Effect of Patulin from Penicillium vulpinum on the Activity of Glutathione-S-Transferase and Selected Antioxidative Enzymes in Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Culture Conditions
2.3. PAT Extraction and Quantification
2.4. Preparation of PAT and Fungal Culture Filtrate Concentrations
2.5. Plant Treatment
2.6. Enzyme Assays
2.7. Data Analysis
3. Results
3.1. Effect of PAT and Culture Filtrate of P. vulpinum CM1 on GST Activities
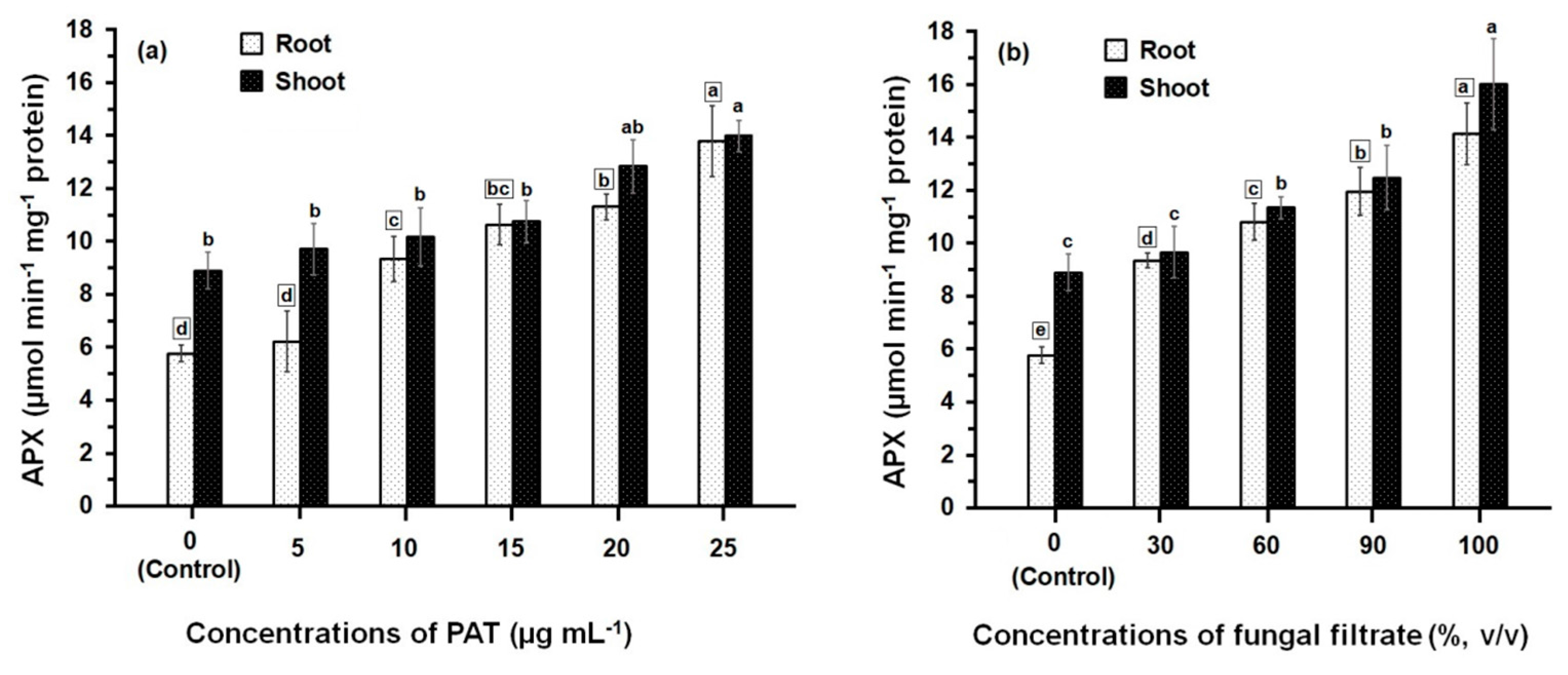
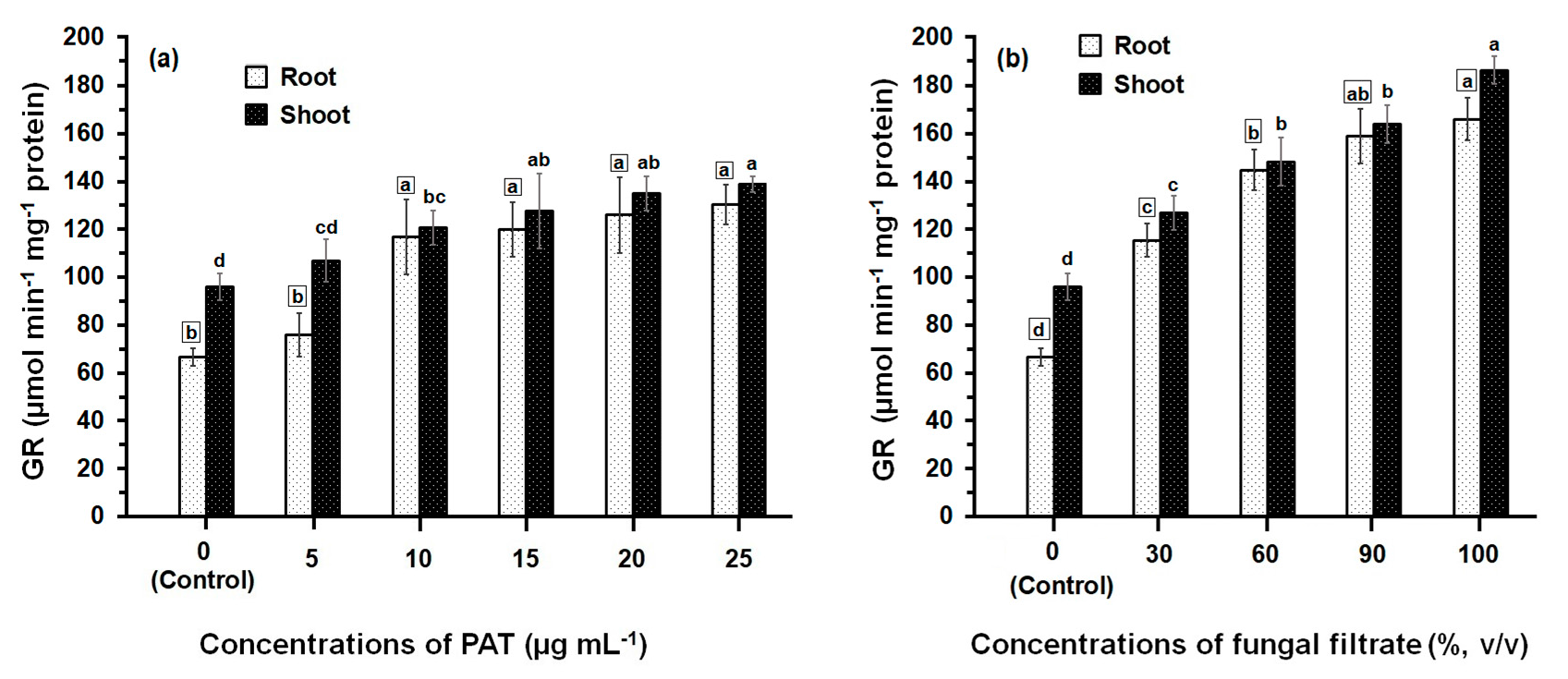
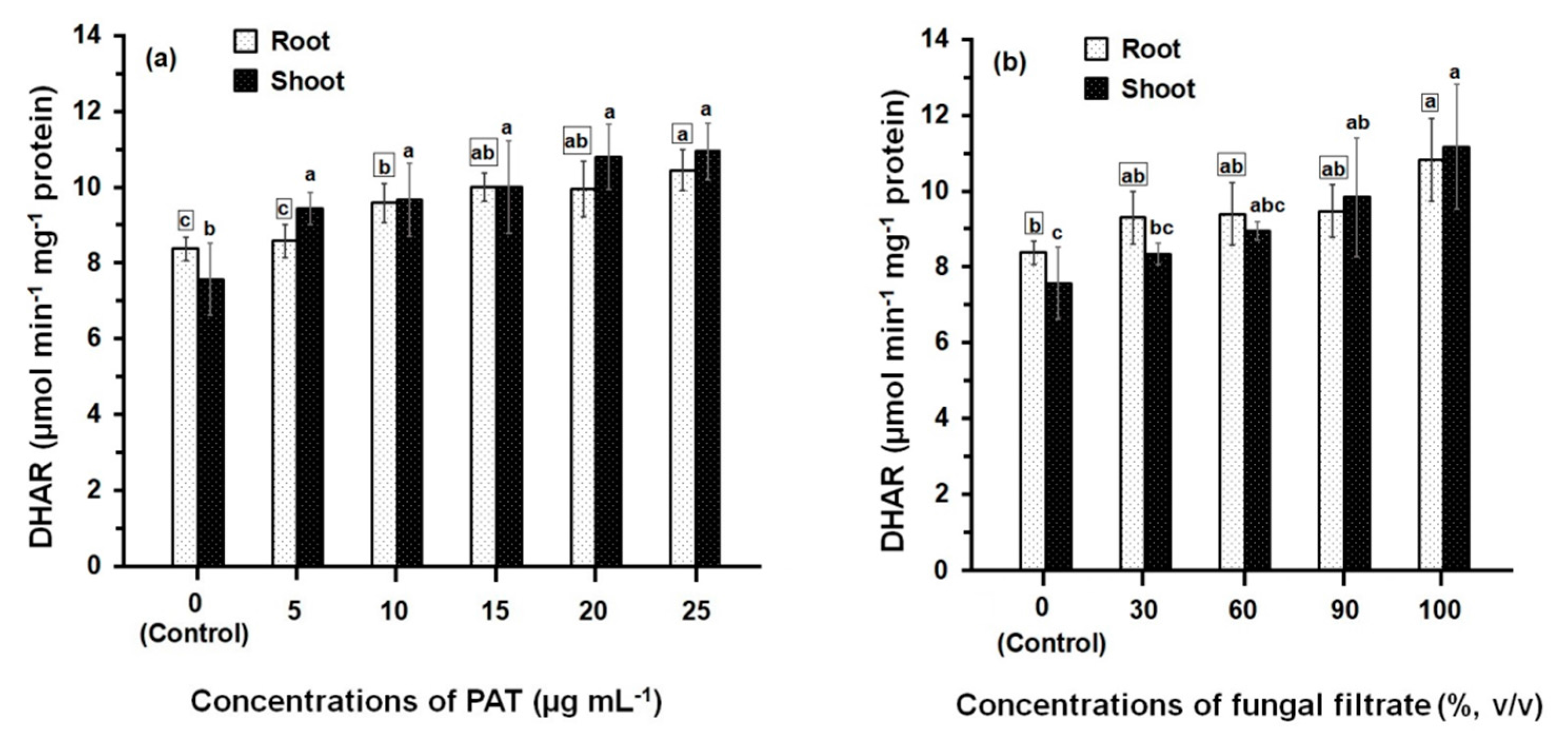
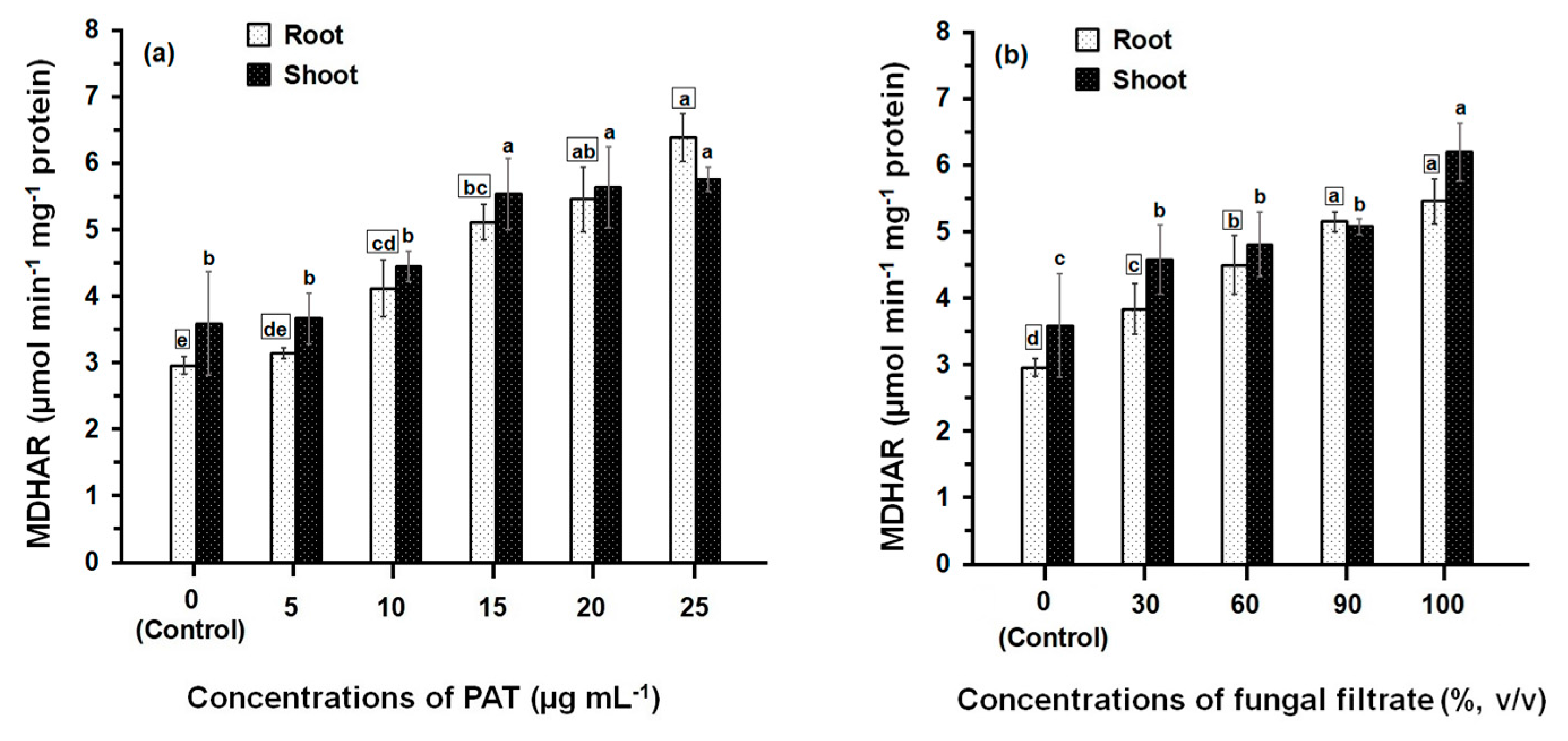
3.2. Response of Antioxidant Enzyme Activities
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Speijers, G.J.A. Patulin. In Mycotoxins in Food—Detection and Control; Magan, N., Olsen, M., Eds.; CRC Press: Cambridge, UK, 2004; pp. 339–352. [Google Scholar]
- Bugbee, W.M. Penicillium claviforme and Penicillium variabile: Pathogens of stored sugar beets. Phytopathology 1975, 65, 926–927. [Google Scholar] [CrossRef]
- Čatská, V.; Vančura, V.; Přikryl, Z.; Hudská, G. Artificial induction of the apple replant problem by Penicillium claviforme inoculation. Plant Soil 1988, 107, 127–136. [Google Scholar] [CrossRef]
- Kozlovskii, A.G.; Vinokurova, N.G.; Zhelifonova, V.P. Mycotoxin production profiles of Penicillium vulpinum (Cooke & Massee) Seifert & Samson strains. Microbiology 2000, 69, 36–39. [Google Scholar]
- Morales, H.; Marin, S.; Rovira, A.; Ramos, A.J.; Sanchis, V. Patulin accumulation in apples by Penicillium expansum during postharvest stages. Lett. Appl. Microbiol. 2007, 44, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.A.; Bassyouni, R.H.; Kamel, Z.; Gabr, S.M. Detoxification of patulin by kombucha tea culture. CyTA J. Food 2016, 14, 271–279. [Google Scholar] [CrossRef]
- Betina, V. Bioactive secondary metabolites of microorganisms. In Progress in Industrial Biology; Elsevier & Ister Science Press: Bratislava, Slovac Republic, 1994; Volume 30, pp. 169–170. [Google Scholar]
- Liu, B.H.; Wu, T.S.; Yu, F.Y.; Su, C.C. Induction of oxidative stress response by the mycotoxin patulin in mammalian cells. Toxicol. Sci. 2007, 95, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, H.; Shanmugasundaram, K.R.; Shanmugasundaram, E.R.B. Neurotoxic effect of patulin. Indian J. Exp. Biol. 1982, 20, 230–231. [Google Scholar] [PubMed]
- Alves, I.; Oliveira, N.G.; Laires, A.; Rodrigues, A.S.; Rueff, J. Induction of micronuclei and chromosomal aberrations by the mycotoxin patulin in mammalian cells: Role of ascorbic acid as a modulator of patulin clastogenicity. Mutagenesis 2000, 15, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, A.S.; Rosenthal, A.; Massaguer, P.R. The fate of patulin in apple juice processing: A review. Food Res. Int. 2008, 41, 441–453. [Google Scholar] [CrossRef]
- Selmanoglu, G.; Koçkaya, E.A. Investigation of the effects of patulin on thyroid and testis, and hormone levels in growing male rats. Food Chem. Toxicol. 2004, 42, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Ellis, J.R.; McCalla, T.M. Effects of patulin and method of application on growth stages of wheat. Appl. Microbiol. 1973, 25, 562–566. [Google Scholar] [PubMed]
- Ismaiel, A.A.; Papenbrock, J. The effects of patulin from Penicillium vulpinum on seedling growth, root tip ultrastructure and glutathione content of maize. Eur. J. Plant Pathol. 2014, 139, 497–509. [Google Scholar] [CrossRef]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int. Rev. Hydrobiol. 2015, 101, 3–19. [Google Scholar] [CrossRef]
- Tang, K.; Zhan, J.-C.; Yang, H.-R.; Huang, W.-D. Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Gil, S.; Estebaranz-Yubero, M.; Medel-Cuesta, D.; Millan, R.; Hernández, L.E. Influence of nitrate fertilization on Hg uptake and oxidative stress parameters in alfalfa plants cultivated in a Hg-polluted soil. Environ. Exp. Bot. 2012, 75, 16–24. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Moons, A. Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs). Vitam. Horm. 2005, 72, 155–202. [Google Scholar] [PubMed]
- Schumacher, D.M.; Müller, C.; Metzler, M.; Lehmann, L. DNA–DNA cross-links contribute to the mutagenic potential of the mycotoxin patulin. Toxicol. Lett. 2006, 166, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Polacco, J.C.; Sands, D.C. The mycotoxin patulin inhibits respiration of higher plant cells. Plant Sci. Lett. 1977, 9, 121–128. [Google Scholar] [CrossRef]
- Ashoor, S.H.; Chu, F.S. Inhibition of alcohol and lactic dehydrogenases by patulin and penicillic acid in vitro. Food Cosmet. Toxicol. 1973, 11, 617–624. [Google Scholar] [CrossRef]
- Ashoor, S.H.; Chu, F.S. Inhibition of muscle aldolase by penicillic acid and patulin in vitro. Food Cosmet. Toxicol. 1973, 11, 995–1000. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Diwald, T.T.; Metzler, M. Patulin reduces glutathione level and enzyme activities in rat liver slices. Mol. Nutr. Food Res. 2005, 49, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Schebb, N.H.; Faber, H.; Maul, R.; Heus, F.; Kool, J.; Irth, H.; Karst, U. Analysis of glutathione adducts of patulin by means of liquid chromatography (HPLC) with biochemical detection (BCD) and electrospray ionization tandem mass spectrometry (ESI-MS/MS). Anal. Bioanal. Chem. 2009, 394, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Sydenham, E.W.; Vismer, H.F.; Marasas, W.F.O.; Brown, N.; Schlechter, M.; Van Der Westhuizen, L.; Rheeder, J.P. Reduction of patulin in apple juice samples–influence of initial processing. Food Control 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Subramanian, T. Colorimetric determination of patulin produced by Penicillium patulum. J. Assoc. Off. Anal. Chem. 1982, 65, 5–7. [Google Scholar] [PubMed]
- Marcacci, S.; Raveton, M.; Ravanel, P.; Schwitzguébel, J. Conjugation of atrazine in vetiver (Chrysopogon zizanioides Nash) grown in hydroponics. Environ. Exp. Bot. 2006, 56, 205–215. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. In Plant Stress Tolerance, Methods in Molecular Biology; Sunkar, R., Ed.; Springer: Berlin, Germany, 2010; Volume 639, pp. 273–281. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Arafat, W.; Kern, D.; Dirheimer, G. Inhibition of aminoacyl-tRNA synthetases by the mycotoxin patulin. Chem. Biol. Interact. 1985, 56, 333–349. [Google Scholar] [CrossRef]
- Fliege, R.; Metzler, M. The mycotoxin patulin induces intra- and intermolecular protein crosslinks in vitro involving cysteine, lysine, and histidine side chains, and α-amino groups. Chem. Biol. Interact. 1999, 123, 85–103. [Google Scholar] [CrossRef]
- Opacka, B.B.; Escoula, L. Production of patulin in a liquid medium by moulds belonging to the genera: Aspergillus and Penicillium. Ann. Rech. Vet. 1977, 8, 129–133. [Google Scholar]
- Lovett, T.; Thompson, R.G., Jr. Patulin production by species of Aspergillus and Penicillium at 1.7, 7.2, and 12.8 C. J. Food Prot. 1978, 41, 195–197. [Google Scholar] [CrossRef]
- Steiman, R.; Seigle-Murandi, F.; Sage, L.; Krivobok, S. Production of patulin by Micromycetes. Mycopathologia 1989, 105, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, M.; Chen, I.; Huang, C.; Chao, L.; Hsieh, H. A Glutathione S-transferase regulated by light and hormones participates in the modulation of Arabidopsis seedling development. Plant Physiol. 2010, 154, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, B.; Keeler, S.J.; Lau, S.C.; Koeppe, M.K.; O’Keefe, D.P. A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 2000, 124, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.; Kausar, M.A.; Kumar, R.; Dhawan, A.; Dwivedi, P.D.; Das, M. Patulin causes DNA damage leading to cell cycle arrest and apoptosis through modulation of Bax, p53 and p21/WAF1 proteins in skin of mice. Toxicol. Appl. Pharmacol. 2009, 234, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E.J.; Notton, B.A.; Afridi, M.M.R.K. Comparative effects of some antimetabolites on ribonucleic acid synthesis and induction of nitrate reductase in cauliflower leaf tissues. Plant Cell Physiol. 1967, 8, 385–397. [Google Scholar]
- Hatey, F.; Moulé, Y. Protein synthesis inhibition in rat liver by the mycotoxin patulin. Toxicology 1979, 13, 223–231. [Google Scholar] [PubMed]
- Arafat, W.; Musa, M.N. Patulin-induced inhibition of protein synthesis in hepatoma tissue culture. Res. Commun. Mol. Pathol. Pharmacol. 1995, 87, 177–186. [Google Scholar] [PubMed]
- Smith, A.P.; Nourizadeh, S.D.; Peer, W.A.; Xu, J.; Bandyopadhyay, A.; Murphy, A.S.; Goldsbrough, P.B. Arabidopsis AtGSTF2 is regulated by ethylene and auxin, and encodes a glutathione S-transferase that interacts with flavonoids. Plant J. 2003, 36, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Jablonkai, I.; Hatzios, K.K. Role of glutathione and glutathione S-transferase in the selectivity of acetochlor in maize and wheat. Pestic. Biochem. Physiol. 1991, 41, 221–231. [Google Scholar] [CrossRef]
- Dalton, D.A.; Boniface, C.; Turner, Z.; Lindahl, A.; Kim, H.J.; Jelinek, L.; Govindarajulu, M.; Finger, R.E.; Taylor, C.G. Physiological roles of glutathione S-transferases in soybean root nodules. Plant Physiol. 2009, 150, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Welinder, K.G. Superfamily of plant, fungal and bacterial peroxidases. Curr. Opin. Struct. Biol. 1992, 2, 388–393. [Google Scholar] [CrossRef]
- Ghisla, S.; Massey, V. Mechanisms of flavoprotein-catalyzed reactions. Eur. J. Biochem. 1989, 181, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, T.; Maki, Y.; Sano, S.; Koshiba, K.; Asada, K.; Tsuji, H. Induction of enzymes involved in the ascorbate-dependent antioxidative system, namely, ascorbate peroxidase, monodehydroascorbate reductase and dehydroascorbate reductase, after exposure to air of rice (Oryza sativa) seedlings germinated under water. Plant Cell Physiol. 1997, 38, 541–549. [Google Scholar] [CrossRef]
- Hossain, M.A.; Asada, K. Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J. Biol. Chem. 1985, 260, 12920–12926. [Google Scholar] [PubMed]
- Iwahashi, Y.; Hosoda, H.; Park, J.H.; Lee, J.H.; Suzuki, Y.; Kitagawa, E.; Murata, S.M.; Jwa, N.M.; Gu, M.B.; Iwahashi, H. Mechanisms of patulin toxicity under conditions that inhibit yeast growth. J. Agric. Food Chem. 2006, 54, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, G.R.; Burchett, M.D. Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar. Pollut. Bull. 2001, 42, 233–240. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Wang, Y.S.; Lou, Z.P.; Dong, J.D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Jiang, X.; Liu, T.; Polle, A.; Liang, Z.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol. Plant. 2011, 143, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Junior, R.A.; Moldes, C.A.; Delite, F.S.; Pompeu, G.B.; Gratao, P.L.; Mazzafera, P.; Lea, P.J.; Azevedo, R.A. Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere 2006, 65, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.; Ibrahim, M.M.; Bagheri, R.; Ahmad, J.; Arif, I.A.; Baig, M.A.; Qureshi, M.I. Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in Indian mustard. AoB Plants 2015, 7, plv001. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Panneerselvam, R. Antioxidative potentials as a protective mechanism in Catharanthus roseus (L.) G. Don plants under salinity stress. Turk. J. Bot. 2007, 31, 245–251. [Google Scholar]

| PAT Conc. (μg·mL−1) | Root | Shoot | ||
|---|---|---|---|---|
| GST (nmol−1·min−1·mg−1 Protein) | Reduction (%) in GST Activity | GST (nmol−1·min−1·mg−1 Protein) | Reduction (%) in GST Activity | |
| 0.0 (control) | 272.68 ± 23.89 a | 0.00 | 113.28 ± 14.59 a | 0.00 |
| 5.0 | 144.31 ± 15.70 b | 47.0 | 70.42 ± 9.67 b | 37.8 |
| 10 | 120.71 ± 17.63 b,c | 55.7 | 64.11 ± 10.99 b | 43.4 |
| 15 | 117.28 ± 9.52 b,c | 57.0 | 59.45 ± 9.42 b | 47.5 |
| 20 | 89.75 ± 15.98 b,c | 67.1 | 56.73 ± 13.03 b | 50.2 |
| 25 | 71.38 ± 9.84 c | 73.8 | 45.19 ± 8.14 b | 60.1 |
| Fungal Filtrate Conc. (%, v/v) | Root | Shoot | ||
|---|---|---|---|---|
| GST (nmol−1·min−1·mg−1 Protein) | Reduction (%) in GST Activity | GST (nmol−1·min−1·mg−1 Protein) | Reduction (%) in GST Activity | |
| 0.0 (control) | 272.68 ± 23.89 a | 0.00 | 113.28 ± 14.59 a | 0.00 |
| 30 | 151.44 ± 19.03 b | 44.5 | 110.12 ± 12.09 a | 2.80 |
| 60 | 124.00 ± 15.89 c | 54.5 | 99.38 ± 12.04 a | 12.3 |
| 90 | 106.94 ± 10.26 c | 60.8 | 95.63 ± 11.56 a | 15.6 |
| 100 | 65.55 ± 4.41 d | 76.0 | 43.33 ± 2.19 b | 61.74 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismaiel, A.A.; Papenbrock, J. Effect of Patulin from Penicillium vulpinum on the Activity of Glutathione-S-Transferase and Selected Antioxidative Enzymes in Maize. Int. J. Environ. Res. Public Health 2017, 14, 825. https://doi.org/10.3390/ijerph14070825
Ismaiel AA, Papenbrock J. Effect of Patulin from Penicillium vulpinum on the Activity of Glutathione-S-Transferase and Selected Antioxidative Enzymes in Maize. International Journal of Environmental Research and Public Health. 2017; 14(7):825. https://doi.org/10.3390/ijerph14070825
Chicago/Turabian StyleIsmaiel, Ahmed A., and Jutta Papenbrock. 2017. "Effect of Patulin from Penicillium vulpinum on the Activity of Glutathione-S-Transferase and Selected Antioxidative Enzymes in Maize" International Journal of Environmental Research and Public Health 14, no. 7: 825. https://doi.org/10.3390/ijerph14070825






