Degrading Enzymes. Marine Drugs, 23(3), 97. https://doi.org/10.3390/md23030097






