Resistance of Escherichia coli in Turkeys after Therapeutic or Environmental Exposition with Enrofloxacin Depending on Flooring
Abstract
:1. Introduction
2. Materials and Methods
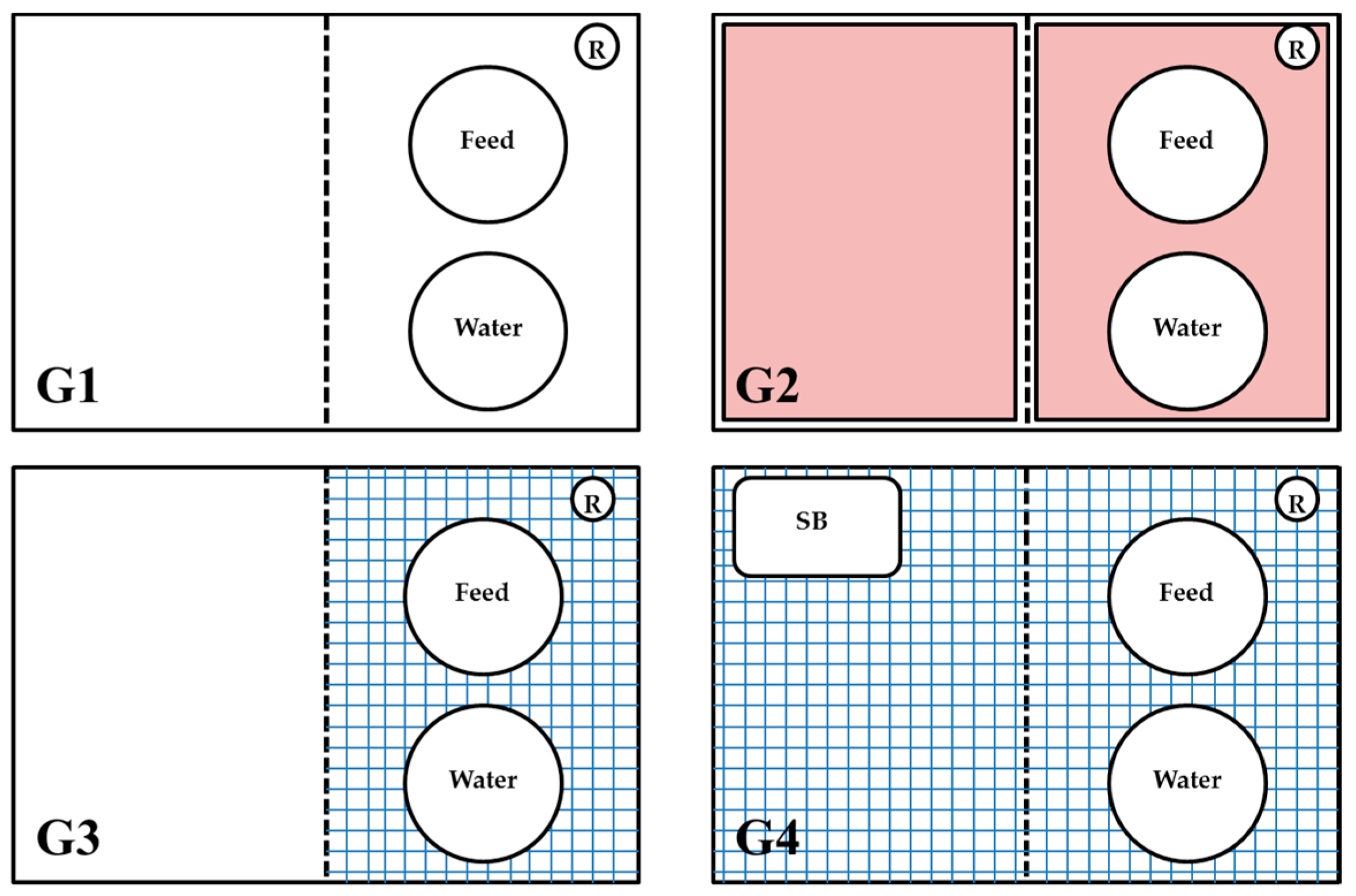
2.1. Design of Experiments
2.2. Collection of Cloacal Swabs and Manure Samples
2.3. Bacteriological Analyses and E. coli Isolation
2.4. Antibacterial Susceptibility Testing
2.5. Screening of Antibacterial Agents in Water Using High Performance Liquid Chromatography
2.6. Statistical Analyses
3. Results
3.1. Differences in Resistance to Antibacterial Agents in E. coli between Sampling Points as Well as between Trials
3.2. Testing the Effect of Different Flooring Designs on the Resistance to Antibacterial Agents in E. coli
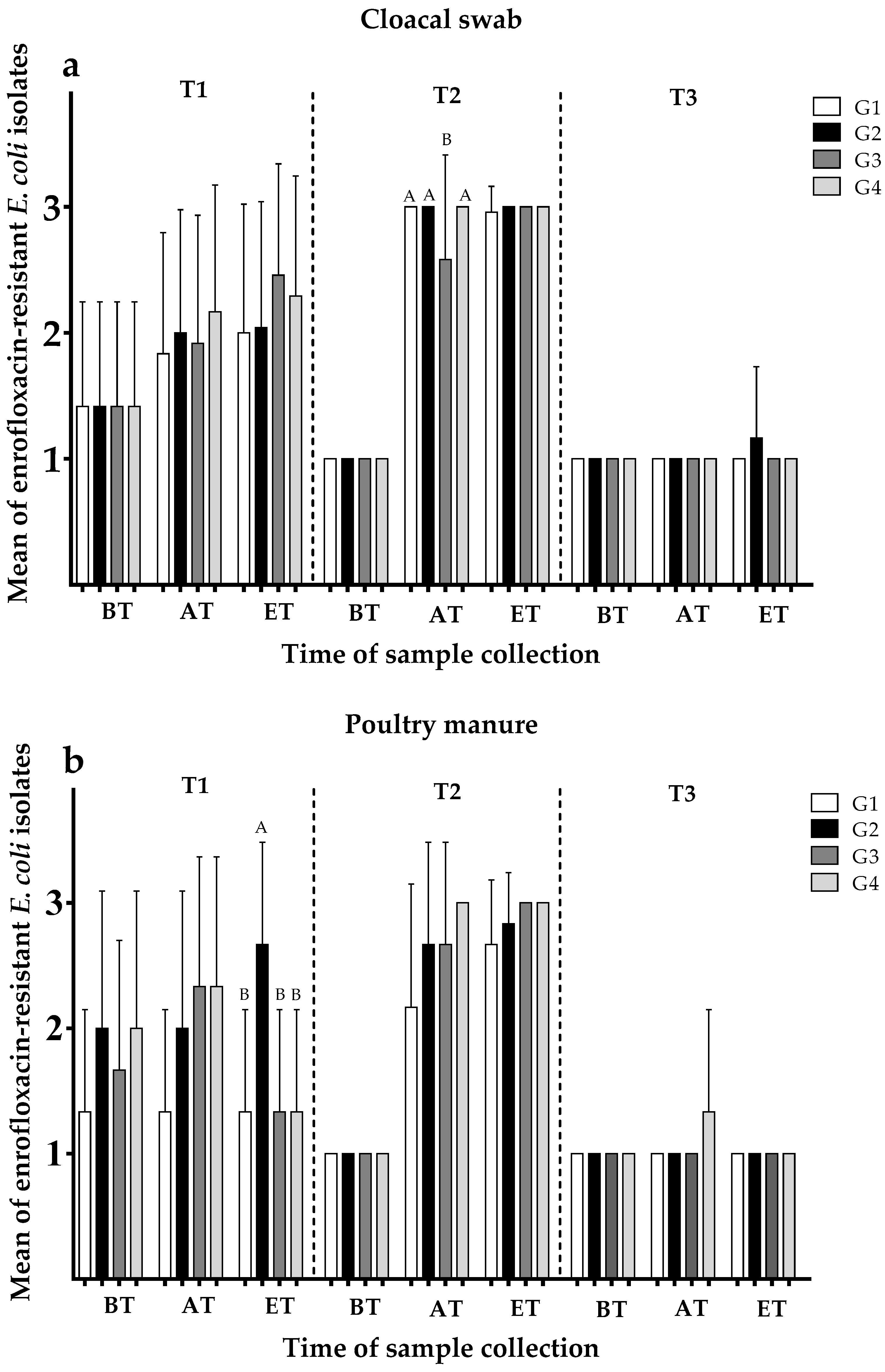
3.2.1. Development of Enrofloxacin Resistance Depending on Group
3.2.2. Development of Ampicillin Resistance Depending on Group
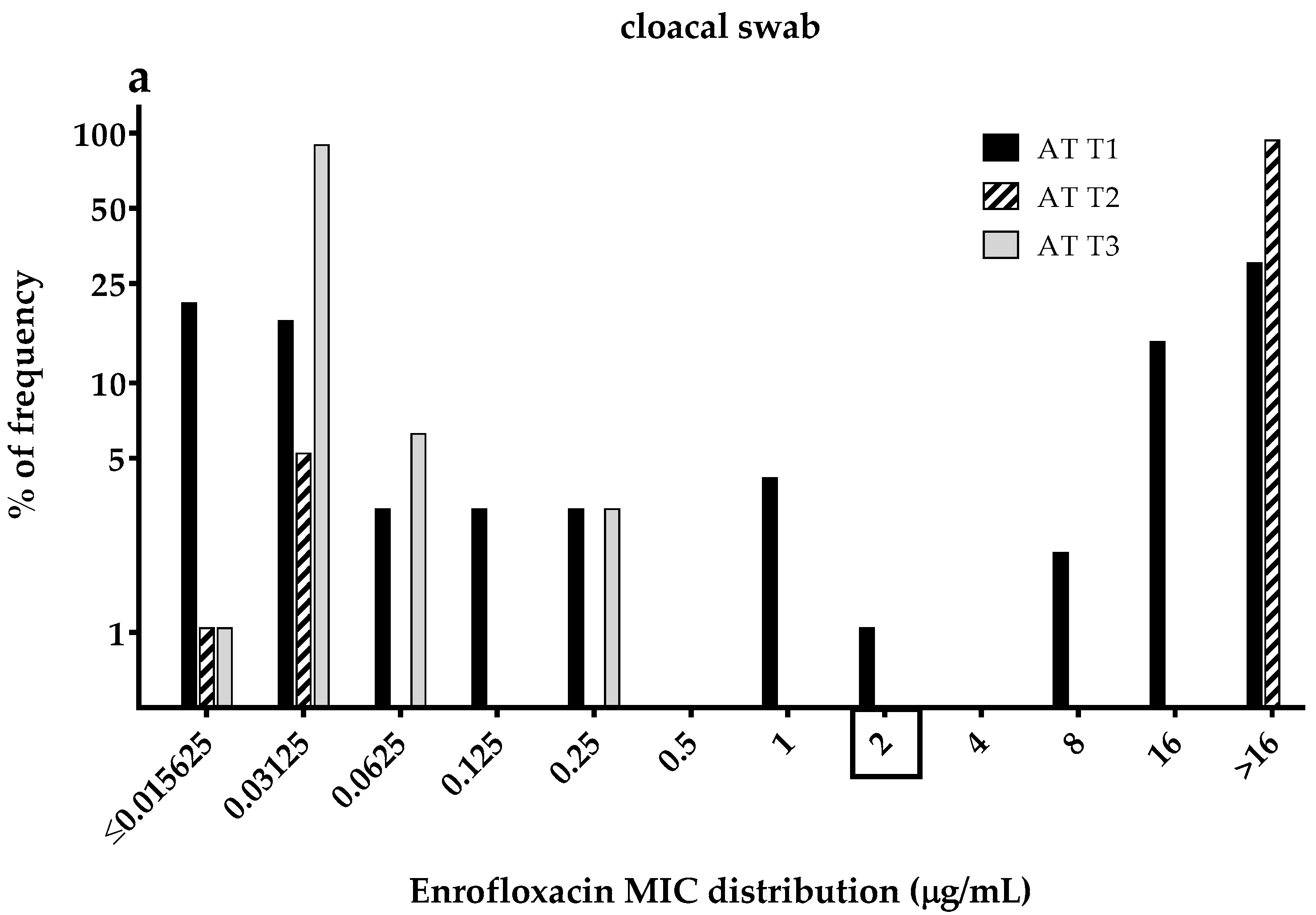
3.3. Enrofloxacin MICs Distributions of the Commensal E. coli Isolates
4. Discussion
4.1. Consequences of the Oral Administration of Antibacterial Agents
4.2. Effects of the Development of Resistance to Antibacterial Agents in E. coli by Water Loss Simulation (Indirect Administration)
4.3. Effect of Different Types of Flooring Design on the Development of Resistant E. coli
4.4. Natural Resistance to Antibacterial Agents Found in Day-Old Chickens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaesbohrer, A.; Schroeter, A.; Tenhagen, B.A.; Alt, K.; Guerra, B.; Appel, B. Emerging antimicrobial resistance in commensal Escherichia coli with public health relevance. Zoonoses Public Health 2012, 59, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, A.; Kaesbohrer, A. German Antimicrobial Resistance Situation in the Food Chain–DARlink; Bundesinstitut fur Risikobewertung (BfR): Berlin, Germany, 2010; pp. 127–133. [Google Scholar]
- ECDC; EFSA; EMA. Second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals—joint interagency antimicrobial consumption and resistance analysis (JIACRA) report. EFSA J. 2017, 15, 4872. [Google Scholar]
- WHO. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization, 2014. Available online: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf (accessed on 29 March 2018).
- Kumar, K.; Gupta, S.C.; Chander, Y.; Singh, A.K. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar]
- Janusch, F.; Scherz, G.; Mohring, S.A.; Hamscher, G. Determination of fluoroquinolones in chicken feces—A new liquid–liquid extraction method combined with LC–MS/MS. Environ. Toxicol. Pharmacol. 2014, 38, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Riaz, L.; Mahmood, T.; Khalid, A.; Rashid, A.; Siddique, M.B.A.; Kamal, A.; Coyne, M.S. Fluoroquinolones (FQs) in the environment: A review on their abundance, sorption and toxicity in soil. Chemosphere 2018, 191, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Sifakis, F.; Harbarth, S.; Schrijver, R.; van Mourik, M.; Voss, A.; Sharland, M.; Rajendran, N.B.; Rodríguez-Baño, J.; Bielicki, J. Surveillance for control of antimicrobial resistance. Lancet Infect. Dis. 2017, 18, E99–E106. [Google Scholar] [CrossRef]
- Projahn, M.; Daehre, K.; Semmler, T.; Guenther, S.; Roesler, U.; Friese, A. Environmental adaptation and vertical dissemination of esbl-/pampc-producing Escherichia coli in an integrated broiler production chain in the absence of an antibiotic treatment. Microb. Biotechnol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Rajić, A.; McFall, M.E.; Reid-Smith, R.J.; Deckert, A.E.; Pearl, D.L.; Avery, B.P.; Checkley, S.L.; McEwen, S.A. Comparison of antimicrobial resistance in generic Escherichia coli and Salmonella spp. Cultured from identical fecal samples in finishing swine. Can. J. Vet. Res. 2008, 72, 181–187. [Google Scholar] [PubMed]
- EFSA. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2013. EFSA J. 2015, 13, 4036. [Google Scholar]
- ECDC; EFSA; EMA. First joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. 2015, 13, 4006. [Google Scholar]
- European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Sales of Veterinary Antimicrobial Agents in 30 European Countries in 2015. 2017. Available online: https://publications.europa.eu/en/publication-detail/-/publication/7656bd79-d970-11e7-a506-01aa75ed71a1/language-en (accessed on 29 March 2018).
- GERMAP. Report on the Consumption of Antimicrobials and the Spread of Antimicrobial Resistance in Human and Veterinary Medicine in Germany; Antiinfectives Intelligence: Rheinbach, Germany, 2016. [Google Scholar]
- Kreienbrock, L. Ergebnisse aus VetCAb, Antibiotikaresistenz in der Lebensmittelkette; Bundesinstitut für Risikobewertung (BfR): Berlin, Germany, 2013; pp. 52–54. [Google Scholar]
- Diarra, M.S.; Malouin, F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Furtula, V.; Farrell, E.; Diarrassouba, F.; Rempel, H.; Pritchard, J.; Diarra, M. Veterinary pharmaceuticals and antibiotic resistance of Escherichia coli isolates in poultry litter from commercial farms and controlled feeding trials. Poult. Sci. 2010, 89, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Dandachi, I.; Sokhn, E.S.; Dahdouh, E.A.; Azar, E.; El-Bazzal, B.; Rolain, J.-M.; Daoud, Z. Prevalence and characterization of multi-drug-resistant gram-negative bacilli isolated from Lebanese poultry: A nationwide study. Front. Microbiol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2013, 69, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Werckenthin, C. Escherichia coli Strains from Northwestern Lower Saxony Obtained as Part of the Zoonosis Monitoring (General Administrative Regulation on Zoonoses in the Food Chain). In GERMAP 2015—Report on the Consumption of Antimicrobials and the Spread of Antimicrobial Resistance in Human and Veterinary Medicine in Germany; Antiinfectives Intelligence: Rheinbach, Germany, 2016; pp. 141–142. [Google Scholar]
- Font-Palma, C. Characterisation, kinetics and modelling of gasification of poultry manure and litter: An overview. Energy Convers. Manag. 2012, 53, 92–98. [Google Scholar] [CrossRef]
- Kamphues, J.; Youssef, I.; El-Wahab, A.A.; Üffing, B.; Witte, M.; Tost, M. Influences of feeding and housing on foot pad health in hens and turkeys. Übers. Tierernährg 2011, 39, 147–193. [Google Scholar]
- Bhushan, C.; Khurana, A.; Sinha, R.; Nagaraju, M. Antibiotic Resistance in Poultry Environment: Spread of Resistance from Poultry Farm to Agricultural Field; CSE: New Delhi, India, 2017. [Google Scholar]
- Wu, Y.; Wang, Z.; Liao, X. Study on the excretion of enrofloxacin in chicken and its degradation in chicken feces. Acta Vet. Zootech. Sin. 2005, 36, 1069. [Google Scholar]
- Chuppava, B.; Keller, B.; Meißner, J.; Kietzmann, M.; Visscher, C. Effects of different types of flooring design on the development of antimicrobial resistance in commensal Escherichia coli in fattening turkeys. Vet. Microbiol. 2018, 217, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Annex I of Council Regulation. European Commission (EC) No. 1099/2009 on the Protection of Animals at the Time of Killing. 2009, pp. 1–84. Available online: https://eur-lex.europa.eu/eli/reg/2009/1099/oj (accessed on 29 March 2018).
- Scherz, G. Carryover of Subtherapeutic Antimicrobial Dosages of Enrofloxacin and the Influence on the Development of Antibiotic Resistance of Commensal Escherichia Coli in the Intestine of Poultry. Ph.D. Thesis, University of Veterinary Medicine, Hannover, Germany, 2013. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard-Fourth Edition; CLSI document Vet01-A4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Witte, W. Medical consequences of antibiotic use in agriculture. Science 1998, 279, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, M.; Baeumer, W. Oral medication via feed and water pharmacological aspects. Dtsch. Tierarztl. Wochenschr. 2009, 116, 204–208. [Google Scholar] [PubMed]
- Da Costa, P.M.; Belo, A.; Gonçalves, J.; Bernardo, F. Field trial evaluating changes in prevalence and patterns of antimicrobial resistance among Escherichia coli and Enterococcus spp. isolated from growing broilers medicated with enrofloxacin, apramycin and amoxicillin. Vet. Microbiol. 2009, 139, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Furtula, V.; Jackson, C.R.; Farrell, E.G.; Barrett, J.B.; Hiott, L.M.; Chambers, P.A. Antimicrobial resistance in Enterococcus spp. isolated from environmental samples in an area of intensive poultry production. Int. J. Environ. Res. Public Health 2013, 10, 1020–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, J.; Vázquez, B.; Fente, C.; Barros-Velázquez, J.; Cepeda, A.; Franco, C. Evolution of resistance in poultry intestinal Escherichia coli during three commonly used antimicrobial therapeutic treatments in poultry. Poult. Sci. 2008, 87, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Jurado, S.; Medina, A.; Ruiz-Santa-Quiteria, J.A.; Orden, J.A. Resistance to non-quinolone antimicrobials in commensal Escherichia coli isolates from chickens treated orally with enrofloxacin. Jpn. J. Vet. Res. 2015, 63, 195–200. [Google Scholar] [PubMed]
- Schwarz, S.; Chaslus-Dancla, E. Use of antimicrobials in veterinary medicine and mechanisms of resistance. Vet. Res. 2001, 32, 201–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, A.; Hafez, H.M.; Böttner, A.; Gangl, A.; Hartmann, K.; Kaske, M.; Kehrenberg, C.; Kietzmann, M.; Klarmann, D.; Klein, G. Application of antibiotics in poultry. Tierärztl. Praxis Großtiere 2009, 37, 321–329. [Google Scholar]
- Apajalahti, J.; Kettunen, A.; Graham, H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. Worlds Poult. Sci. J. 2004, 60, 223–232. [Google Scholar] [CrossRef]
- Moro, M.; Beran, G.; Griffith, R.; Hoffman, L. Effects of heat stress on the antimicrobial drug resistance of Escherichia coli of the intestinal flora of swine. J. Appl. Microbiol. 2000, 88, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Oloso, N.O.; Fagbo, S.; Garbati, M.; Olonitola, S.O.; Awosanya, E.J.; Aworh, M.K.; Adamu, H.; Odetokun, I.A.; Fasina, F.O. Antimicrobial Resistance in Food Animals and the Environment in Nigeria: A Review. Int. J. Environ. Res. Public Health 2018, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Esaki, H.; Takemoto, K.; Ikeda, A.; Nakatani, Y.; Someya, A.; Hirayama, N.; Murase, T. Antimicrobial resistance in fecal Escherichia coli isolated from growing chickens on commercial broiler farms. Vet. Microbiol. 2011, 150, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Belenguer, A.; Doménech, E.; Villagrá, A.; Fenollar, A.; Ferrús, M.A. Antimicrobial resistance of Escherichia coli isolated in newly-hatched chickens and effect of amoxicillin treatment during their growth. Avian Pathol. 2016, 45, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Dierikx, C.M.; van der Goot, J.A.; Smith, H.E.; Kant, A.; Mevius, D.J. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: A descriptive study. PLoS ONE 2013, 8, e79005. [Google Scholar] [CrossRef] [PubMed]
- Persoons, D.; Haesebrouck, F.; Smet, A.; Herman, L.; Heyndrickx, M.; Martel, A.; Catry, B.; Berge, A.; Butaye, P.; Dewulf, J. Risk factors for ceftiofur resistance in Escherichia coli from belgian broilers. Epidemiol. Infect. 2011, 139, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef] [PubMed]



| Time of Sample Collection ** | Enrofloxacin * | |||||
|---|---|---|---|---|---|---|
| Cloacal Swab (N = 648) *** | Manure (N = 216) *** | |||||
| BT | AT | ET | BT | AT | ET | |
| T1 | 1.42 A,b | 1.98 B,a | 2.20 B,a | 1.75 A,a | 2.00 B,a | 1.67 B,a |
| T2 | 1.00 B,b | 2.90 A,a | 2.99 A,a | 1.00 B,b | 2.63 A,a | 2.92 A,a |
| T3 | 1.00 B,a | 1.00 C,a | 1.04 C,a | 1.00 B,a | 1.08 C,a | 1.00 C,a |
| Time of Sample Collection ** | Ampicillin * | |||||
|---|---|---|---|---|---|---|
| Cloacal Swab (N = 648) *** | Manure (N = 216) *** | |||||
| BT | AT | ET | BT | AT | ET | |
| T1 | 1.33 A,a | 1.80 B,a | 1.52 B,a | 1.42 A,a | 1.33 B,a | 1.00 B,a |
| T2 | 1.00 B,b | 1.31 C,a | 2.00 A,a | 1.00 B,b | 2.13 A,a | 1.67 A,a |
| T3 | 1.00 B,b | 2.08 A,a | 1.73 B,a | 1.25 AB,a | 1.17 B,a | 1.83 A,a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuppava, B.; Keller, B.; El-Wahab, A.A.; Meißner, J.; Kietzmann, M.; Visscher, C. Resistance of Escherichia coli in Turkeys after Therapeutic or Environmental Exposition with Enrofloxacin Depending on Flooring. Int. J. Environ. Res. Public Health 2018, 15, 1993. https://doi.org/10.3390/ijerph15091993
Chuppava B, Keller B, El-Wahab AA, Meißner J, Kietzmann M, Visscher C. Resistance of Escherichia coli in Turkeys after Therapeutic or Environmental Exposition with Enrofloxacin Depending on Flooring. International Journal of Environmental Research and Public Health. 2018; 15(9):1993. https://doi.org/10.3390/ijerph15091993
Chicago/Turabian StyleChuppava, Bussarakam, Birgit Keller, Amr Abd El-Wahab, Jessica Meißner, Manfred Kietzmann, and Christian Visscher. 2018. "Resistance of Escherichia coli in Turkeys after Therapeutic or Environmental Exposition with Enrofloxacin Depending on Flooring" International Journal of Environmental Research and Public Health 15, no. 9: 1993. https://doi.org/10.3390/ijerph15091993
APA StyleChuppava, B., Keller, B., El-Wahab, A. A., Meißner, J., Kietzmann, M., & Visscher, C. (2018). Resistance of Escherichia coli in Turkeys after Therapeutic or Environmental Exposition with Enrofloxacin Depending on Flooring. International Journal of Environmental Research and Public Health, 15(9), 1993. https://doi.org/10.3390/ijerph15091993





