The Effect of UV Aging on Antimicrobial and Mechanical Properties of PLA Films with Incorporated Zinc Oxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PLA Films Preparation and Antimicrobial Properties Analysis
2.3. Accelerated UV-A and Q-SUN Irradiation
2.4. Fourier Transform Infrared Analysis
2.5. Mechanical Analysis
2.6. Statistical Analysis
3. Results
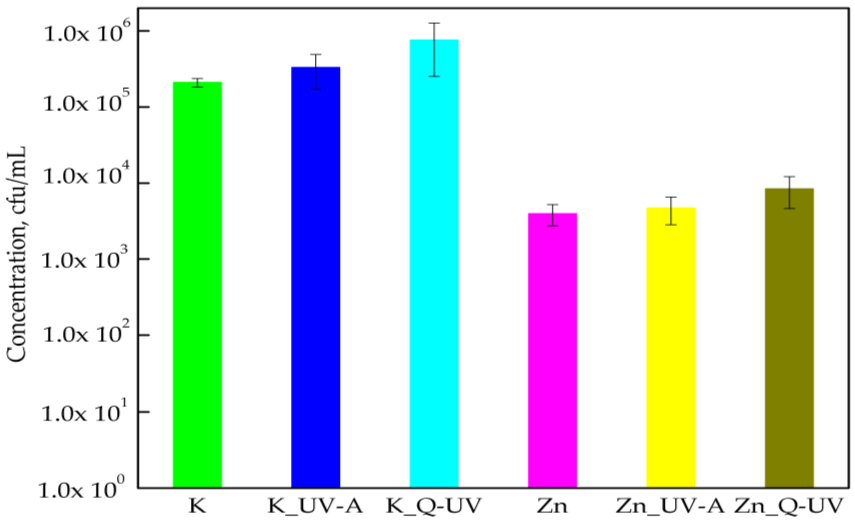
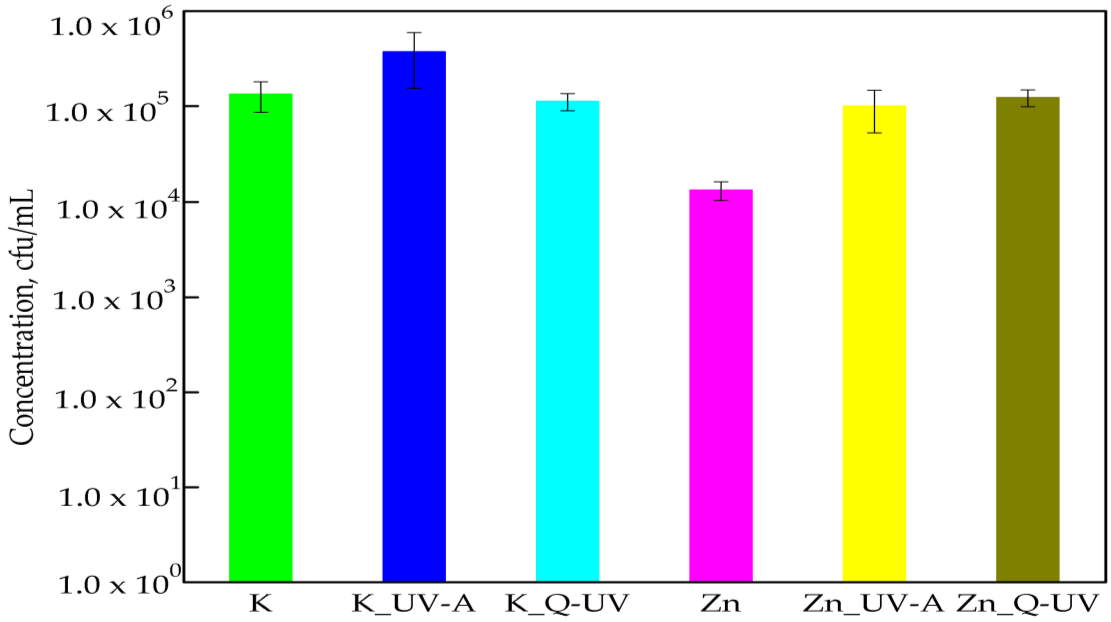
3.1. The Activity of PLA Films against Chosen Microorganisms
3.2. Fourier Transform Infrared Spectroscopy
3.3. Mechanical Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rhim, J.W.; Park, H.M.; Chang-Sik Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Winickoff, J.P.; Ivanoff, B.; Clemens, J.D.; Swerdlow, D.L.; Sansonetti, P.J.; Adak, G.K.; Levine, M.M. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 1999, 77, 651–666. [Google Scholar] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready to-eat poultry meat. Food Control 2014, 38, 88–95. [Google Scholar] [CrossRef]
- Marra, A.; Rollo, G.; Cimmino, S.; Silvestre, C. Assessment on the effects of ZnO and Coated ZnO particles on iPP and PLA properties for application in food packaging. Coatings 2017, 7, 29. [Google Scholar] [CrossRef]
- Mizielińska, M.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The influence of accelerated UV-A and Q-SUN irradiation on the antimicrobial properties of coatings containing ZnO nanoparticles. Molecules 2017, 22, 1156. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Lisiecki, S.; Jotko, M.; Chodzyńska, I.; Bartkowiak, A. The antimicrobial properties of polylactide films covered with ZnO nanoparticles-containing layers. Przem. Chem. 2015, 94, 1205–1208. [Google Scholar]
- Bartkowiak, A.; Mizielińska, M.; Sumińska, P.; Romanowska-Osuch, A.; Lisiecki, S. Innovations in food packaging materials. In Emerging and Traditional Technologies for Safe, Healthy and Quality Food; Nedovic, V., Raspor, P., Lević, J., Tumbas, V., Barbosa-Canovas, G.V., Eds.; Springer: Berlin, Germany, 2016. [Google Scholar]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S.; Najafi, A.; Ahmad, M. Fabrication and characterization of novel semolina-based antimicrobial films derived from the combination of ZnO nanorods and nanokaolin. J. Food Sci. Technol. 2017, 54, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, J.; Xu, Q.; Zhang, J. Fabrication of antibacterial casein-based ZnO nanocomposite for flexible coatings. Mater. Des. 2017, 113, 240–245. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Oprea, A.E.; Pandel, L.M.; Dumitrescu, A.M.; Andronescu, E.; Grumezescu, V.; Chifiriuc, M.C.; Mogoantă, L.; Bălşeanu, T.-A.; Mogoşanu, G.D.; Socol, G.; et al. Bioactive ZnO coatings deposited by maple—An appropriate strategy to produce efficient anti-biofilm surfaces. Molecules 2016, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A. Synthesis, antibacterial and thermal studies of cellulose nanocrystal stabilized ZnO-Ag heterostructure nanoparticles. Molecules 2013, 18, 6269–6280. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- Shi, L.E.; Li, Z.H.; Zheng, W.; Zhao, Y.F.; Jin, Y.F.; Tang, Z.X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. Part A 2014, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, C.; Duraccio, D.; Marra, A.; Strongone, V.; Cimmino, S. Development of antimicrobial composite films based on isotactic polypropylene and coated ZnO particles for active food packaging. Coatings 2016, 6, 4. [Google Scholar] [CrossRef]
- Castro-Mayorgaa, J.L.; Fabraa, M.J.; Pourrahimib, A.M.; Olssonb, R.T.; Lagarona, J.M. The impact of zinc oxide particle morphology as anantimicrobial and when incorporated inpoly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for food packaging and food contact surfacesapplications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lledo, M.L.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. Food Sci. Technol. 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K. Flexible chitosan-nano ZnO antimicrobial pouches as a new material for extending the shelf life of raw meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Espita, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; de Andrade, N.J.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. Part A 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Dhapte, V.; Gaikwad, N.; More, P.V.; Banerjee, S.; Dhapte, V.V.; Kadam, S.; Khanna, P.K. Transparent ZnO/polycarbonate nanocomposite for food packaging applications. Nanocomposites 2015, 1, 106–112. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Cao, Y.; Lan, T.; Chen, H.; Qin, Y. Effects of PLA film incorporated with ZnO nanoparticle on the quality attributes of fresh-cut apple. Nanomaterials 2017, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Fortunati, E.; Vargas, M.; Chiralt, A.; Kenny, J.M. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. Food Eng. 2013, 119, 236–243. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef] [PubMed]
- Esmailzadeh, H.; Sangpour, P.; Shahraz, F.; Hejazi, J.; Khaksar, R. Effect of nanocomposite packaging containing ZnO on growth of Bacillus subtilis and Enterobacter aerogenes. Mater. Sci. Eng. 2016, 58, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Nafchi, A.M.; Jokar, M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nguyena, T.V.; Dao, P.H.; Khanh Linh Duong, K.L.; Duong, Q.H.; Vu, Q.T.; Nguyena, A.H.; Mac, V.P.; Lea, T.L. Effect of R-TiO2 and ZnO nanoparticles on the UV-shielding efficiency of water-borne acrylic coating. Prog. Org. Coat. 2017, 110, 114–121. [Google Scholar] [CrossRef]
- El-Feky, O.M.; Hassan, E.A.; Fadel, S.M.; Hassan, M.L. Use of ZnO nanoparticles for protecting oil paintings on paper support against dirt, fungal attack, and UV aging. J. Cult. Herit. 2014, 15, 165–172. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A.; Hallaj, R. Influence of nano-ZnO on microbial growth, bioactive content and postharvest quality of strawberries during storage. Innov. Food. Sci. Emerg. Technol. 2016, 35, 168–176. [Google Scholar] [CrossRef]
- ASTM Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials; E 2180-01; ASTM: West Conshohocken, PA, USA, 2002.
- Nichols, M.; Boisseau, J.; Pattison, L.; Campbell, D.; Quill, J.; Zhang, J.; Smith, D.; Henderson, K.; Seebergh, J.; Berry, D.; et al. An improved accelerated weathering protocol to anticipate Florida exposure behavior of coatings. J. Coat. Technol. Res. 2013, 10, 153–173. [Google Scholar] [CrossRef]
- Plastics—Determination of Tensile Properties—Part 3: Test Conditions. PN-EN ISO 527–3:1998. Available online: http://sklep.pkn.pl/pn-en-iso-527-3-1998p.html (accessed on 13 September 2017).
- Iconaru, S.L.; Prodan, A.M.; Buton, N.; Predoi, D. Structural characterization and antifungal studies of zinc-doped hydroxyapatite coatings. Molecules 2017, 22, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. Biol. 2013, 128, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gunalan, S.; Sivaraj, R.; Rajendranb, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surf. B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.H.; Djurišić, A.B.; Chan, C.M.N.; Xi, Y.Y.; Tse, C.W.; Leung, Y.H.; Chan, W.K.; Leung, F.C.C.; Au, D.W.T. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films 2008, 516, 6167–6174. [Google Scholar] [CrossRef]
- Aysa, N.H.; Salman, D.H. Antibacterial activity of modified zinc oxide nanoparticles against Pseudomonas aeruginosa isolates of burn infections. World Sci. News 2016, 33, 1–14. [Google Scholar]
- Liu, Y.; Kim, H.I. Characterization and antibacterial properties of genipin-crosslinked chitosan/poly(ethylene glycol)/ZnO/Ag nanocomposites. Carbohydr. Polym. 2012, 89, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Natesan Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for food packaging. Polym. Adv. Technol. 2017, 28, 20–27. [Google Scholar] [CrossRef]
- Gandhi, R.R.; Gowri, S.; Suresh, J.; Sundrarajan, M. Ionic liquids assisted synthesis of ZnO nanostructures: Controlled size, morphology and antibacterial properties. J. Mater. Sci. Technol. 2013, 29, 533–538. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, S.; Kaitha, B.S.; Rajputa, J.; Kaur, M. Synthesis of ZnO nanoparticles using surfactant free in-air and microwave method. Appl. Surf. Sci. 2011, 257, 9661–9672. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of antimicrobial poly (lactic acid)/nano-composite films with silver and zinc oxide nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [PubMed]





| Sample | Fmax | F at Break | Elongation at Break | |
|---|---|---|---|---|
| N | N | % | ||
| Not irradiated | Machine direction | 143.13 ± 15.23 | 26.80 ± 4.50 | 109.26 ± 3.87 |
| Transverse direction | 97.93 ± 14.91 | 22.60 ± 3.66 | 102.36 ± 0.67 | |
| Irradiated with UV-A | Machine direction | 140.13 ± 22.59 | 24.68 ± 5.92 | 106.12 ± 1.20 |
| Transverse direction | 105.78 ± 7.64 | 18.78 ± 2.63 | 103.05 ± 0.44 | |
| Irradiated with Q-UV | Machine direction | 141.50 ± 18.07 | 27.37 ± 2.91 | 105.69 ± 2.04 |
| Transverse direction | 93.43 ± 10.13 | 21.40 ± 1.46 | 102.36 ± 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizielińska, M.; Kowalska, U.; Jarosz, M.; Sumińska, P.; Landercy, N.; Duquesne, E. The Effect of UV Aging on Antimicrobial and Mechanical Properties of PLA Films with Incorporated Zinc Oxide Nanoparticles. Int. J. Environ. Res. Public Health 2018, 15, 794. https://doi.org/10.3390/ijerph15040794
Mizielińska M, Kowalska U, Jarosz M, Sumińska P, Landercy N, Duquesne E. The Effect of UV Aging on Antimicrobial and Mechanical Properties of PLA Films with Incorporated Zinc Oxide Nanoparticles. International Journal of Environmental Research and Public Health. 2018; 15(4):794. https://doi.org/10.3390/ijerph15040794
Chicago/Turabian StyleMizielińska, Małgorzata, Urszula Kowalska, Michał Jarosz, Patrycja Sumińska, Nicolas Landercy, and Emmanuel Duquesne. 2018. "The Effect of UV Aging on Antimicrobial and Mechanical Properties of PLA Films with Incorporated Zinc Oxide Nanoparticles" International Journal of Environmental Research and Public Health 15, no. 4: 794. https://doi.org/10.3390/ijerph15040794
APA StyleMizielińska, M., Kowalska, U., Jarosz, M., Sumińska, P., Landercy, N., & Duquesne, E. (2018). The Effect of UV Aging on Antimicrobial and Mechanical Properties of PLA Films with Incorporated Zinc Oxide Nanoparticles. International Journal of Environmental Research and Public Health, 15(4), 794. https://doi.org/10.3390/ijerph15040794







