Effects of Acid Mine Drainage on Calcareous Soil Characteristics and Lolium perenne L. Germination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Stimulated Acid Mine Drainage (AMD) Preparation
2.3. Interface Behavior of the Major Ions of AMD in Soil and Water after AMD is Added to Calcareous Soil
2.4. Effects of AMD on Calcareous Soil Characteristics
2.5. Effect of AMD-Polluted Calcareous Soil on Lolium Perenne Seed Germination Efficiency
2.6. Analytical Methods
2.7. Statistical Analyses
3. Results and Discussion
3.1. Acid Buffering Properties of the Soil Collected from Different Shanxi Regions
3.2. Total Fe, Fe2+, and SO42− Concentrations in AMD when It Reacts with the Calcareous Soil
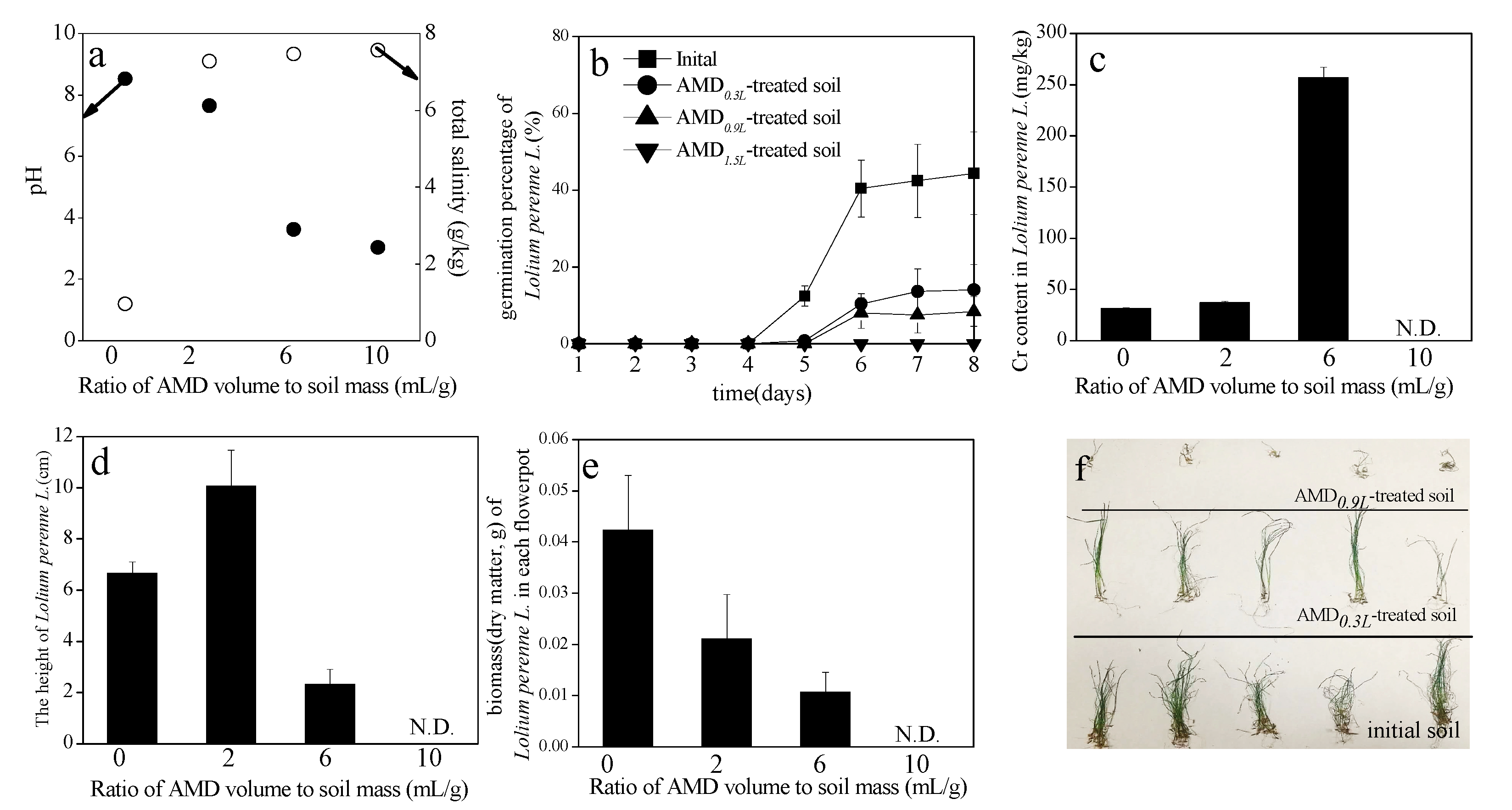
3.3. Effects of AMD on Calcareous Soil Characteristics
3.4. Effects of AMD on Heavy Metal Contents and Oxidation States in Calcareous Soil
3.5. Effects of AMD-Polluted Calcareous Soil on Lolium Perenne L. Seed Germination
4. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papaioannou, D.; Kalavrouziotis, I.K.; Koukoulakis, P.H.; Papadopoulos, F.; Psoma, P. Interrelationships of metal transfer factor under wastewater reuse and soil pollution. J. Environ. Manag. 2018, 216, 328–336. [Google Scholar] [CrossRef]
- Ye, S.J.; Zeng, G.M.; Wu, H.P.; Zhang, C.; Dai, J.; Liang, J.; Yu, J.F.; Ren, X.Y.; Yi, H.; Cheng, M.; et al. Co-occurrence and interactions of pollutants, and their impacts on soil remediation—A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1528–1553. [Google Scholar] [CrossRef]
- Ye, S.J.; Zeng, G.M.; Wu, H.P.; Zhang, C.; Liang, J.; Dai, J.; Liu, Z.F.; Xiong, W.P.; Wan, J.; Xu, P.; et al. Biological technologies for the remediation of cocontaminated soil. Crit. Rev. Biotechnol. 2017, 37, 1062–1076. [Google Scholar] [CrossRef]
- Sun, H.W.; Zhou, L.Z.; Zhou, D.M. POLSOIL: Research on soil pollution in China. Environ. Sci. Pollut. Rese. 2018, 25, 1–3. [Google Scholar] [CrossRef]
- Ye, S.J.; Zeng, G.M.; Wu, H.P.; Liang, J.; Zhang, C.; Dai, J.; Xiong, W.P.; Song, B.; Wu, S.H.; Yu, J.F. The effects of activated biochar addition on remediation efficiency of cocomposting with contaminated wetland soil. Resour. Conserv. Recycl. 2019, 140, 278–285. [Google Scholar] [CrossRef]
- Costa, M.C.; Duarte, J.C. Bioremediation of acid mine drainage using acidic soil and organic wastes for promoting sulphate-reducing bacteria activity on a column reactor. Water Air Soil Pollut. 2005, 165, 325–345. [Google Scholar] [CrossRef]
- Valente, T.; Grande, J.A.; Torre, M.L.; Gomes, P.; Santisteban, M.; Borrego, J.; Sequeira Braga, M.A. Mineralogy and geochemistry of a clogged mining reservoir affected by historical acid mine drainage in an abandoned mining area. J. Geochem. Explor. 2015, 157, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Mapanda, F.; Nyamadzawo, G.; Nyamangara, J.; Wuta, M. Effects of discharging acid-mine drainage into evaporation ponds lined with clay on chemical quality of the surrounding soil and water. Phys. Chem. Earth 2007, 32, 1366–1375. [Google Scholar] [CrossRef]
- Schmidt, A.; Haferburg, G.; Sineriz, M.; Merten, D.; Büchel, G.; Kothe, E. Heavy metal resistance mechanisms in actinobacteria for survival in AMD contaminated soils. Chemie der Erde 2005, 65, 131–144. [Google Scholar] [CrossRef]
- Zhou, J.M.; Dang, Z.; Cai, M.F.; Liu, C.Q. Soil heavy metal pollution around the Dabaoshan mine, Guangdong Province, China. Pedosphere 2007, 17, 588–594. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Bhattacharyya, P.; Tripathy, S.; Equeenuddin, S.M.; Panigrahi, M.K. Influence of different forms of acidities on soil microbiological properties an enzyme activities at an acid mine drainage contaminated site. J. Hazard. Mater. 2010, 179, 966–975. [Google Scholar] [CrossRef]
- Johnston, S.G.; Burton, E.D.; Aaso, T.; Tuckerman, G. Sulfur, iron and carbon cycling following hydrological restoration of acidic freshwater wetlands. Chem. Geol. 2014, 371, 9–26. [Google Scholar] [CrossRef]
- Karimian, N.; Johnston, S.G.; Burton, E.D. Iron and sulfur cycling in acid sulfate soil wetlands under dynamic redox conditions: A review. Chemosphere 2018, 197, 803–816. [Google Scholar] [CrossRef]
- Qu, L.; Xie, Y.Y.; Lu, G.N.; Yang, C.F.; Zhou, J.N.; Yi, X.Y.; Dang, Z. Distribution, fractionation, and contamination assessment of heavy metals in paddy soil related to acid mine drainage. Paddy Water Environ. 2017, 15, 553–562. [Google Scholar] [CrossRef]
- State Environmental Protection Agency of China (SEPAC). Environmental Quality Standard for Soils; State Environmental Protection Administration: Beijing, China, 1995. [Google Scholar]
- Li, Y.T.; Becquer, T.; Dai, J.; Quantin, C.; Benedetti, M.F. Ion activity and distribution of heavy metals in acid mine drainage polluted subtropical soils. Environ. Pollut. 2009, 157, 1249–1257. [Google Scholar] [CrossRef]
- Yang, C.F.; Lu, G.N.; Chen, M.Q.; Xie, Y.Y.; Guo, C.L.; Reinfelder, J.; Yi, X.Y.; Wang, H.; Dang, Z. Spatial and temporal distributions of sulfur species in paddy soils affected by acid mine drainage in Dabaoshan sulfide mining area, South China. Geoderma 2016, 281, 21–29. [Google Scholar] [CrossRef]
- Liao, J.B.; Wen, Z.W.; Ru, X.; Chen, J.D.; Wu, H.Z.; Wei, C.H. Distribution and migration of heavy metals in soil and crops affected by acid mine drainage: Public health implications in Guangdong Province, China. Ecotoxicol. Environ. Saf. 2016, 124, 460–469. [Google Scholar] [CrossRef]
- Zhao, F.H.; Cong, Z.Y.; Sun, H.F.; Ren, D.Y. The geochemistry of rare earth elements (REE) in acid mine drainage from the Sitai coal mine, Shanxi Province, North China. Int. J. Coal Geol. 2007, 70, 184–192. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, F.; Zhang, M.; Li, J. Behavior of rare earth elements in acid coal mine drainage in Shanxi Province, China. Environ. Earth Sci. 2012, 67, 205–213. [Google Scholar] [CrossRef]
- Liu, F.W.; Qiao, X.X.; Zhou, L.X.; Zhang, J. Migration and fate of acid mine drainage pollutants in calcareous soil. Int. J. Environ. Res. Public Health 2018, 15, 1759. [Google Scholar] [CrossRef]
- Fawcett, J.K. The semi-micro Kjeldahl method for the determination of nitrogen. J. Med. Lab. Technol. 1954, 12, 1–22. [Google Scholar] [PubMed]
- Wang, H.M.; Wang, W.J.; Chen, H.; Zhang, Z.; Mao, Z.; Zu, Y.G. Temporal changes of soil physic-chemical properties at different soil depths during larch afforestation by multivariate analysis of covariance. Ecol. Evol. 2014, 4, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Liu, M.D.; Yu, D.; He, H.H.; Chen, H. Effect of ecological orchard construction on soil N, P, K content. Southwest China J. Agric. Sci. 2010, 2, 444–448. [Google Scholar]
- Qiao, R.; Guo, G. Determination of calcium oxide, magnesium oxide and silicon dioxide in dolomite and limestone by X-ray fluorescence spectrometry. Metal. Anal. 2014, 34, 75–78. [Google Scholar]
- Liu, F.W.; Zhou, J.; Jin, T.J.; Zhang, S.S.; Liu, L.L. Effect of calcium oxide on the efficiency of ferrous ion oxidation and total iron precipitation during ferrous ion oxidation in simulated acid mine drainage treatment with inoculation of Acidithiobacillus ferrooxidans. Water Sci. Technol. 2016, 73, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Y.; Cui, Y.S.; Duan, J.; Tang, L. Pilot study of temporal variations in lead bioaccessibility and chemical fractionation in some Chinese soils. J. Hazard. Mater. 2008, 160, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, J.D.; Lesch, S.M.; Shouse, P.J.; Alves, W.J. New calibrations for determining soil electrical conductivity—Depth relations from electromagnetic measurements. Soil Sci. Soc. Am. J. 1989, 53, 74–79. [Google Scholar] [CrossRef]
- Cai, X.B.; Peng, Y.L.; Yu, B.Z. Soil aggregates organic carbon change and its influence in Tibetan alpine steppe. Trans. Chin. Soc. Agric. Eng. 2013, 29, 92–99. [Google Scholar]
- Liu, F.W.; Zhou, J.; Zhou, L.X.; Zhang, S.S.; Liu, L.L.; Wang, M. Effect of neutralized solid waste generated in lime neutralization on the ferrous ion bio-oxidation process during acid mine drainage. J. Hazard. Mater. 2015, 299, 404–411. [Google Scholar] [CrossRef]
- Long, J.; Chen, R.; Zhang, N. Importance of temperature control in hydrometer method used for measurement of soil texture. Geology of Anhui. Geol. Anhui 2014, 3, 63–65. [Google Scholar]
- Zhang, X.H.; Ding, H.; Zhou, Z.X.; He, Y.N. Effects of wheat/alfalfa intercropping on soil nutrients. Sch. J. Agric. Vet. Sci. 2016, 3, 346–350. [Google Scholar]
- Wu, Y.P.; Li, Y.F.; Zheng, C.Y.; Zhang, Y.F.; Sun, Z.J. Organic amendment application influence soil organism abundance in saline alkali soil. Eur. J. Soil Biol. 2013, 54, 32–40. [Google Scholar] [CrossRef]
- Chaudhari, U.E.; Wanjari, A.K.; Kumre, N.D.; Ahmad, A.N.M.; Barde, M.P. Assessment of macro and micro nutrients in alkaline soil from Satpuda Region Orange Belt, Maharashtra, India. Adv. Appl. Sci. Res. 2016, 7, 175–178. [Google Scholar]
- Tapadar, S.A.; Jha, D.K. Seasonal and temporal dynamics of physicochemical and biological properties of chronosequence coal mine spoil soils. Clean-Soil Air Water 2016, 44, 1405–1413. [Google Scholar] [CrossRef]
- Nelson, P.N.; Su, N.H. Soil pH buffering capacity: A descriptive function and its application to some acidic tropical soils. Aust. J. Soil Res. 2010, 48, 201–207. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zhang, J.Y. Soil in Shanxi Province; Science Press: Beijing, China, 1992. [Google Scholar]
- Magdoff, F.R.; Bartlett, R.J. Soil pH buffering revisited. Soil Sci. Soc. Am. J. 1985, 49, 145–148. [Google Scholar] [CrossRef]
- Xu, S.L.; Duan, W.H.; Liu, S.J.; Zheng, X.J.; She, G.H. A study on the ferrous ion oxidized by the air in aqueous solution. J. Yunnan Univ. 1986, 8, 191–197. [Google Scholar]
- Blodau, C. A review of acidity generation and consumption in acidic coal mine lakes and their watersheds. Sci. Total Environ. 2006, 369, 307–332. [Google Scholar] [CrossRef]
- Karimian, N.; Johnston, S.G.; Burton, E.D. Effect of cyclic redox oscillations on water quality in freshwater acid sulfate soil wetlands. Sci. Total Environ. 2017, 581–582, 314–327. [Google Scholar] [CrossRef]
- Tian, W.J.; Zhao, F.N.; He, X.W.; Li, F.S. Effects of simulated acid rain on the release of polycyclic aromatic hydrocarbons from top soils of an industrially contaminated site. Environ. Chem. 2012, 31, 497–503. [Google Scholar]
- Mullins, G. Phosphorus, Agriculture & the Environment; Virginia Cooperative Extension Publication, Virginia Tech: Blacksburg, VA, USA, 2009. [Google Scholar]
- Akinrinde, E.A.; Obigbesan, G.O. Quantity-intensity parameters of potassium in relation to uptake by guinea-corn in representative soils of the ecological zones of Nigeria. Commun. Soil Sci. Plant Anal. 1999, 30, 2695–2710. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, J.P.; Liu, S.P.; Wang, C.W.; Liu, Y.H. Effect of simulated acid rain on nitrogen transplant and acidifying potential in Litchi Orchard soils. J. Soil Water Conserv. 2006, 20, 18–21. [Google Scholar]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Zhu, J.; Pigna, M.; Cozzolino, V.; Caporale, A.G.; Violante, A. Competitive sorption of copper(II), chromium(III) and lead(II) on ferrihydrite and two organomineral complexes. Geoderma 2010, 159, 409–416. [Google Scholar] [CrossRef]
- Chen, F.X.; Zhou, L.X. Adsorption of Cr(VI) in wastewater by catalytic biosynthesis Schwertmannite. China Environ. Sci. 2006, 26, 11–15. [Google Scholar]
- Strobel, B.W.; Hansen HC, B.; Borggaard, O.K.; Andersen, M.K.; Rasmussen, K.R. Cadmium and copper release kinetics in relation to afforestation of cultivated soil. Geochimica et Cosmochimica Acta 2001, 65, 1233–1242. [Google Scholar] [CrossRef]
- Laghmouchi, Y.; Belmehdi, O.; Bouyahya, A.; Senhaji, N.S.; Abrini, J. Effect of temperature, salt stress and pH on seed germination of medicinal plant origanum compactum. Biocatal. Agric. Biotechnol. 2017, 10, 156–169. [Google Scholar] [CrossRef]
- Peerzada, A.M.; Naeem, M. Germination ecology of Cenchrus biflorus Roxb.: Effects of environmental factors on seed germination. Rangel. Ecol. Manag. 2018, 71, 424–432. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, J.; Zhao, L. Effect of ammonia in the sediment on the growth and physiological characteristics of submerged macrophytes. Ecol. Environ. 2006, 15, 914–920. [Google Scholar]
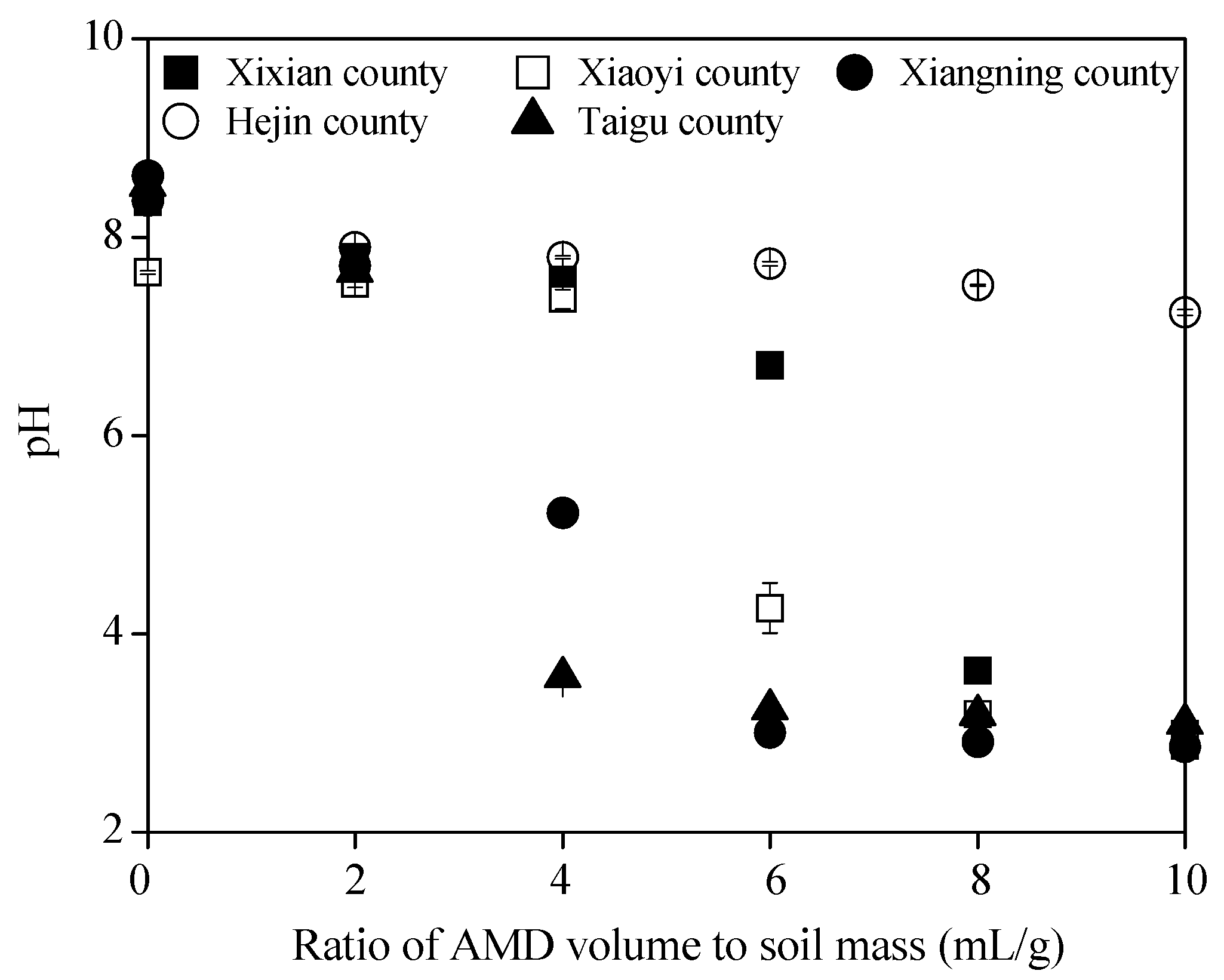
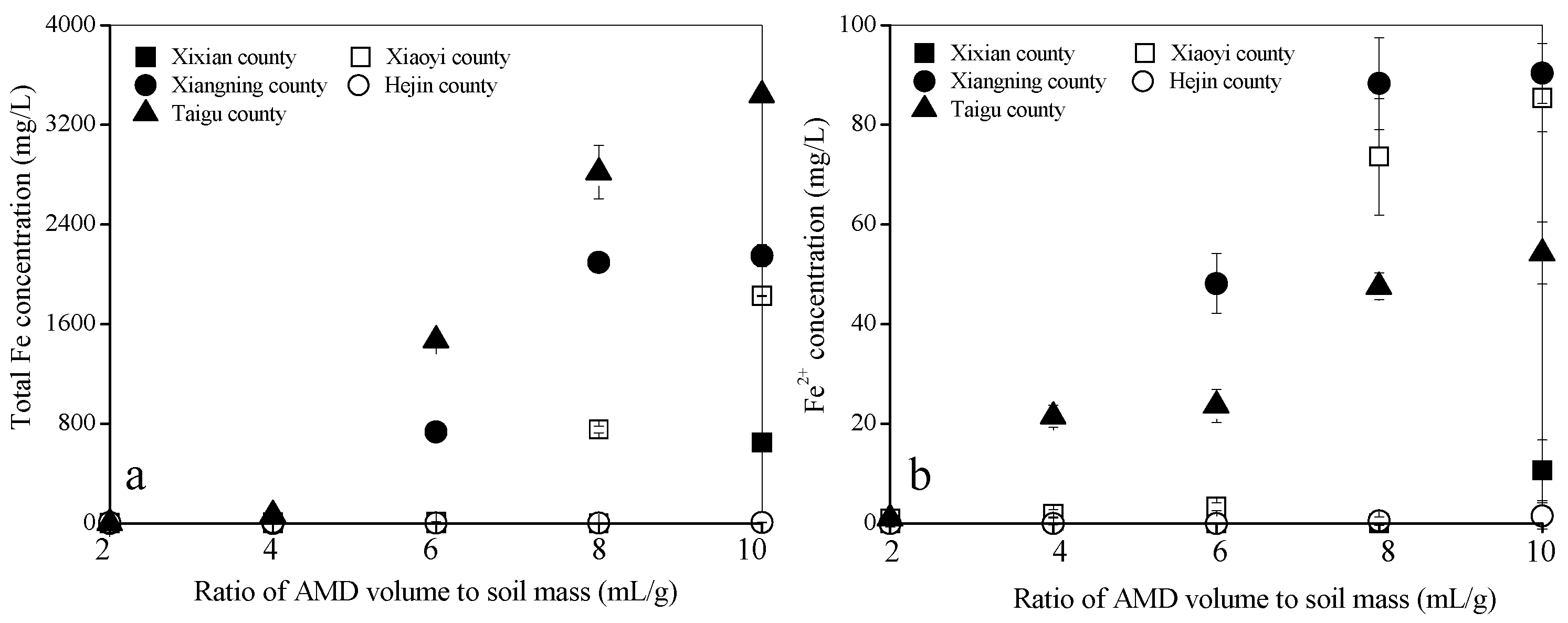


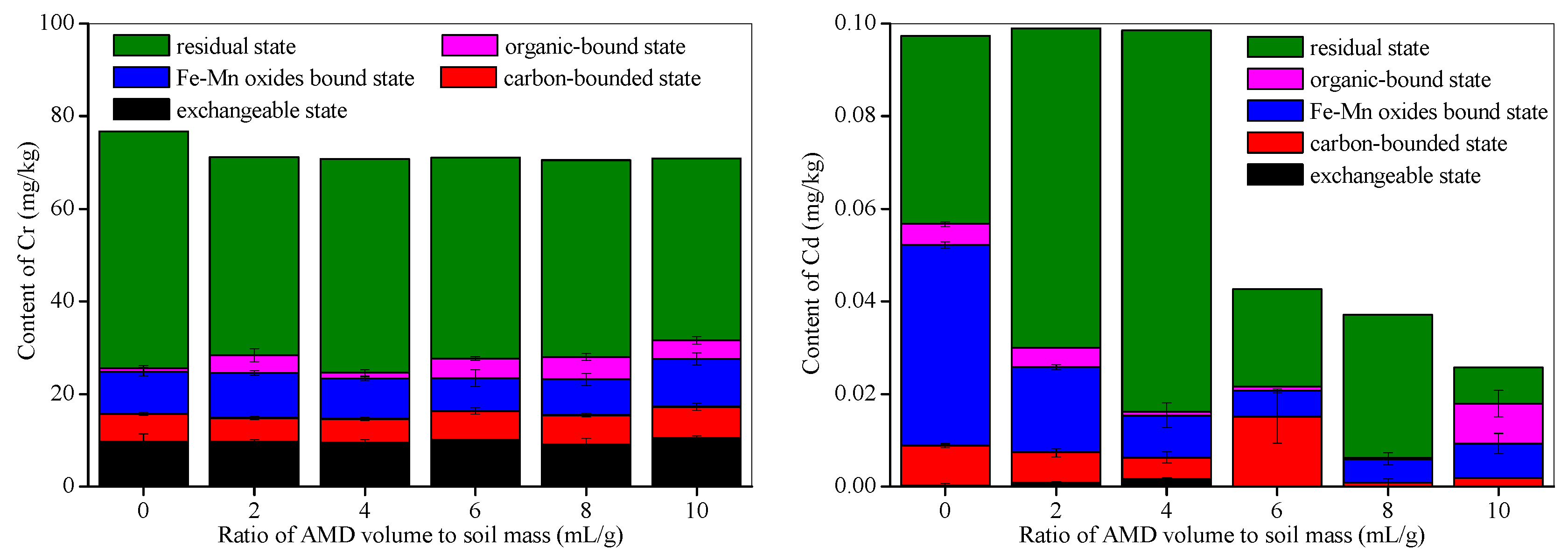
| Soil Sample | pH | Organic Matter (g/kg d.w.) | Total N (g/kg d.w.) | Total P (g/kg d.w.) | Total K (g/kg d.w.) | Available N (mg/kg d.w.) | Available P (mg/kg d.w.) | Available K (mg/kg d.w.) | CaO (% d.w.) | MgO (% d.w.) | Physical Clay Content (<0.01 mm) (% d.w.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Taigu County | 8.52 | 18.32 | 0.93 | 0.68 | 26.94 | 30.71 | 18.45 | 218.23 | 4.17 | 2.25 | 19.87 |
| Xiaoyi County | 7.64 | 24.99 | 1.12 | 0.58 | 21.43 | 45.55 | 44.85 | 127.40 | 5.74 | 1.89 | 27.42 |
| Xiangning County | 8.62 | 19.15 | 1.05 | 0.08 | 19.42 | 51.77 | 7.08 | 153.37 | 4.76 | 1.96 | 39.66 |
| Hejin County | 8.37 | 26.59 | 0.80 | 0.20 | 16.92 | 37.93 | 17.57 | 316.13 | 11.36 | 2.87 | 19.46 |
| Xixian County | 8.36 | 16.96 | 1.05 | 0.56 | 18.67 | 37.32 | 43.22 | 90.83 | 7.28 | 2.24 | 25.58 |
| CaO | CaO + MgO | MgO | Organic Matter | Clay Content | Available N | Available P | Available K | Total N | Total P | Total K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil pH reduction | −0.906 * | −0.913* | −0.847 | −0.817 | 0.531 | 0.183 | 0.135 | −0.812 | 0.752 | 0.356 | 0.505 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Liu, F.; Qiao, X.; Zhou, L.; Bi, W. Effects of Acid Mine Drainage on Calcareous Soil Characteristics and Lolium perenne L. Germination. Int. J. Environ. Res. Public Health 2018, 15, 2742. https://doi.org/10.3390/ijerph15122742
Dong Y, Liu F, Qiao X, Zhou L, Bi W. Effects of Acid Mine Drainage on Calcareous Soil Characteristics and Lolium perenne L. Germination. International Journal of Environmental Research and Public Health. 2018; 15(12):2742. https://doi.org/10.3390/ijerph15122742
Chicago/Turabian StyleDong, Yan, Fenwu Liu, Xingxing Qiao, Lixiang Zhou, and Wenlong Bi. 2018. "Effects of Acid Mine Drainage on Calcareous Soil Characteristics and Lolium perenne L. Germination" International Journal of Environmental Research and Public Health 15, no. 12: 2742. https://doi.org/10.3390/ijerph15122742
APA StyleDong, Y., Liu, F., Qiao, X., Zhou, L., & Bi, W. (2018). Effects of Acid Mine Drainage on Calcareous Soil Characteristics and Lolium perenne L. Germination. International Journal of Environmental Research and Public Health, 15(12), 2742. https://doi.org/10.3390/ijerph15122742





