Association of Plasma Lipids and Polar Metabolites with Low Bone Mineral Density in Singaporean-Chinese Menopausal Women: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Study Population, Inclusion and Exclusion Criteria
2.3. Blood Collection
2.4. Analysis of Blood Parameters
2.5. Bone Mineral Density
2.6. Metabolomic Analysis
2.6.1. Sample Preparation
2.6.2. LC-MS Conditions and Metabolite Identification
2.6.3. Metabolite Identification and Processing
2.7. Statistical Analyses
2.7.1. Univariate Analysis
2.7.2. Multivariate Analysis
3. Results
3.1. Characteristics of the Menopausal Women Bone Status
3.1.1. Entire Cohort
3.1.2. Subset
3.2. Untargeted Analysis Metabolomic Approach
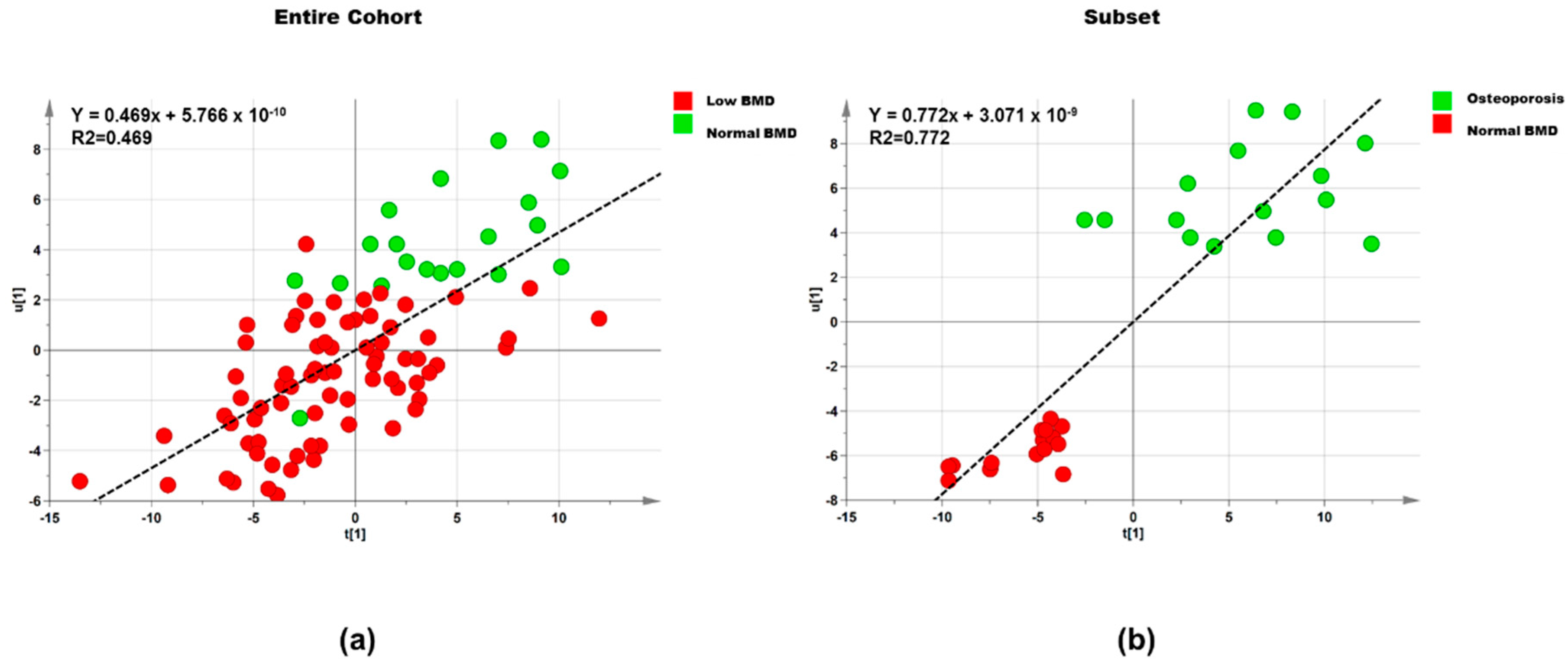
3.2.1. Lipids
Entire Cohort
Subset
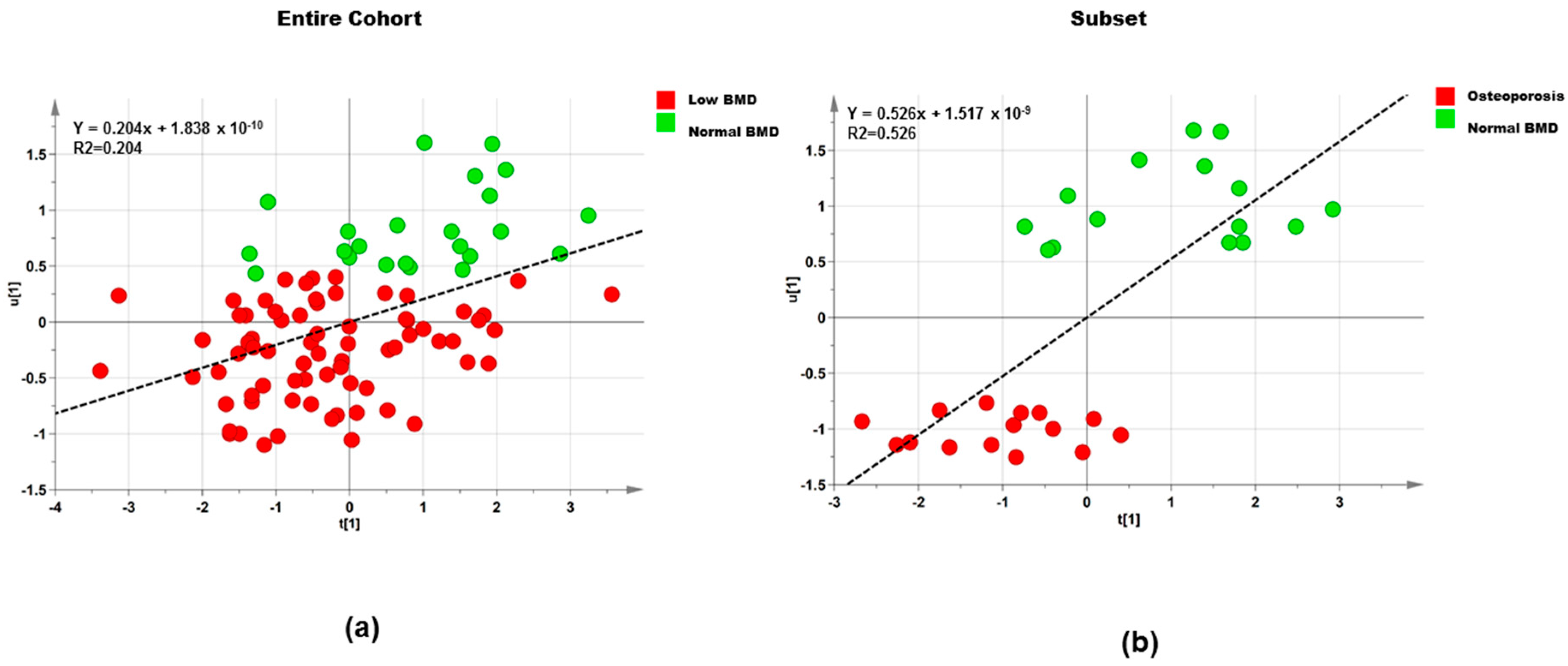
3.2.2. Polar Metabolites
Entire Cohort
Subset
4. Discussion
4.1. Lipids
4.2. Polar Metabolites
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| BMD | Bone mineral density |
| BMI | Body mass index |
| BMUs | Basic multicellular units |
| CTx-1 | c-terminal telopeptide of type I collagen |
| DG | Diacylglycerol |
| DXA | Dual X-ray absorptiometry |
| EDTA | Ethylenediamine tetraacetic acid |
| HILIC LC-MS | Hydrophilic interaction chromatography liquid chromatography mass spectrometry |
| IL-1 | Interleukin-1 |
| IK-6 | Interleukin-6 |
| CerP | Ceramide-1-phosphate |
| S1P | Sphingosine-1-phosphate |
| M-CSF | Macrophage colony-stimulating factor |
| MSCs | Mesenchymal stem cells |
| OPLS | Orthogonal partial least squares |
| OVX | Ovariectomized |
| PA | Phosphatidic acid |
| PI | Phosphatidylinositol |
| Plasmenyl-PE | Plasmenylphosphatidylethanolamine |
| PE | Phosphatidylethanolamine |
| PPARy | Peroxisome proliferator-activated receptor |
| PS | Phosphatidylserine |
| PTH | parathyroid hormone |
| ROS | Reactive oxygen species |
| RP (LC-MS) | Reverse-phase liquid chromatography mass spectrometry |
| SC | Singaporean-Chinese |
| TNF-α | Tumor necrosis factor-α |
| QC | Quality control |
| WHO | World Health Organization |
References
- Aaseth, J.; Boivin, G.; Andersen, O. Osteoporosis and trace elements—An overview. J. Trace Elem. Med. Biol. 2012, 26, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Cole, Z.A.; Holroyd, C.R.; Earl, S.C.; Harvey, N.C.; Dennison, E.M.; Melton, L.J.; Cummings, S.R.; Kanis, J.A. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos. Int. 2011, 22, 1277. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked Decrease in Plasma Antioxidants in Aged Osteoporotic Women: Results of a Cross-Sectional Study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Riggs, B.L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Investig. 2000, 106, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Hangoc, G.; Girasole, G.; Passeri, G.; Williams, D.C.; Abrams, J.S.; Boyce, B.; Broxmeyer, H.; Manolagas, S.C. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 1992, 257, 88. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-R.; Huang, R.-Q.; Xiao, B.-K.; Yang, J.-Y.; Dong, J.-X. 1H NMR metabolic profiling analysis offers evaluation of Nilestriol treatment in ovariectomised rats. Mol. Cell. Endocrinol. 2014, 387, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-R.; Xiao, B.-K.; Yang, J.-Y.; Guo, C.-H.; Shen, S.-J.; Tang, Z.-S.; Dong, J.-X.; Huang, R.-Q. 1H-NMR and HPLC–MS/MS-based global/targeted metabolomic evaluation of Hypericum perforatum L. intervention for menopause. J. Funct. Foods 2015, 17, 722–741. [Google Scholar] [CrossRef]
- Iida, M.; Harada, S.; Kurihara, A.; Fukai, K.; Kuwabara, K.; Sugiyama, D.; Takeuchi, A.; Okamura, T.; Akiyama, M.; Nishiwaki, Y.; et al. Profiling of plasma metabolites in postmenopausal women with metabolic syndrome. Menopause 2016, 23, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Hou, Y.; Zhang, H.; Yang, K.; Wang, J.; Guo, B.; Zhang, F.; Li, H.; Zhou, X.; Li, Y.; et al. Plasma Metabolic Profiles in Women are Menopause Dependent. PLoS ONE 2015, 10, e0141743. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, J.; Zhang, Q.; Ying, H.; Jiye, A.; Sun, J.; Wu, D.; Wang, Y.; Li, J.; Liu, Y. Metabolomic profiles delineate signature metabolic shifts during estrogen deficiency-induced bone loss in rat by GC-TOF/MS. PLoS ONE 2013, 8, e54965. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Kim, H.Y.; Singh, D.; Yeo, S.H.; Baek, S.Y.; Park, Y.K.; Lee, C.H. Metabolite profiling reveals the effect of dietary Rubus coreanus vinegar on ovariectomy-induced osteoporosis in a rat model. Molecules 2016, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, Y.; Liu, L.; Zhao, L.; Han, T.; Zhang, Q.; Qin, L. A 1HNMR-Based Metabonomics Study of Postmenopausal Osteoporosis and Intervention Effects of Er-Xian Decoction in Ovariectomized Rats. Int. J. Mol. Sci. 2011, 12, 7635–7651. [Google Scholar] [CrossRef] [PubMed]
- Long, W.-F.; Li, L.; Chen, H.-Q.; Tang, Y.; He, X.-L.; Jing, R.-Z. 1H-NMR-based metabonomics analysis of plasma from osteoporotic rats induced by ovariectomy. J. Sichuan Univ. Med. Sci. Ed. 2009, 40, 843–847. [Google Scholar]
- Miyamoto, T.; Hirayama, A.; Sato, Y.; Koboyashi, T.; Katsuyama, E.; Kanagawa, H.; Miyamoto, H.; Mori, T.; Yoshida, S.; Fujie, A.; et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone 2017, 95, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Lu, X.; Zheng, S.; Li, F.; Xiong, Z. Metabonomic study on the anti-osteoporosis effect of Rhizoma Drynariae and its action mechanism using ultra-performance liquid chromatography–tandem mass spectrometry. J. Ethnopharmacol. 2012, 139, 311–317. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-S.; Lin, C.-Y.; Liang, H.-J.; Lee, S.-H.; Tsai, K.-S.; Chiou, J.-M.; Chen, Y.-C.; Tsao, C.-K.; Chen, J.-H. Association Between the Metabolome and Low Bone Mineral Density in Taiwanese Women Determined by 1H NMR Spectroscopy. J. Bone Miner. Res. 2014, 29, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, Z.; Wright, D.J.; Rainbow, S.J. Routine isotopedilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin. Chem. 2005, 51, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group [Meeting Held in Rome from 22 to 25 June 1992]; WHO: Geneva, Switzerland, 1994. [Google Scholar]
- Xu, J.; Begley, P.; Church, S.J.; Patassini, S.; Hollywood, K.A.; Jüllig, M.; Curtis, M.A.; Waldvogel, H.J.; Faull, R.L.M.; Unwin, R.D.; et al. Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: Snapshot of a pervasive metabolic disorder. Biochim. Biophys. Acta 2016, 1862, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a Robust and Repeatable UPLC−MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Maille, G.O.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A Metabolite Mass Spectral Database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liland, K.H. Multivariate methods in metabolomics—From pre-processing to dimension reduction and statistical analysis. TrAC Trends Anal. Chem. 2011, 30, 827–841. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. 2010, 21, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Ann. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.-H.; Shin, M.-H.; Chung, E.-K.; Lee, Y.-H.; Kweon, S.-S.; Park, K.-S.; Choi, J.-S. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos. Int. 2005, 16, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; O’Brien, C.A. Basic Biology of Skeletal Aging: Role of Stress Response Pathways. J. Gerontol. Ser. A 2013, 68, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-W.; Gao, Z.-L.; Wang, Y.; Mei, H.; Li, Y.-L. Differentiation of Human Mesenchymal Stem Cells: The Potential Mechanism for Estrogen-Induced Preferential Osteoblast Versus Adipocyte Differentiation. Am. J. Med. Sci. 2011, 341, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Ambrogini, E.; Han, L.; Manolagas, S.C.; Jilka, R.L. Increased Lipid Oxidation Causes Oxidative Stress, Increased Peroxisome Proliferator-activated Receptor-γ Expression, and Diminished Pro-osteogenic Wnt Signaling in the Skeleton. J. Biol. Chem. 2009, 284, 27438–27448. [Google Scholar] [CrossRef] [PubMed]
- Dieudonne, M.N.; Pecquery, R.; Leneveu, M.C.; Giudicelli, Y. Opposite Effects of Androgens and Estrogens on Adipogenesis in Rat Preadipocytes: Evidence for Sex and Site-Related Specificities and Possible Involvement of Insulin-Like Growth Factor 1 Receptor and Peroxisome Proliferator-Activated Receptorγ 21. Endocrinology 2000, 141, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Namba, N.; Zhao, J.J.; Jiang, Y.; Genant, H.K.; Silva, M.J.; Brodt, M.D.; Helgason, C.D.; Kalesnikoff, J.; Rauh, M.J.; et al. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat. Med. 2002, 8, 943. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.M.; Maciel, E.; Moreira, A.S.P.; Sousa, B.; Melo, T.; Domingues, P.; Curado, L.; Antunes, B.; Domingues, M.R.M.; Santos, F. Lipidomics of Mesenchymal Stromal Cells: Understanding the Adaptation of Phospholipid Profile in Response to Pro-Inflammatory Cytokines. J. Cell. Physiol. 2016, 231, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, L.; Tigistu-Sahle, F.; Oja, S.; Greco, D.; Parmar, A.; Saavalainen, P.; Nikkilä, J.; Korhonen, M.; Lehenkari, P.; Käkelä, R.; et al. Aging bone marrow mesenchymal stromal cells have altered membrane glycerophospholipid composition and functionality. J. Lipid Res. 2013, 54, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Maté, I.; Naudí, A.; Mota-Martorell, N.; Portero-Otín, M.; de la Fuente, M.; Pamplona, R. Human Aging Is a Metabolome-related Matter of Gender. J. Gerontol. Ser. A 2016, 71, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Vinayavekhin, N.; Sueajai, J.; Chaihad, N.; Panrak, R.; Chokchaisiri, R.; Sangvanich, P.; Suksamrarn, A.; Piyachaturawat, P. Serum lipidomics analysis of ovariectomized rats under Curcuma comosa treatment. J. Ethnopharmacol. 2016, 192, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Brownbill, R.A.; Ilich, J.Z. Lipid Profile and Bone Paradox: Higher Serum Lipids Are Associated with Higher Bone Mineral Density in Postmenopausal Women. J. Women’s Health 2006, 15, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Makovey, J.; Chen, J.S.; Hayward, C.; Williams, F.M.K.; Sambrook, P.N. Association between serum cholesterol and bone mineral density. Bone 2009, 44, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Brown, C.; Puscheck, E.; Friedrich, E.; Slatopolsky, E.; Maggio, D.; McCracken, R.; Avioli, L.V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc. Natl. Acad. Sci. USA 1991, 88, 5134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zilberman, Y.; Wassermann, K.; Bain, S.D.; Sadovsky, Y.; Gazit, D. Estrogen modulates estrogen receptor α and β expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J. Cell. Biochem. 2001, 81, 144–155. [Google Scholar] [CrossRef]
- Zheng, S.X.; Vrindts, Y.; Lopez, M.; de Groote, D.; Zangerle, P.F.; Collette, J.; Franchimont, N.; Geenen, V.; Albert, A.; Reginster, J.Y. Increase in cytokine production (IL-1β, IL-6, TNF-α but not IFN-γ, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas 1997, 26, 63–71. [Google Scholar] [CrossRef]
- Arana, L.; Gangoiti, P.; Ouro, A.; Trueba, M.; Gómez-Muñoz, A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Muñoz, A. Ceramide-1-phosphate: A novel regulator of cell activation. FEBS Lett. 2004, 562, 5–10. [Google Scholar] [CrossRef]
- Gangoiti, P.; Granado, M.H.; Wang, S.W.; Kong, J.Y.; Steinbrecher, U.P.; Gómez-Muñoz, A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell. Signal. 2008, 20, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Muñoz, A.; Kong, J.Y.; Salh, B.; Steinbrecher, U.P. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J. Lipid Res. 2004, 45, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.-Y.; Lee, Y.-S.; Kim, B.-J.; Lim, K.-H.; Cho, E.-H.; Kim, S.-W.; Koh, J.-M.; Kim, G.S. Higher Circulating Sphingosine 1-Phosphate Levels Are Associated with Lower Bone Mineral Density and Higher Bone Resorption Marker in Humans. J. Clin. Endocrinol. Metab. 2012, 97, E1421–E1428. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Bao, J.; An, G.; Ouyang, G.; Zhang, P.; Wang, C.; Ying, H.; Ouyang, P.; Ma, B.; Zhang, Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. BioSyst. 2016, 12, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.M. Arginine: Beyond protein1–4. Am. J. Clin. Nutr. 2006, 83, 508S–512S. [Google Scholar] [CrossRef] [PubMed]
- Chevalley, T.; Rizzoli, R.; Manen, D.; Caverzasio, J.; Bonjour, J.P. Arginine increases insulin-like growth factor-I production and collagen synthesis in osteoblast-like cells. Bone 1998, 23, 103–109. [Google Scholar] [CrossRef]
- Wood, S.L.; Westbrook, J.A.; Brown, J.E. Omic-profiling in breast cancer metastasis to bone: Implications for mechanisms, biomarkers and treatment. Cancer Treat. Rev. 2014, 40, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, J.B.J.; Dhonukshe-Rutten, R.A.M.; Pluijm, S.M.F.; van der Klift, M.; de Jonge, R.; Lindemans, J.; de Groot, L.C.P.G.M.; Hofman, A.; Witteman, J.C.M.; van Leeuwen, J.P.T.M.; et al. Homocysteine Levels and the Risk of Osteoporotic Fracture. N. Engl. J. Med. 2004, 350, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Turecek, C.; Fratzl-Zelman, N.; Rumpler, M.; Buchinger, B.; Spitzer, S.; Zoehrer, R.; Durchschlag, E.; Klaushofer, K.; Paschalis, E.P. Collagen Cross-Linking Influences Osteoblastic Differentiation. Calcif. Tissue Int. 2008, 82, 392–400. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Entire Cohort (n = 95) | Subset (n = 30) | ||||
|---|---|---|---|---|---|---|
| Normal BMD (n = 23) | Low BMD (n = 72) | p-Value | Normal BMD (n = 15) | Osteoporosis (n = 15) | p-Value | |
| Age (years) | 59.4 (4.19) | 61.3 (4.19) | 0.06 | 58 (3.42) | 61 (3.42) | 0.02 * |
| BMI (kg/m2) | 23.8 (2.61) | 22.5 (2.61) | 0.04 * | 23.8 (2.25) | 20.7 (2.25) | <0.001 * |
| PTH (pmol/L) | 4.7 (1.35) | 4.5 (1.32) | 0.29 | 4.8 (1.59) | 4.3 (1.59) | 0.08 |
| CTx-1 (ug/L) | 0.44 (0.21) | 0.55 (0.20) | 0.02 * | 0.41 (0.26) | 0.64 (0.26) | 0.04 * |
| Vitamin D (nmol/L) | 57.4 (15.24) | 60.1 (14.87) | 0.23 | 56.5 (16.84) | 54.8 (16.84) | 0.81 |
| Femoral neck BMD (g/cm2) | 0.75 (0.05) | 0.60 (0.05) | <0.001 * | 0.78 (0.04) | 0.51 (0.04) | <0.001 * |
| Dataset | Step | Component | R2X | R2Y | Q2 |
|---|---|---|---|---|---|
| Entire cohort (n = 95) | 1 | 1P and O1 | 0.395 | 0.302 | 0.042 |
| Entire cohort (n = 95) | 2 | 1P and O2 | 0.678 | 0.469 | 0.233 |
| Subset (n = 30) | 1 | 1P and O1 | 0.434 | 0.601 | 0.209 |
| Subset (n = 30) | 2 | 1P and O1 | 0.540 | 0.773 | 0.540 |
| Lipid | Normal BMD a (n = 23) | Low BMD a (n = 72) | p-Value | log2 (FC) | Correlation b |
|---|---|---|---|---|---|
| PS 31:6; [M + H]+ | 0.893 (0.427–1.871) | 0.867 (0.675–1.114) | 0.939 | −0.455 | −0.027 |
| PS 33:6; [M + H]+ | 0.857 (0.408–1.800) | 0.876 (0.681–1.127) | 0.955 | −0.373 | −0.070 |
| PS 29:6; [M + H]+ | 0.906 (0.428–1.918) | 0.921 (0.714–1.188) | 0.967 | −0.296 | −0.086 |
| DG 42:4; [M + NH4]+ | 0.850 (0.395–1.828) | 0.947 (0.725–1.219) | 0.804 | −0.176 | −0.075 |
| Plasmenyl-PE 38:4; [M + H]+ | 0.745 (0.353–1.571) | 0.841 (0.653–1.083) | 0.760 | −0.174 | −0.01 |
| Lipid | Normal BMD a (n = 15) | Osteoporosis a (n = 15) | p-Value | log2 (FC) | Correlation b |
|---|---|---|---|---|---|
| PA 34:4; [M − H]− | 0.531 (0.321–0.878) | 1.617 (0.822–3.182) | 0.005 * | 0.412 | −0.403 |
| CerP 38:1; [M + H]+ | 1.871 (1.196–2.927) | 0.605 (0.3319–1.105) | 0.002 * | −0.637 | −0.384 |
| PS 20:4; [M − H]− | 0.547 (0.330–0.906) | 1.585 (0.804–3.124) | 0.008 * | 0.395 | 0.274 |
| DG 40:0; [M + NH4]+ | 1.545 (0.907–2.630) | 0.425 (0.207–0.870) | 0.0029 * | 0.729 | −0.270 |
| PS 33:6; [M + H]+ | 1.435 (0.908–2.267) | 0.504 (0.277–0.933) | 0.0046 * | −0.560 | −0.363 |
| PS 31:6; [M + H]+ | 1.423 (0.886–2.286) | 0.506 (0.267–0.958) | 0.0065 * | −0.656 | −0.377 |
| PS 32:6; [M + H]+ | 1.414 (0.885–2.259) | 0.515 (0.274–0.967) | 0.007 * | −0.573 | −0.359 |
| PI 14:0; [M − H]− | 0.608 (0.357–1.036) | 1.572 (0.767–3.220) | 0.022 * | 0.327 | 0.165 |
| DG 42:4; [M + NH4]+ | 1.510 (0.976–2.336) | 0.574 (0.319–1.033) | 0.005 * | −0.327 | −0.374 |
| CerP 24:0; [M − H]− | 1.254 (0.728–2.160) | 0.418 (0.201–0.869) | 0.010 * | −0.265 | 0.008 |
| DG 47:5; [M + NH4]+ | 1.670 (0.920–3.032) | 0.854 (0.382–1.905) | 0.135 | −0.351 | −0.014 |
| PE 42:1; [M − H]− | 0.592 (0.346–1.013) | 1.246 (0.605–2.568) | 0.069 | 0.585 | 0.034 |
| Dataset | Step | Component | R2X | R2Y | Q2 |
|---|---|---|---|---|---|
| Entire cohort (n = 95) | 1 | 1P and O1 | 0.483 | 0.223 | −0.371 |
| Entire cohort (n = 95) | 2 | 1P and O1 | 0.512 | 0.205 | 0.035 |
| Subset (n = 30) | 1 | 1P and O1 | 0.437 | 0.749 | −0.399 |
| Subset (n = 30) | 2 | 1P and O1 | 0.514 | 0.526 | 0.247 |
| Polar Metabolite | Normal BMD a (n = 23) | Low BMD a (n = 72) | p-Value | Log2 (FC) | Correlation b |
|---|---|---|---|---|---|
| 4-Aminobutyric acid | 1.400 (0.909–2.155) | 0.874 (0.679–1.125) | 0.062 | −0.185 | −0.013 |
| Threonine | 1.315 (0.806–2.011) | 0.843 (0.658–1.081) | 0.073 | −0.159 | −0.172 |
| Asn–Gly–Cys | 0.794 (0.512–1.230) | 1.014 (0.512–1.230) | 0.337 | 0.058 | 0.059 |
| Turanose | 1.319 (0.858–2.028) | 0.845 (0.657–1.087) | 0.076 | −0.174 | −0.039 |
| Polar Metabolite | Normal BMD a (n = 15) | Osteoporosis a (n = 15) | p-Value | Log2 (FC) | Correlation b |
|---|---|---|---|---|---|
| Proline | 1.581 (0.928–2.692) | 0.786 (0.384–1.609) | 0.084 | −0.234 | −0.295 |
| Aminopropionitrile | 0.729 (0.418–1.272) | 1.848 (0.875–3.905) | 0.03 * | −0.270 | −0.315 |
| Threonine | 1.561 (0.911–2.674) | 0.765 (0.371–1.579) | 0.081 | −0.219 | −0.170 |
| Methionine | 1.534 (0.894–2.632) | 0.758 (0.366–1.567) | 0.085 | 0.141 | 0.494 |
| Asn-Gly-Cys | 1.428 (0.782–2.607) | 0.593 (0.264–1.334) | 0.056 | 0.142 | −0.038 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, D.; Kruger, M.; Wolber, F.M.; Roy, N.C.; Totman, J.J.; Henry, C.J.; Cameron-Smith, D.; Fraser, K. Association of Plasma Lipids and Polar Metabolites with Low Bone Mineral Density in Singaporean-Chinese Menopausal Women: A Pilot Study. Int. J. Environ. Res. Public Health 2018, 15, 1045. https://doi.org/10.3390/ijerph15051045
Cabrera D, Kruger M, Wolber FM, Roy NC, Totman JJ, Henry CJ, Cameron-Smith D, Fraser K. Association of Plasma Lipids and Polar Metabolites with Low Bone Mineral Density in Singaporean-Chinese Menopausal Women: A Pilot Study. International Journal of Environmental Research and Public Health. 2018; 15(5):1045. https://doi.org/10.3390/ijerph15051045
Chicago/Turabian StyleCabrera, Diana, Marlena Kruger, Frances M. Wolber, Nicole C. Roy, John J. Totman, Christiani Jeyakumar Henry, David Cameron-Smith, and Karl Fraser. 2018. "Association of Plasma Lipids and Polar Metabolites with Low Bone Mineral Density in Singaporean-Chinese Menopausal Women: A Pilot Study" International Journal of Environmental Research and Public Health 15, no. 5: 1045. https://doi.org/10.3390/ijerph15051045
APA StyleCabrera, D., Kruger, M., Wolber, F. M., Roy, N. C., Totman, J. J., Henry, C. J., Cameron-Smith, D., & Fraser, K. (2018). Association of Plasma Lipids and Polar Metabolites with Low Bone Mineral Density in Singaporean-Chinese Menopausal Women: A Pilot Study. International Journal of Environmental Research and Public Health, 15(5), 1045. https://doi.org/10.3390/ijerph15051045








