Study of the Influence of the Orientation of a 50-Hz Magnetic Field on Fetal Exposure Using Polynomial Chaos Decomposition
Abstract
:1. Introduction
2. Material and Methods
2.1. Polynomial Chaos Expansion of the System Output Y
| Probability Density Function | Support * | Polynomial π |
|---|---|---|
| Gaussian | Hermite | |
| Uniform | (−1,1) | Legendre |
| Gamma | (0,+∞) | Laguerre |
| Chebyshev | (−1,1) | Chebyshev |
| Beta | (−1,1) | Jacobi |
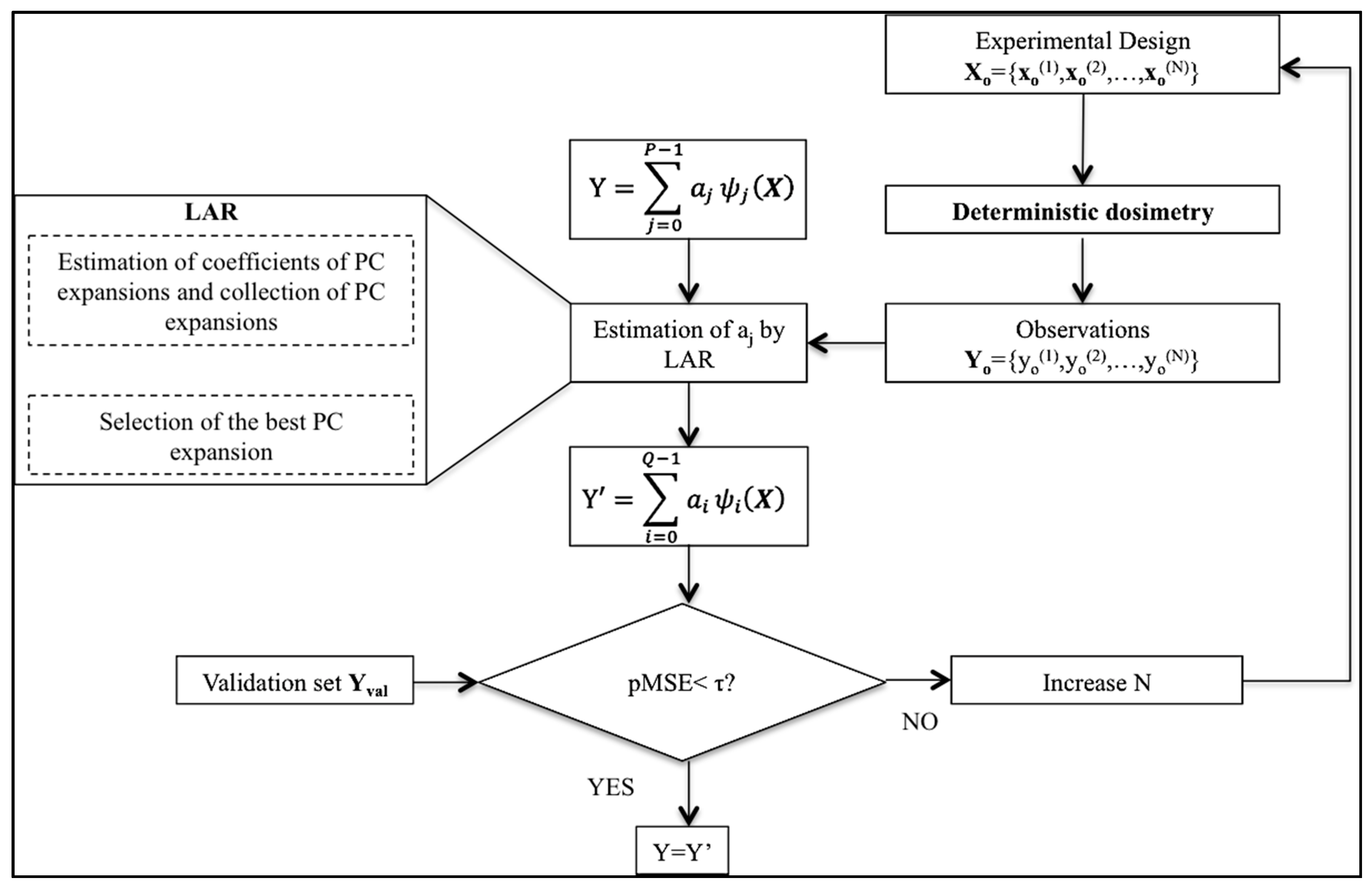
2.2. Validation of the PC Expansion
2.3. Application of the PC Theory to the Analysis of the Fetal Exposure Varying B-Field Orientation
2.3.1. Definition of the Input Random Vector X and the System Output Y
2.3.2 The Observation Set Yo

2.4. Analysis of the Fetal Exposure
3. Results and Discussion
3.1. Validation of the PC Expansion in Each Fetal Tissue
3.2. Estimation of the Statistical Moments


3.3. Analysis of the Fetal Exposure Respect to the Limits




4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- SCENIHR. Health Effects on EMF—Preliminary Opinion on Potential Health Effects of Exposure to Electromagnetic Fields (EMF). 2014. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_041.pdf (accessed on 19 May 2015).
- ICNIRP. Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–522. [Google Scholar]
- ICNIRP. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar]
- Grellier, J.; Ravazzani, P.; Cardis, E. Potential health impacts of residential exposures to extremely low frequency magnetic fields in Europe. Environ. Int. 2014, 62, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wiart, J.; Conil, E.; Hadjem, A.; Jala, M.; Kersaudy, P.; Varsier, N. Handle variability in numerical exposure assessment: the challenge of the stochastic dosimetry. In Proceedings of European Conference on Antennas and Propagation (EUCAP), Gothenburg, Germany, 8–12 April 2013.
- Gabriel, C. Dielectric properties of biological tissue: Variation with age. Bioelectromagnetics 2005, 7, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A.; Gabriel, C.; Grant, E.H.; Vermeeren, G.; Martens, L. Variation of the dielectric properties of tissues with age: the effect on the values of SAR in children when exposed to walkie-talike devices. Phys. Med. Biol. 2009, 54, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A. Dielectric properties of tissues; variation with age and their relevance in exposure of children to electromagnetic field; state of knowledge. Progr. Biophys. Mol. Biol. 2011, 107, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A.; Gabriel, C. Dielectric properties of rat embryo and fetus as a function of gestation. Phys. Med. Biol. 2012, 57, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Hirata, A. Fast multigrid-based computation of induced electric field for transcranial magnetic stimulation. Phys. Med. Biol. 2012, 57, 7753–7765. [Google Scholar] [CrossRef] [PubMed]
- Blatman, G. Adaptive sparse polynomial chaos expansions for uncertainty propagation and sensitivity analysis. Ph.D. Thesis, Université Blaise Pascal, Clermont-Ferrand, France, 2009. [Google Scholar]
- Ghanem, R.; Spanos, P. Stochastic finite elements—A spectral approach; Springer Verlag: Mineola, NY, USA, 1991. [Google Scholar]
- Soize, C.; Ghanem, R. Physical systems with random uncertainties: Chaos representations with arbitrary probability measure. SIAM J. Sci. Comput. 2004, 26, 395–410. [Google Scholar] [CrossRef]
- Wiener, N. The homogeneous chaos. Amer. J. Math. 1938, 60, 897–936. [Google Scholar] [CrossRef]
- Xiu, D.; Karniadakis, G.E. The Wiener-Askey polynomial chaos for stochastic differential equations. SIAM J. Sci. Comput. 2002, 24, 619–644. [Google Scholar] [CrossRef]
- Voyer, D.; Musy, F.; Nicolas, L.; Perrussel, R. Probabilistic methods applied to 2D electromagnetics numerical dosimetry. Int. J. Comput. Math. Electr. Electron. Eng. 2008, 27, 651–667. [Google Scholar] [CrossRef] [Green Version]
- Wiart, J.; Kersaudy, P.; Ghanmi, A.; Varsier, N.; Hadhem, A.; Odile, P.; Sudret, B.; Mittra, R. Stochastic Dosimetry to Manage Uncertainty in Numerical EMF Exposure Assessment. Forum for Electromagnetic Research Methods and Application Technologies (FERMAT). Available online: http://www.e-fermat.org (accessed on 13 May 2015).
- Gaignaire, R.; Scorretti, R.; Sabariego, R.V.; Geuzaine, C. Stochastic uncertainty quantification of eddy currents in the human body by polynomial chaos decomposition. IEEE Trans. Magnet. 2012, 48, 451–454. [Google Scholar] [CrossRef]
- Schmidt, C.; Grant, P.; Lowery, M.; van Rienen, U. Influence of uncertainties in the material properties of brain tissue on the probabilistic volume of tissue activated. IEEE Trans. Biomed. Eng. 2013, 60, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Dimbylow, P. Development of pregnant female, hybrid voxel-mathematical models and their application to the dosimetry of applied magnetic and electric fields at 50 Hz. Phys. Med. Biol. 2006, 51, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Cech, R.; Leitgeb, N.; Pediaditis, M. Fetal exposure to low frequency electric and magnetic fields. Phys. Med. Biol. 2007, 52, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Zupanic, A; Valic, B.; Miklavcic, D. Numerical assessment of induced current densities for pregnant women exposed to 50 Hz electromagnetic field. Int. Feder. Med. Biol. Eng. Proc. 2007, 16, 226–229. [Google Scholar]
- Dimbylow, P.; Findlay, R. The effects of body posture, anatomy, age and pregnancy on the calculation of induced current densities at 50 Hz. Radiat. Protect. Dosim. 2010, 139, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Liorni, I.; Parazzini, M.; Fiocchi, S.; Douglas, M.; Capstick, M.; Gosselin, M.C.; Kuster, N.; Ravazzani, P. Dosimetric study of fetal exposure to uniform magnetic fields at 50 Hz. Bioelectromagnetics 2014, 35, 580–597. [Google Scholar] [CrossRef] [PubMed]
- Dimbylow, P. Development of the female voxel phantom, NAOMI, and its application to calculations of induced current densities and electric fields from applied low frequency magnetic and electric fields. Phys. Med. Biol. 2005, 50, 1047–1070. [Google Scholar] [CrossRef] [PubMed]
- Blatman, G.; Sudret, B. Adaptive sparse polynomial chaos expansion based on least angle regression. J. Comput. Phy. 2011, 230, 2345–2367. [Google Scholar] [CrossRef]
- Field, R.V.; Grigoriu, M. On the accuracy of the polynomial chaos approximation. Probab. Eng. Mech. 2004, 19, 65–80. [Google Scholar] [CrossRef]
- Choi, S.K.; Grandhi, R.V.; Canfield, R.A.; Pettitt, C.L. Polynomial chaos expansion with Latin hypercube sampling for estimating response variability. AIAA J. 2004, 45, 1191–1198. [Google Scholar] [CrossRef]
- Berveiller, M.; Sudret, B.; Lemaire, M. Stochastic finite elements: A non intrusive approach by regression. Eur. J. Comput. Mech. 2006, 15, 81–92. [Google Scholar] [CrossRef]
- Nobile, F.; Tempone, R.; Webster, C. A Sparse Grid Stochastic Collocation Method for Elliptic Partial Differential Equations with Random Input Data; Politecnico di Milano: Milano, Italy, 2006. [Google Scholar]
- Blatman, G.; Sudret, B. Sparse polynomial chaos expansions and adaptive stochastic finite elements using a regression approach. C R Mécanique 2008, 336, 518–523. [Google Scholar] [CrossRef]
- Efron, B.; Hastie, T.; Johnstone, I.; Tibshirani, R. Least angle regression. Ann. Statist. 2004, 32, 407–499. [Google Scholar]
- Weisberg, S. Applied Linear Regression; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Stone, M. Cross-validatory choice and the assessment of statistical predictions (with discussion). J. Roy. Statist. Soc. 1974, 36, 111–133. [Google Scholar]
- Geisser, S. A predictive sample reuse method with applications. J. Amer. Statist. Assoc. 1975, 70, 320–328. [Google Scholar] [CrossRef]
- OpenTURNS. Version 1.3. Available online: http://trac.openturns.org (accessed on 30 September 2014).
- Niederreiter, H. Random Number Generation and Quasi-Monte Carlo Methods; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1994. [Google Scholar]
- Morokoff, W.; Caflisch, R. Quasi-Monte Carlo integration. J. Comput. Phys. 1995, 122, 21–230. [Google Scholar] [CrossRef]
- Christ, A.; Kainz, W.; Hahn, E.G.; Honegger, K.; Zefferer, M.; Neufeld, E.; Rascher, W.; Janka, R.; Bautz, W.; Chen, J.; et al. The Virtual Family-development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys. Med. Biol. 2010, 55, N23–N38. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Guldimann, R.; Buehlmann, B.; Zefferer, M.; Bakker, J.F.; van Rohn, G.C.; Kuster, N. Exposure of the human body to professional and domestic induction cooktops compared to the basic restrictions. Bioelectromagnetics 2012, 33, 695–705. [Google Scholar] [CrossRef] [PubMed]
- SEMCAD X v. 14.8.4. Available online: http://www.speag.com (accessed on 13 May 2015).
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: 1. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249, 2251–2269, 2271–2293. [Google Scholar] [CrossRef] [PubMed]
- Hasgall, P.A.; Neufeld, E.; Gosselin, M.C.; Klingenböck, A.; Kuster, N. It’is Database for thermal and electromagnetic parameters of biological tissues. Version 2.2. 11 July 2012. Available online: http://www.itis.ethz.ch/database (accessed on 28 February 2014).
- Parazzini, M.; Fiocchi, S.; Rossi, E.; Paglialonga, A.; Ravazzani, P. Transcranial direct current stimulation: Estimation of the electric field and of the current density in an anatomical human head model. IEEE Trans. Biomed. Eng. 2011, 58, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Varsier, N.; Dahdouh, S.; Serruries, A.; de la Plata, J.-P.; Anquez, J.; Angelini, E.D.; Bloch, I.; Wiart, J. Influence of pregnancy stage and fetus position on the whole-body and local exposure of the fetus to RF-EMF. Phys. Med. Biol. 2014, 59, 4913–4926. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liorni, I.; Parazzini, M.; Fiocchi, S.; Ravazzani, P. Study of the Influence of the Orientation of a 50-Hz Magnetic Field on Fetal Exposure Using Polynomial Chaos Decomposition. Int. J. Environ. Res. Public Health 2015, 12, 5934-5953. https://doi.org/10.3390/ijerph120605934
Liorni I, Parazzini M, Fiocchi S, Ravazzani P. Study of the Influence of the Orientation of a 50-Hz Magnetic Field on Fetal Exposure Using Polynomial Chaos Decomposition. International Journal of Environmental Research and Public Health. 2015; 12(6):5934-5953. https://doi.org/10.3390/ijerph120605934
Chicago/Turabian StyleLiorni, Ilaria, Marta Parazzini, Serena Fiocchi, and Paolo Ravazzani. 2015. "Study of the Influence of the Orientation of a 50-Hz Magnetic Field on Fetal Exposure Using Polynomial Chaos Decomposition" International Journal of Environmental Research and Public Health 12, no. 6: 5934-5953. https://doi.org/10.3390/ijerph120605934







