Trypanosoma brucei Inhibition by Essential Oils from Medicinal and Aromatic Plants Traditionally Used in Cameroon (Azadirachta indica, Aframomum melegueta, Aframomum daniellii, Clausena anisata, Dichrostachys cinerea and Echinops giganteus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Isolation of Essential Oil
2.3. Chemicals
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis of Essential Oils
2.5. T. brucei and Mammalian Cell Culture and Growth Inhibition Assay
3. Results and Discussion
3.1. Chemical Composition of Essential Oils from Cameroonian Plants
3.2. Inhibition of Trypanosoma brucei Proliferation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- World Health Organization. Control and surveillance of human African trypanosomiasis. World Health Organ. Tech. Rep. Ser. 2013, 984, 1–237. [Google Scholar]
- Kennedy, P.G. The continuing problem of human African trypanosomiasis (sleeping sickness). Ann. Neurol. 2008, 64, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.; Yukich, J.O.; Sutherland, C.S.; Woods, G.; Tediosi, F. Human African trypanosomiasis prevention, treatment and control costs: A systematic review. Acta Trop. 2015, 150, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Mohamed, A.; Isah, M.B.; Aliyu, A.B. Anti-trypanosomal activity of African medicinal plants: A review update. J. Ethnopharmacol. 2014, 154, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.; Kandela, N.B.; Chilton, P.J.; Lilford, R.J. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan. 2016, 31, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural product as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Nour, A.M.; Khalid, S.A.; Kaiser, M.; Brun, R.; Abdallah, W.E.; Schmidt, T.J. The antiprotozoal activity of sixteen Asteraceae species native to Sudan and bioactivity-guided isolation of xanthanolides from Xanthuim brasilicum. Planta Med. 2009, 75, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Bockman, M.R.; Kalinda, A.S.; Petrelli, R.; De la Mora-Rey, T.; Tiwari, D.; Liu, F.; Dawadi, S.; Nandakumar, M.; Rhee, K.Y.; Schnappinger, D.; et al. Targeting Mycobacterium tuberculosis Biotin Protein Ligase (MtBPL) with Nucleoside-Based Bisubstrate Adenylation Inhibitors. J. Med. Chem. 2015, 58, 7349–7369. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; No, J.H.; Lee, G.Y.; Li, W.; Yang, S.Y.; Yang, G.; Schmidt, T.J.; Kang, J.S.; Kim, Y.H. Phenolic constituents of medicinal plants with activity against Trypanosoma brucei. Molecules 2016, 21, 480. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Mehlhorn, H. Declining malaria, rising dengue and Zika virus: Insights for mosquito vector control. Parasitol. Res. 2016, 115, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Halldor, T. Lipids and Essential Oils as Antimicrobial Agents, 1st ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2011; pp. 1–334. [Google Scholar]
- Pavela, R.; Benelli, G. Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Tapanelli, S.; Chianese, G.; Lucantoni, L.; Yerbanga, R.S.; Habluetzel, A.; Tagliatela-Scafati, O. Transmission blocking effects of Neem (Azadirachta indica) seed kernel limonoids on Plasmodium berghei early sporogonic development. Fitoterapia 2016, 114, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Igbal, Z.; Lateef, M.; Jabbar, A.; Gilani, A.H. In vivo anthelmintic activity of Azadirachta indica A. Juss seeds against gastrointestinal nematodes of sheep. Vet. Parasitol. 2010, 168, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Noorul, A.; Gayathri. Beneficial effects of Neem oil—An updated review. J. Pharm. Sci. Res. 2016, 8, 756–758. [Google Scholar]
- Benelli, G.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Conti, B.; Nicoletti, M. Old ingredients for a new recipe? Neem cake, a low-cost botanical by-product in the fight against mosquito-borne diseases. Parasitol. Res. 2015, 114, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Canale, A.; Toniolo, C.; Higuchi, A.; Murugan, K.; Pavela, R.; Nicoletti, M. Neem (Azadirachta indica): Towards the ideal insecticide? Nat. Prod. Res. 2017, 31, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Mbaya, A.W.; Ibrahim, U.I.; God, O.T.; Ladi, S. Toxicity and potential anti-trypanosomal activity of ethanolic extract of Azadirachta indica (Meliacea) stem bark: An in vivo and in vitro approach using Trypanosoma brucei. J. Ethnopharmacol. 2010, 128, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Ilic, N.; Schmidt, B.M.; Pouley, A.; Raskin, I. Toxicological evaluation of grains of paradise (Aframomum melegueta) [Roscoe] K. Schum. J. Ethnopharmacol. 2010, 127, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Adefegha, O.M.; Henle, T. Alligator pepper/Grain of paradise (Aframomum melegueta) modulates Angiotensin-I converting enzyme activity, lipid profile and oxidative imbalances in a rat model of hypercholesterolemia. Pathophysiology 2016, 23, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Ukeh, D.A.; Birkett, M.A.; Pickett, J.A.; Bowman, A.S.; Luntz, A.J. Repellent activity of alligator pepper, Aframomum melegueta, and ginger, Zingiber officinale, against the maize weevil, Sitophilus zeamais. Phytochemistry 2009, 70, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, G.O.; Skura, B.J. Nutritional profile and antimicrobial spectrum of the spice Aframomum danielli K. Schum. Plant Foods Hum. Nutr. 1994, 45, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Maggi, F.; Mbuntcha, H.; Woguem, V.; Fogang, H.P.; Womeni, H.M.; Tapondjou, L.A.; Barboni, L.; Nicoletti, M.; Canale, A.; et al. Traditional herbal remedies and dietary spices from Cameroon as novel sources of larvicides against against filariasis mosquitoes? Parasitol. Res. 2016, 115, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, O.A.; Houghton, P.J.; Raman, A. Lipoxygenase inhibitors in the seeds of Aframomum danielli K. Schum (Zingiberaceae). Phytomedicine 1999, 6, 251–256. [Google Scholar] [CrossRef]
- Fasoyiro, S.B. Preservative property of Aframomum danielli fractions in stored grains. Afr. J. Biotechnol. 2007, 6, 235–237. [Google Scholar]
- Moshi, M.J.; Kagaste, G.A.B.; Mbwambo, Z.H. Plants used to treat epilepsy by Tanzanian traditional healers. J. Ethnopharmacol. 2005, 97, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Innocent, E.; Hassanali, A. Constituents of EOs from Three Plant Species Used in Traditional Medicine and Insect Control in Tanzania. J. Herbs Spices Med. Plants 2015, 21, 219–229. [Google Scholar] [CrossRef]
- Menut, C.; Lamaty, G.; Weyerstahl, P.; Marschall, H.; Seelmann, I.; Amvam Zollo, P.H. Aromatic plants of tropical Central Africa. Part XXXI. Tricyclic sesquiterpenes from the root essential oil of Echinops giganteus var. lelyi C. D. Adams. Flavour Fragr. J. 1997, 12, 415–421. [Google Scholar] [CrossRef]
- Tene, M.; Tane, P.; Sondengam, B.L.; Connolly, J.D. Lignans from the roots of Echinops giganteus. Phytochemistry 2004, 65, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- Fankam, A.G.; Kuete, V.; Voukeng, I.K.; Kuiate, J.R.; Pages, J.-M. Antibacterial activities of selected Cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complement. Altern. Med. 2011, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Tchuenguem, R.T.; Kuiate, J.R.; Teke, G.N.; Kechia, F.A.; Kuete, V. In vitro and in vivo antifungal activities of selected Cameroonian dietary spices. BMC Complement. Altern. Med. 2014, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, W.R.; Schumacher, M.; Teiten, M.-H.; Dicato, M.; Diederich, M. Traditional West African pharmacopeia, plants and derived compounds for cancer therapy. Biochem. Pharmacol. 2012, 84, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Krusche, B.; Youns, M.; Voukeng, I.; Fankam, A.G.; Tankeo, S.; Lacmata, S.; Efferth, T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 2011, 134, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Sandjo, L.P.; Wiench, B.; Efferth, T. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J. Ethnopharmacol. 2013, 149, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, T.A.; Gbolade, A.A.; Olaifa, J.I. Comparative study of toxicity of some essential oils to larvae of three mosquito species. Niger. J. Nat. Prod. Med. 1999, 3, 74–76. [Google Scholar] [CrossRef]
- Ahua, K.M.; Ioset, J.R.; Ioset, K.N.; Diallo, D.; Mauel, J.; Hostettmann, K. Antileishmanial activities associated with plants used in the Malian traditional medicine. J. Ethnopharmacol. 2007, 110, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Karunamoorthi, K.; Hailu, T. Insect repellent plants traditional usage practices in the Ethiopian malaria epidemic-prone setting: An ethnobotanical survey. J. Ethnobiol. Ethnomed. 2014, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST 08. National Institute of Standards and Technology. Mass Spectral Library (NIST/EPA/NIH); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008.
- FFNSC 2. Flavors and Fragrances of Natural and Synthetic Compounds. Mass Spectral Database; Shimadzu Corps: Kyoto, Japan, 2012. [Google Scholar]
- Petrelli, R.; Orsomando, G.; Sorci, L.; Maggi, F.; Ranjbarian, F.; Biapa Nya, P.C.; Petrelli, D.; Vitali, L.A.; Lupidi, G.; Quassinti, L.; et al. Biological Activities of the Essential Oil from Erigeron floribundus. Molecules 2016, 21, 1065. [Google Scholar] [CrossRef] [PubMed]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.-S.; El-Tantawy, M.E.; Rabeh, M.A.; Badr, W.K. Chemical composition and biological activities of essential oils of Azadirachta indica A. Juss. Int. J. Appl. Res. Nat. Prod. 2013, 6, 33–42. [Google Scholar]
- Dastan, D.; Pezhmanmehr, M.; Askari, N.; Ebrahimi, S.N.; Hadian, J. Essential oil compositions of the leaves of Azadirachta indica A. Juss from Iran. J. Essent. Oil-Bear. Plants 2010, 13, 357–361. [Google Scholar] [CrossRef]
- Lamaty, G.; Menut, C.; Koudou, J.; Régnier, P.; Bessière, J.-M. Aromatic Plants of Tropical Central Africa. XI. Essential Oils of Leaf and Seed of Aframomum melegueta (Roscoe) K. Schum. from Central African Republic. J. Essent. Oil Res. 1993, 5, 81–83. [Google Scholar] [CrossRef]
- Menut, C.; Lamaty, G.; Amvam Zollo, P.H.; Atogho, B.M.; Abondo, R.; Bessière, J.M. Aromatic plants of tropical central Africa. V. volatile components of three Zingiberaceae from Cameroon: Aframomum melegueta (roscoe) K. Schum., A. daniellii (hook. f.) K. Schum. and A. sulcatum (oliv. and hanb.) K. Schum. Flavour Fragr. J. 1997, 6, 183–186. [Google Scholar] [CrossRef]
- Bohlmann, F.; Jakupovic, J. Neue Sesquiterpen-Kohlenwasserstoffe mit anomalen Kohlenstoffgerüst aus Silphium-arten. Phytochemistry 1980, 19, 259–265. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; Jakupovic, J.; Robinson, H.; King, R.M. Eriolanolides, eudesmanolides and a rearranged sesquiterpene from Eriophyllum species. Phytochemistry 1981, 20, 2239–2244. [Google Scholar] [CrossRef]
- Taber, D.F.; Nelson, C.G. Aliphatic C-H to C-C Conversion: Synthesis of (−)-Cameroonan-7α-ol. J. Org. Chem. 2011, 76, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Maggi, F.; Petrelli, R.; Canale, A.; Nicoletti, M.; Rakotosaona, R.; Rasoanaivo, P. Not ordinary antimalarial drugs: Madagascar plant decoctions potentiating the chloroquine action against Plasmodium parasites. Ind. Crops Prod. 2017, 103, 19–38. [Google Scholar] [CrossRef]
- Petrelli, R.; Ranjbarian, F.; Dall’Acqua, S.; Papa, F.; Iannarelli, R.; Ngahang Kamte, S.L.; Vittori, S.; Benelli, G.; Maggi, F.; Hofer, A.; et al. An overlooked horticultural crop, Smyrnium olusatrum, as a potential source of compounds effective against African trypanosomiasis. Parasitol. Int. 2017, 66, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Mikus, J.; Harkenthal, M.; Steverding, D.; Reichling, J. In vitro effect of essential oils and isolated mono-and sesquiterpenes on Leishmania major and Trypanosoma brucei. Planta Med. 2000, 66, 366–368. [Google Scholar] [CrossRef] [PubMed]
| No. | Component a | RI calc. b | RI lit. c | % d | ID f | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Azadirachta indica | Aframomum melegueta | Aframomum daniellii e | Clausena anisata | Dichrostachys cinerea e | Echinops giganteus e | |||||
| 1 | isopentyl acetate | 873 | 869 | tr g | RI,MS | |||||
| 2 | 2-methyl butyl acetate | 876 | 875 | tr | RI,MS | |||||
| 3 | 2-heptanone | 891 | 892 | tr | RI,MS | |||||
| 4 | 2-heptanol | 901 | 894 | 0.2 | RI,MS | |||||
| 5 | α-thujene | 916 | 924 | tr | 1.0 | 0.1 | RI,MS | |||
| 6 | α-pinene | 921 | 932 | 2.0 | 2.4 | 0.2 | tr | Std | ||
| 7 | α-fenchene | 938 | 945 | 0.1 | RI,MS | |||||
| 8 | camphene | 939 | 946 | 0.3 | tr | RI,MS | ||||
| 9 | sabinene | 959 | 969 | tr | 43.9 | 0.6 | RI,MS | |||
| 10 | β-pinene | 963 | 974 | 7.1 | 5.8 | 0.3 | 0.1 | Std | ||
| 11 | dehydro-1,8-cineole | 979 | 988 | 0.1 | RI,MS | |||||
| 12 | myrcene | 982 | 988 | 0.2 | 1.5 | 2.0 | 0.3 | tr | Std | |
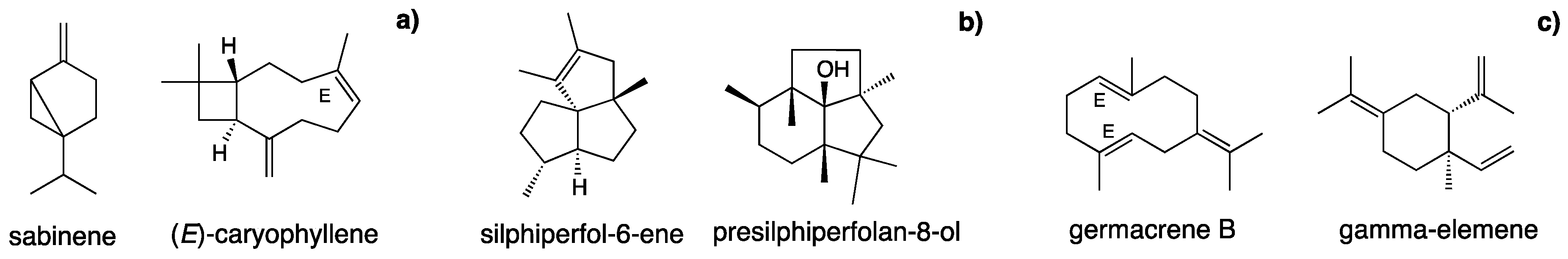
| 13 | α-phellandrene | 996 | 1004 | 0.3 | tr | Std | ||||
| 14 | δ-3-carene | 1003 | 1008 | tr | Std | |||||
| 15 | α-terpinene | 1009 | 1014 | 0.3 | 0.9 | tr | RI,MS | |||
| 16 | p-cymene | 1016 | 1020 | 1.1 | 1.0 | 2.9 | 0.1 | Std | ||
| 17 | limonene | 1020 | 1024 | 1.5 | 0.7 | 0.4 | tr | tr | Std | |
| 18 | 1,8-cineole | 1021 | 1026 | 58.5 | 0.5 | 0.2 | 2.3 | Std | ||
| 19 | ethylhexanol | 1031 | 1030 | 0.2 | RI,MS | |||||
| 20 | (Z)-β-ocimene | 1037 | 1032 | 0.4 | Std | |||||
| 21 | (E)-β-ocimene | 1041 | 1044 | 0.1 | 0.3 | 0.3 | RI,MS | |||
| 22 | γ-terpinene | 1050 | 1054 | 0.9 | 1.9 | 2.4 | Std | |||
| 23 | cis-sabinene hydrate | 1057 | 1065 | 1.1 | RI,MS | |||||
| 24 | cis-linalool oxide | 1071 | 1067 | tr | 0.1 | RI,MS | ||||
| 25 | terpinolene | 1079 | 1086 | 0.8 | 0.4 | 0.1 | tr | Std | ||
| 26 | p-cymenene | 1086 | 1089 | 0.2 | RI,MS | |||||
| 27 | ligustrazin | 1081 | 1083 | 5.1 | RI,MS | |||||
| 28 | trans-sabinene hydrate | 1089 | 1098 | 0.9 | RI,MS | |||||
| 29 | 2-nonanone | 1094 | 1094 | tr | RI,MS | |||||
| 30 | linalool | 1096 | 1095 | tr | 1.8 | tr | 4.0 | Std | ||
| 31 | n-nonanal | 1105 | 1100 | tr | RI,MS | |||||
| 32 | endo-fenchol | 1108 | 1114 | 0.3 | RI,MS | |||||
| 33 | cis-p-menth-2-en-1-ol | 1113 | 1118 | 0.2 | 0.3 | RI,MS | ||||
| 34 | α-campholenal | 1123 | 1122 | tr | 0.1 | RI,MS | ||||
| 35 | trans-pinocarveol | 1128 | 1135 | 0.2 | 0.7 | Std | ||||
| 36 | trans-p-menth-2-en-1-ol | 1131 | 1136 | 0.1 | 0.3 | RI,MS | ||||
| 37 | cis-β-terpineol | 1142 | 1140 | tr | RI,MS | |||||
| 38 | cis-verbenol | 1142 | 1137 | 0.2 | RI,MS | |||||
| 39 | trans-pinocamphone | 1151 | 1158 | 0.4 | RI,MS | |||||
| 40 | pinocarvone | 1152 | 1160 | tr | 0.1 | RI,MS | ||||
| 41 | borneol | 1156 | 1165 | 0.2 | 0.7 | Std | ||||
| 42 | p-mentha-1,5-dien-8-ol | 1158 | 1166 | 1.1 | RI,MS | |||||
| 43 | cis-pinocamphone | 1162 | 1172 | 0.8 | RI,MS | |||||
| 44 | umbelullone | 1166 | 1167 | 3.8 | RI,MS | |||||
| 45 | terpinen-4-ol | 1167 | 1174 | 1.4 | 3.7 | tr | 7.5 | Std | ||
| 46 | cis-pinocarveol | 1175 | 1182 | tr | RI,MS | |||||
| 47 | cryptone | 1183 | 1183 | tr | RI,MS | |||||
| 48 | p-cymen-8-ol | 1178 | 1179 | tr | tr | 0.3 | RI,MS | |||
| 49 | α-terpineol | 1181 | 1186 | 19.4 | 0.2 | tr | 3.3 | Std | ||
| 50 | myrtenal | 1184 | 1195 | 0.2 | 0.1 | 0.2 | Std | |||
| 51 | myrtenol | 1186 | 1194 | 0.2 | 0.4 | 1.1 | Std | |||
| 52 | cis-piperitol | 1199 | 1195 | tr | RI,MS | |||||
| 53 | γ-terpineol | 1195 | 1199 | tr | RI,MS | |||||
| 54 | methyl chavicol | 1196 | 1195 | 2.0 | RI,MS | |||||
| 55 | trans-piperitol | 1205 | 1207 | 0.1 | RI,MS | |||||
| 56 | trans-carveol | 1217 | 1215 | tr | 0.2 | RI,MS | ||||
| 57 | cis-carveol | 1228 | 1226 | tr | RI,MS | |||||
| 58 | thymol methyl ether | 1224 | 1232 | tr | RI,MS | |||||
| 59 | nerol | 1229 | 1227 | 0.2 | Std | |||||
| 60 | citronellol | 1231 | 1223 | 0.3 | Std | |||||
| 61 | carvone | 1240 | 1239 | tr | Std | |||||
| 62 | carvacrol methyl ether | 1237 | 1241 | tr | RI,MS | |||||
| 63 | neral | 1241 | 1235 | 0.2 | Std | |||||
| 64 | piperitone | 1250 | 1249 | 0.3 | RI,MS | |||||
| 65 | (Z)-anethole | 1250 | 1249 | 0.3 | RI,MS | |||||
| 66 | p-anisaldehyde | 1251 | 1247 | 0.7 | RI,MS | |||||
| 67 | geraniol | 1251 | 1249 | 18.2 | Std | |||||
| 68 | trans-ascaridol glycol | 1262 | 1266 | tr | RI,MS | |||||
| 69 | (E)-cinnamaldehyde | 1267 | 1267 | tr | RI,MS | |||||
| 70 | phellandral | 1269 | 1273 | 0.1 | RI,MS | |||||
| 71 | isobornyl acetate | 1276 | 1283 | tr | Std | |||||
| 72 | (E)-anethole | 1287 | 1282 | 64.6 | Std | |||||
| 73 | thymol | 1291 | 1289 | 0.1 | 0.9 | Std | ||||
| 74 | trans-sabinyl acetate | 1291 | 1289 | tr | RI,MS | |||||
| 75 | methyl myrtenate | 1292 | 1293 | 2.0 | RI,MS | |||||
| 76 | carvacrol | 1301 | 1298 | 0.5 | tr | 0.7 | Std | |||
| 77 | cis-pinocarvyl acetate | 1303 | 1311 | 0.1 | RI,MS | |||||
| 78 | myrtenyl acetate | 1316 | 1324 | 1.9 | RI,MS | |||||
| 79 | silphiperfol-5-ene | 1318 | 1326 | 2.1 | RI,MS | |||||
| 80 | δ-elemene | 1326 | 1335 | 0.1 | 0.1 | RI,MS | ||||
| 81 | presilphiperfol-7-ene | 1328 | 1334 | 7.8 | RI,MS | |||||
| 82 | silphinene | 1333 | 1340 | 1.7 | RI,MS | |||||
| 83 | 7-epi-silphiperfol-5-ene | 1336 | 1349 | 3.5 | RI,MS | |||||
| 84 | α-terpinyl acetate | 1341 | 1346 | tr | 0.3 | RI,MS | ||||
| 85 | α-copaene | 1362 | 1374 | 0.2 | 0.2 | tr | Std | |||
| 86 | β-bourbonene | 1369 | 1387 | 0.3 | tr | RI,MS | ||||
| 87 | modheph-2-ene | 1362 | 1382 | 3.0 | RI,MS | |||||
| 88 | silphiperfol-6-ene | 1373 | 1377 | 23.0 | RI,MS | |||||
| 89 | β-bourbonene | 1377 | 1387 | 0.1 | RI,MS | |||||
| 90 | β-cubebene | 1377 | 1387 | tr | RI,MS | |||||
| 91 | α-isocomene | 1379 | 1387 | 2.4 | RI,MS | |||||
| 92 | β-elemene | 1380 | 1389 | 0.9 | tr | 0.1 | Std | |||
| 93 | decanoic acid | 1380 | 1386 | 2.8 | RI,MS | |||||
| 94 | anisyl methyl ketone | 1382 | 1380 | 0.1 | RI,MS | |||||
| 95 | iso-longifolene | 1383 | 1389 | tr | RI,MS | |||||
| 96 | β-isocomene | 1400 | 1407 | 2.1 | RI,MS | |||||
| 97 | α-gurjunene | 1400 | 1409 | tr | Std | |||||
| 98 | (E)-caryophyllene | 1402 | 1417 | 2.4 | tr | 16.6 | 0.8 | 6.3 | Std | |
| 99 | methyl eugenol | 1406 | 1403 | 0.3 | RI,MS | |||||
| 100 | α-trans-bergamotene | 1425 | 1432 | 0.1 | RI,MS | |||||
| 101 | isoamyl benzoate | 1433 | 1433 | tr | RI,MS | |||||
| 102 | γ-elemene | 1427 | 1434 | 18.3 | 0.1 | RI,MS | ||||
| 103 | α-humulene | 1436 | 1452 | 0.4 | tr | 1.5 | 0.8 | 2.0 | Std | |
| 104 | geranyl acetone | 1449 | 1453 | 1.2 | RI,MS | |||||
| 105 | (E)-β-farnesene | 1450 | 1454 | tr | tr | RI,MS | ||||
| 106 | germacrene D | 1465 | 1484 | 0.5 | 0.3 | 2.2 | 0.3 | RI,MS | ||
| 107 | selina-4,11-diene | 1467 | 1474 | 0.1 | RI,MS | |||||
| 108 | β-selinene | 1469 | 1489 | tr | RI,MS | |||||
| 109 | ar-curcumene | 1472 | 1479 | tr | 0.1 | RI,MS | ||||
| 110 | bicyclogermacrene | 1480 | 1500 | 0.1 | 0.1 | RI,MS | ||||
| 111 | benzaldehyde, 3,4-dimethoxy- | 1482 | 1489 | 0.2 | RI,MS | |||||
| 112 | (E)-β-ionone | 1481 | 1487 | 0.5 | Std | |||||
| 113 | epi-cubebol | 1489 | 1493 | 0.1 | RI,MS | |||||
| 114 | α-zingiberene | 1492 | 1493 | 0.1 | RI,MS | |||||
| 115 | (Z)-α-bisabolene | 1493 | 1506 | 0.1 | RI,MS | |||||
| 116 | silphiperfolan-6-α-ol | 1496 | 1507 | 1.0 | RI,MS | |||||
| 117 | β-bisabolene | 1498 | 1505 | 0.9 | RI,MS | |||||
| 118 | (E)-methyl isoeugenol | 1499 | 1491 | 16.1 | RI,MS | |||||
| 119 | cameroonan-7-α-ol | 1500 | 1510 | 7.1 | RI,MS | |||||
| 120 | β-bisabolene | 1506 | 1505 | 0.3 | RI,MS | |||||
| 121 | 7-epi-α-selinene | 1507 | 1520 | tr | RI,MS | |||||
| 122 | (E,E)-α-farnesene | 1508 | 1505 | 0.3 | Std | |||||
| 123 | trans-calamenene | 1508 | 1521 | tr | RI,MS | |||||
| 124 | δ-cadinene | 1510 | 1522 | 0.2 | tr | 0.1 | 0.3 | RI,MS | ||
| 125 | silphiperfolan-7-β-ol | 1510 | 1519 | 2.5 | RI,MS | |||||
| 126 | β-sesquiphellandrene | 1520 | 1521 | tr | RI,MS | |||||
| 127 | selina-3,7(11)-diene | 1531 | 1545 | 0.2 | RI,MS | |||||
| 128 | silphiperfolan-6-β-ol | 1535 | 1546 | 1.7 | RI,MS | |||||
| 129 | hedycaryol | 1536 | 1546 | 1.5 | RI,MS | |||||
| 130 | germacrene B | 1546 | 1559 | 74.0 | 0.3 | RI,MS | ||||
| 131 | elemicin | 1556 | 1555 | 3.0 | RI,MS | |||||
| 132 | (E)-nerolidol | 1556 | 1561 | 0.7 | Std | |||||
| 133 | isoaromadendrene epoxide | 1560 | 1572 | 1.8 | RI,MS | |||||
| 134 | prenopsan-8-ol | 1564 | 1575 | 3.2 | RI,MS | |||||
| 135 | caryophyllene oxide | 1564 | 1582 | 2.2 | 1.1 | Std | ||||
| 136 | (3Z)-hexenyl benzoate | 1566 | 1565 | 0.3 | RI,MS | |||||
| 137 | spathulenol | 1568 | 1576 | 0.1 | RI,MS | |||||
| 138 | presilphiperfolan-8-ol | 1578 | 1585 | 22.7 | MS | |||||
| 139 | guaiol | 1583 | 1600 | 0.5 | RI,MS | |||||
| 140 | humulene epoxide II | 1590 | 1608 | 0.1 | tr | RI,MS | ||||
| 141 | 10-epi-γ-eudesmol | 1600 | 1622 | tr | 0.3 | RI,MS | ||||
| 142 | eremoligenol | 1611 | 1629 | 0.4 | RI,MS | |||||
| 143 | γ-eudesmol | 1615 | 1630 | tr | 0.4 | RI,MS | ||||
| 144 | 1,10-di-epi-cubenol | 1619 | 1618 | 0.1 | RI,MS | |||||
| 145 | β-eudesmol | 1631 | 1649 | 0.2 | 1.5 | RI,MS | ||||
| 146 | α -acorenol | 1628 | 1632 | 1.0 | RI,MS | |||||
| 147 | caryophylla-4(12),8(13)-dien-5-ol h | 1630 | 1639 | 0.2 | tr | RI,MS | ||||
| 148 | epi-α -muurolol | 1635 | 1640 | tr | 0.7 | 0.4 | RI,MS | |||
| 149 | α -muurolol | 1640 | 1644 | 0.1 | RI,MS | |||||
| 150 | α -eudesmol | 1636 | 1652 | 0.5 | RI,MS | |||||
| 151 | intermedeol | 1639 | 1666 | 0.1 | RI,MS | |||||
| 152 | α-cadinol | 1647 | 1652 | tr | 1.4 | 0.4 | RI,MS | |||
| 153 | ageratochromene | 1655 | 1658 | 0.8 | RI,MS | |||||
| 154 | α-bisabolol | 1673 | 1685 | tr | 0.4 | Std | ||||
| 155 | 3-oxo-β-ionone | 1678 | 1685 | 0.9 | RI,MS | |||||
| 156 | cyperotundone | 1688 | 1695 | 0.9 | RI,MS | |||||
| 157 | (2E-6Z)-farnesol | 1709 | 1698 | 2.4 | RI,MS | |||||
| 158 | curcuphenol | 1716 | 1717 | 0.4 | RI,MS | |||||
| 159 | (2E-6Z)-farnesal | 1718 | 1713 | 1.0 | RI,MS | |||||
| 160 | (2E-6E)-farnesal | 1737 | 1740 | 1.7 | RI,MS | |||||
| 161 | n-tricosane | 2300 | 2300 | 0.1 | Std | |||||
| Oil yield (%) | 0.01 | 0.3 | 0.2 | 2.0 | 0.4 | 1.8 | ||||
| Total identified (%) | 98.3 | 99.4 | 99.3 | 99.6 | 76.0 | 94.3 | ||||
| Grouped compounds (%) | ||||||||||
| Monoterpene hydrocarbons | 14.9 | 59.8 | 0.6 | 0.6 | tr | |||||
| Oxygenated monoterpenes | 83.3 | 11.0 | 0.3 | 50.6 | ||||||
| Sesquiterpene hydrocarbons | 97.4 | 0.2 | 20.0 | 5.2 | 54.7 | |||||
| Oxygenated sesquiterpenes | 0.4 | 8.4 | 0.2 | 12.1 | 39.6 | |||||
| Phenylpropanoids | 84.0 | |||||||||
| Others | 0.9 | 0.6 | 0.2 | 12.7 | ||||||
| Treatment | IC50 (µg/mL) | Selectivity Index (SI) | |
|---|---|---|---|
| T. b. brucei (TC221) | Balb/3T3 | ||
| Essential oils | |||
| Aframomum danielli | 7.65 ± 1.1 | >100 | >13.1 |
| Dichrostachys cinerea | >100 | - | - |
| Echinops giganteus | 10.50 ± 1.7 | >100 | >9.52 |
| Azadirachta indica | 15.21 ± 0.97 | >100 | >6.57 |
| Aframomum melegueta | >100 | - | - |
| Clausena anisata | >100 | - | - |
| Pure compounds | µg/mL (µM) | µg/mL (µM) | |
| Sabinene | 5.96 ± 1.3 (43.8) | >100 | >16.7 |
| β-Pinene | 11.4 ± 2.6 (83.7) | >100 | >8.77 |
| 1,8-Cineole | >100 | - | |
| Terpinen-4-ol | >100 | - | |
| (E)-Caryophyllene | 8.25 ± 1.3 (40.4) | >100 | >12.1 |
| Reference drug | µg/mL (µM) | µg/mL (µM) | |
| Suramin | 0.0286 ± 0.0008 (0.0220) | - | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamte, S.L.N.; Ranjbarian, F.; Campagnaro, G.D.; Nya, P.C.B.; Mbuntcha, H.; Woguem, V.; Womeni, H.M.; Ta, L.A.; Giordani, C.; Barboni, L.; et al. Trypanosoma brucei Inhibition by Essential Oils from Medicinal and Aromatic Plants Traditionally Used in Cameroon (Azadirachta indica, Aframomum melegueta, Aframomum daniellii, Clausena anisata, Dichrostachys cinerea and Echinops giganteus). Int. J. Environ. Res. Public Health 2017, 14, 737. https://doi.org/10.3390/ijerph14070737
Kamte SLN, Ranjbarian F, Campagnaro GD, Nya PCB, Mbuntcha H, Woguem V, Womeni HM, Ta LA, Giordani C, Barboni L, et al. Trypanosoma brucei Inhibition by Essential Oils from Medicinal and Aromatic Plants Traditionally Used in Cameroon (Azadirachta indica, Aframomum melegueta, Aframomum daniellii, Clausena anisata, Dichrostachys cinerea and Echinops giganteus). International Journal of Environmental Research and Public Health. 2017; 14(7):737. https://doi.org/10.3390/ijerph14070737
Chicago/Turabian StyleKamte, Stephane L. Ngahang, Farahnaz Ranjbarian, Gustavo Daniel Campagnaro, Prosper C. Biapa Nya, Hélène Mbuntcha, Verlaine Woguem, Hilaire Macaire Womeni, Léon Azefack Ta, Cristiano Giordani, Luciano Barboni, and et al. 2017. "Trypanosoma brucei Inhibition by Essential Oils from Medicinal and Aromatic Plants Traditionally Used in Cameroon (Azadirachta indica, Aframomum melegueta, Aframomum daniellii, Clausena anisata, Dichrostachys cinerea and Echinops giganteus)" International Journal of Environmental Research and Public Health 14, no. 7: 737. https://doi.org/10.3390/ijerph14070737
APA StyleKamte, S. L. N., Ranjbarian, F., Campagnaro, G. D., Nya, P. C. B., Mbuntcha, H., Woguem, V., Womeni, H. M., Ta, L. A., Giordani, C., Barboni, L., Benelli, G., Cappellacci, L., Hofer, A., Petrelli, R., & Maggi, F. (2017). Trypanosoma brucei Inhibition by Essential Oils from Medicinal and Aromatic Plants Traditionally Used in Cameroon (Azadirachta indica, Aframomum melegueta, Aframomum daniellii, Clausena anisata, Dichrostachys cinerea and Echinops giganteus). International Journal of Environmental Research and Public Health, 14(7), 737. https://doi.org/10.3390/ijerph14070737











