Preparation of Iron-Loaded Granular Activated Carbon Catalyst and Its Application in Tetracycline Antibiotic Removal from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Iron-Loaded Granular Activated Carbon Catalyst
2.3. Antibiotic Removal Experiment
2.4. Recycling Experiment
2.5. Characterization of Catalyst
2.6. Measurement of Tetracycline Antibiotics
3. Results and Discussion
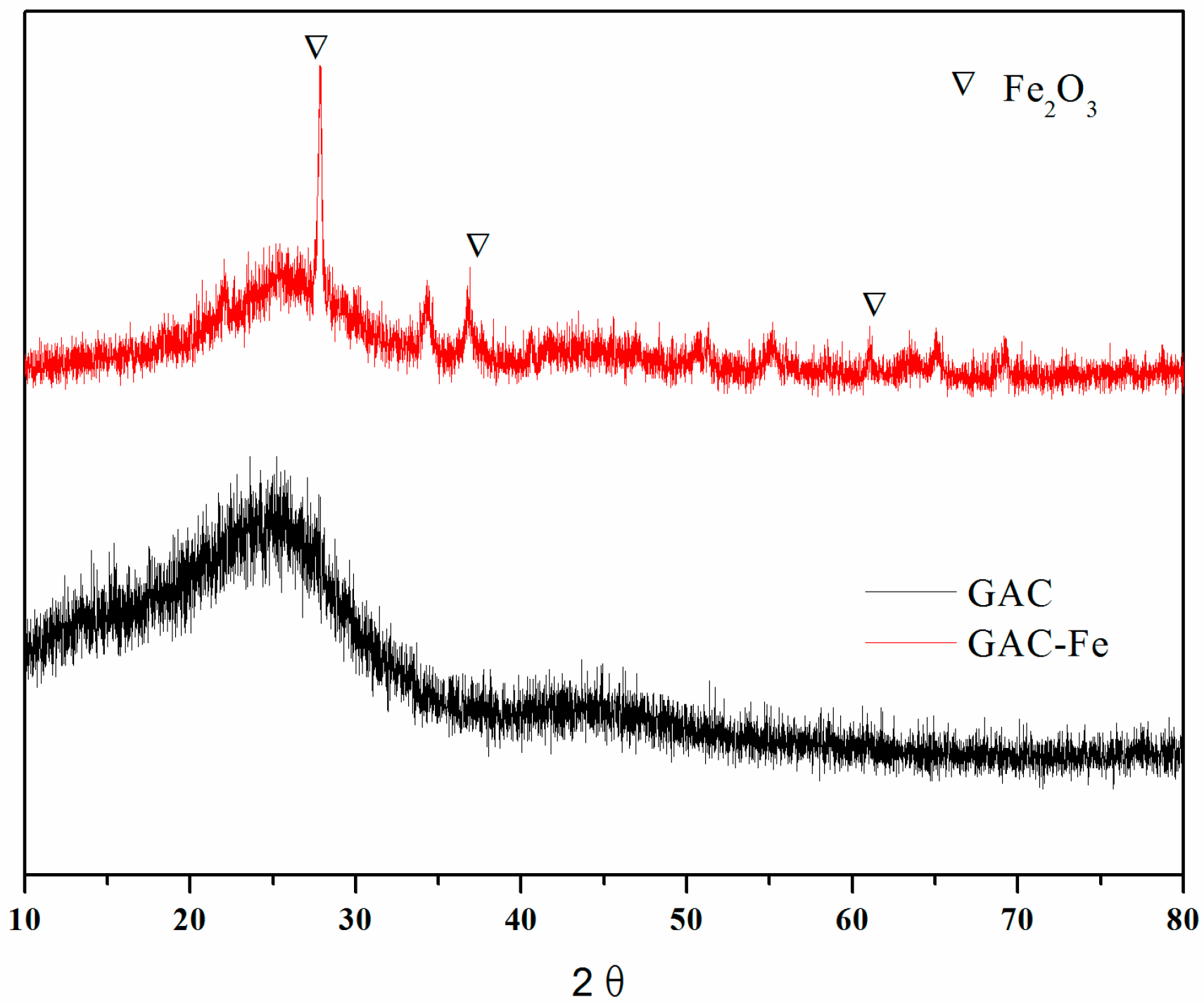
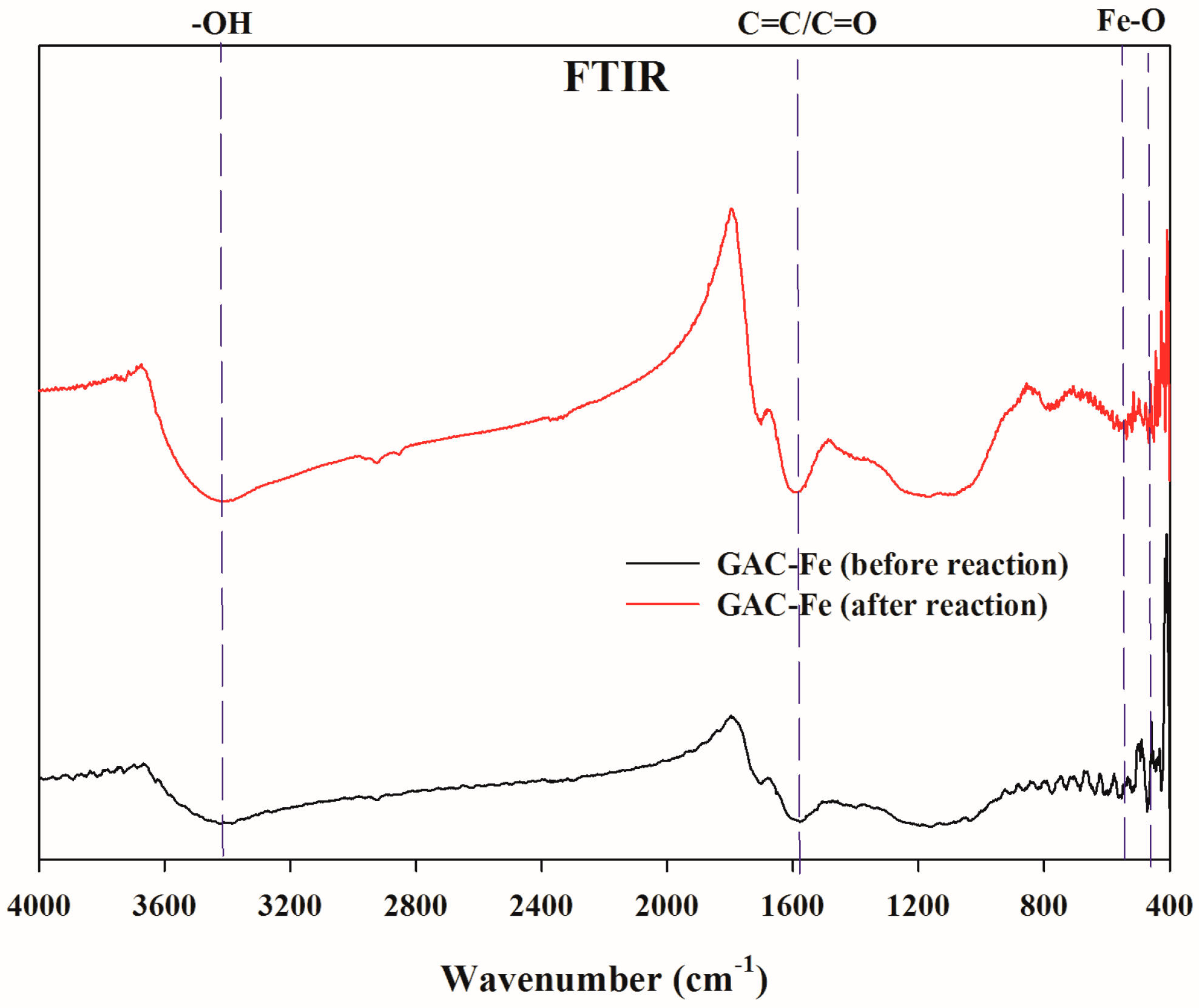
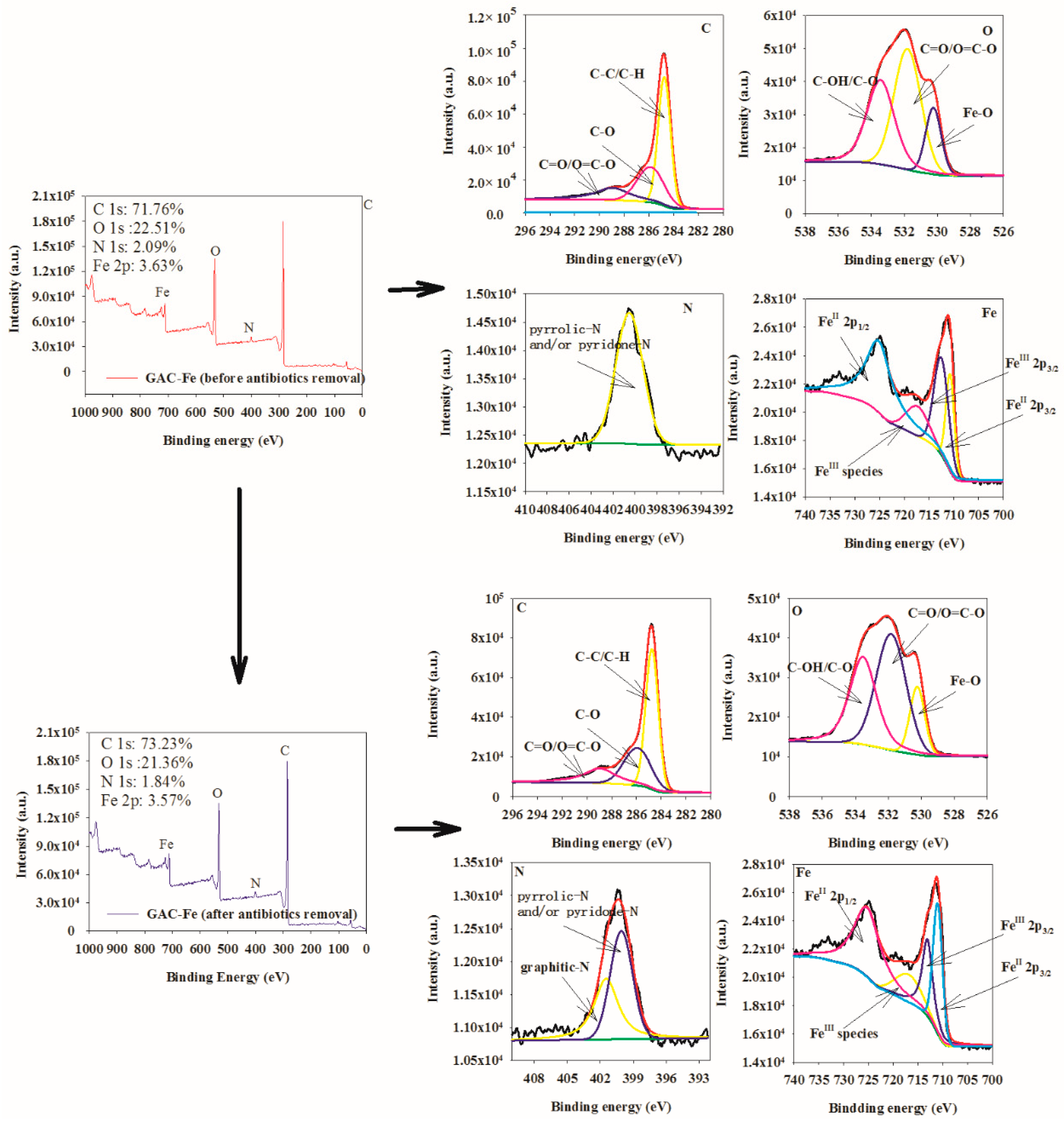
3.1. Characterization of GAC-Fe
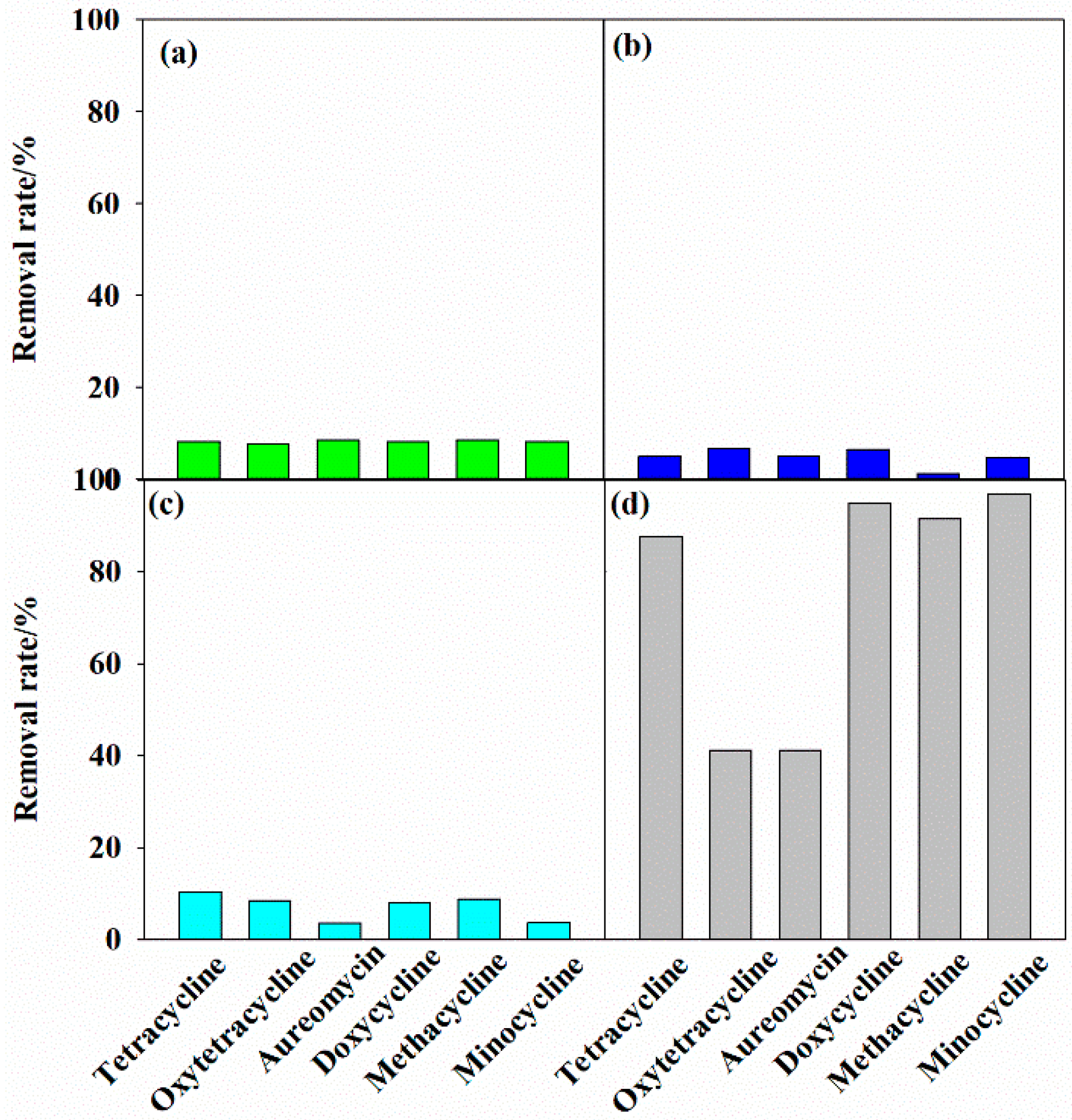
3.2. Removal of Tetracycline Antibiotics under Different Systems
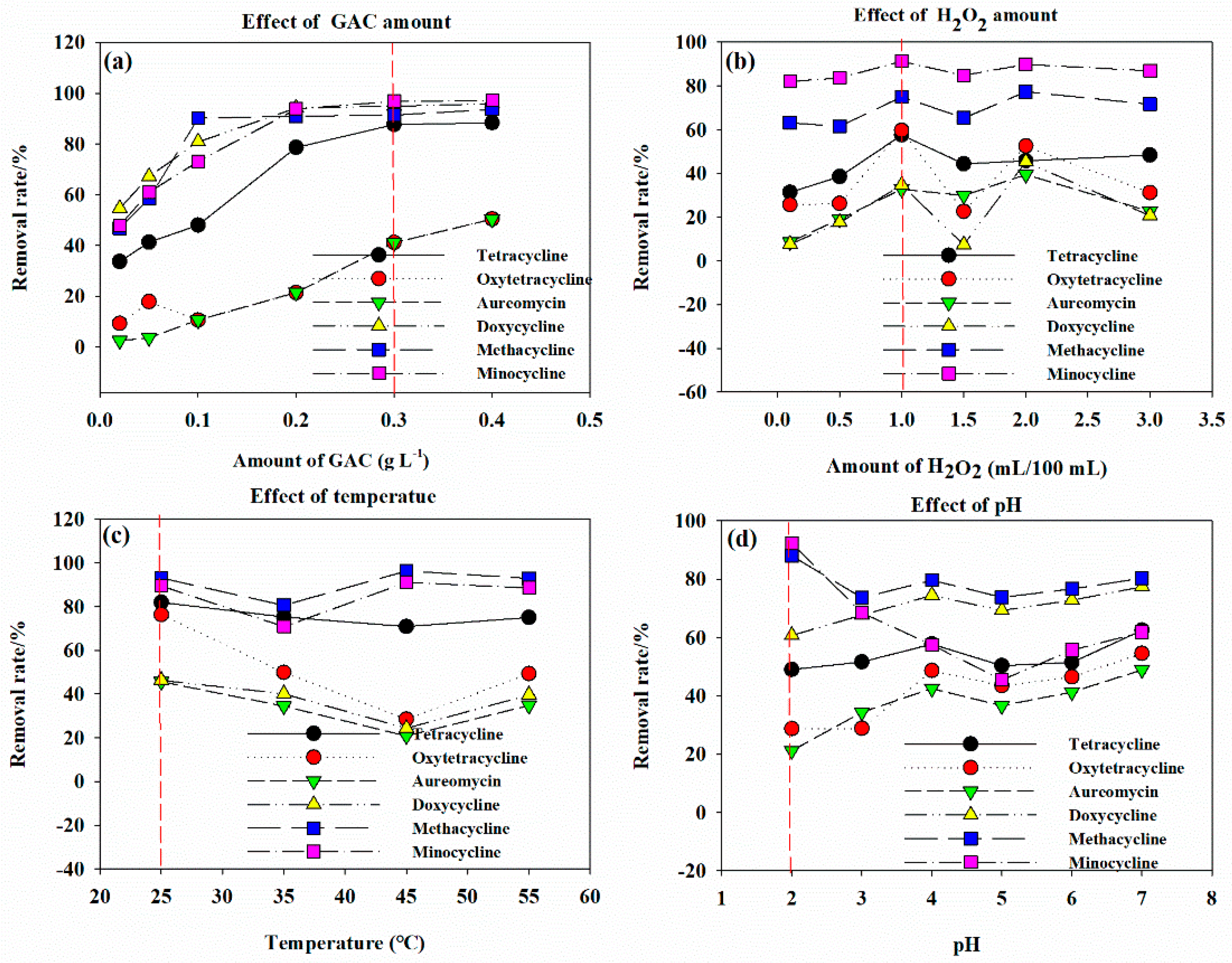
3.3. The Removal Rate of Antibiotics under Different Influencing Factors
3.3.1. Catalyst Dosage
3.3.2. H2O2 Dosage
3.3.3. Environmental Temperature
3.3.4. Initial of pH Solution
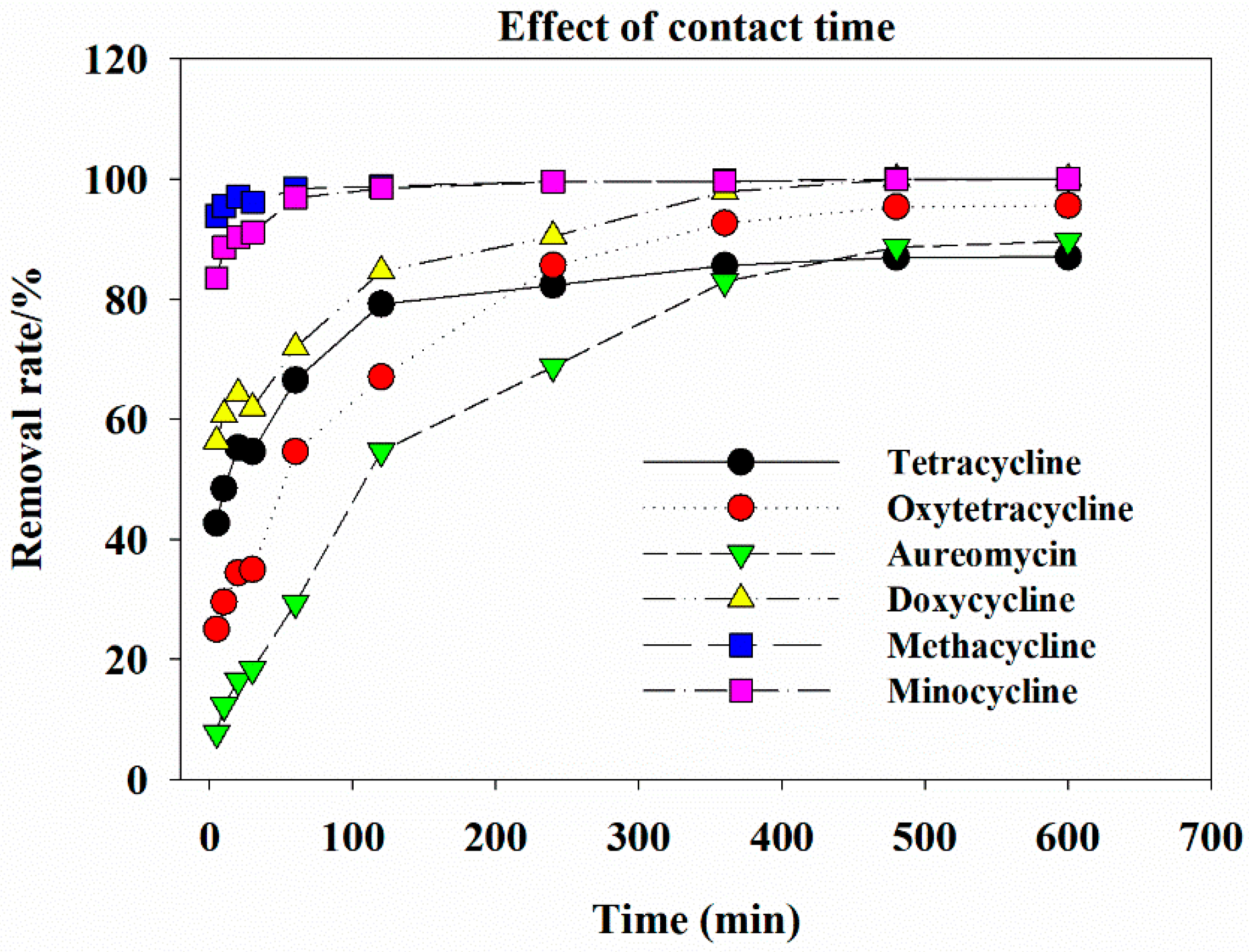
3.4. Effect of Contact Time on Tetracycline Antibiotic Removal by GAC-Fe
3.5. The Mechanism of Tetracycline Removal by GAC-Fe
3.5.1. Iron Ion Leaching and Homogeneous Reaction
3.5.2. Inhibition of Free Radical Reaction
3.5.3. Tetracycline Antibiotic Removal Mechanism by GAC-Fe
3.6. Effect of GAC-Fe Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, C.; Mao, D.; Luo, Y. The occurrence and fate of tetracyclines in two pharmaceutical wastewater treatment plants of northern China. Environ. Sci. Pollut. Res. 2016, 23, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lu, G.; Yan, Z.; Liu, J.; Wang, P.; Wang, Y. Bioaccumulation and trophic transfer of pharmaceuticals in foodwebs froma large freshwater lake. Environ. Pollut. 2017, 222, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, H.; Dong, H.; Ma, B.; Sun, H.; Pan, T.; Jiang, R.; Zhou, R.; Shen, J.; Liu, J.; et al. Occurrence and ecological risk assessment of organic micropollutants in the lower reaches of the Yangtze River, China: A case study of water diversion. Environ. Pollut. 2018, 239, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Tang, J.; Wu, X.; Li, X.; Hua, R. Bioconcentration, metabolism and the effects of tetracycline on multiple biomarkers in Chironomus riparius larvae. Sci. Total Environ. 2019, 649, 1590–1598. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; Marchão, L.; Lucas, M.S.; Peres, J.A. Application of Advanced Oxidation Processes for the Treatment of Recalcitrant Agro-Industrial Wastewater: A Review. Water 2019, 11, 205. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Tu, Y.; Tian, S.; Kong, L.; Xiong, Y. Co-catalytic effect of sewage sludge-derived char as the support of Fenton-like catalyst. Chem. Eng. J. 2012, 185–186, 44–51. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Fan, Y.; Tong, T.; Han, X.; Du, Y. Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate. Chem. Eng. J. 2018, 336, 721–731. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal–Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef]
- Da Costa Evangelista, J.P.; Gondim, A.D.; Souza, L.D.; Araujo, A.S. Alumina-supported potassium compounds as heterogeneous catalysts for biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 59, 887–894. [Google Scholar] [CrossRef]
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197–5211. [Google Scholar] [CrossRef]
- He, Y.; Jiang, D.B.; Jiang, D.Y.; Chen, J.; Zhang, Y.X. Evaluation of MnO2-templated iron oxide-coated diatomites for their catalytic performance in heterogeneous photo Fenton-like system. J. Hazard. Mater. 2018, 344, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yang, C.; Cheng, X.; Xu, S.; Fan, Z.; Wang, G.; Wang, S.; Guan, X.; Sun, X. Specifically enhancement of heterogeneous Fenton-like degradation activities for ofloxacin with synergetic effects of bimetallic Fe-Cu on ordered mesoporous silicon. Sep. Purif. Technol. 2017, 189, 357–365. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Royer, S.; Zhang, H. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J. Hazard. Mater. 2016, 302, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cui, C.; Zhang, D.; Li, L.; Pan, D. Heterogeneous catalytic ozonation of dibutyl phthalate in aqueous solution in the presence of iron-loaded activated carbon. Chemosphere 2015, 119, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Qiang, Z.; Shi, Y.; Zhang, T.; Dong, B. Fe(III)-loaded activated carbon as catalyst to improve omethoate degradation by ozone in water. J. Mol. Catal. A Chem. 2011, 342–343, 23–29. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Guo, X.; Guo, S.; Yan, G. Advanced ozone treatment of heavy oil refining wastewater by activated carbon supported iron oxide. J. Ind. Eng. Chem. 2014, 20, 2782–2791. [Google Scholar] [CrossRef]
- Zhu, S.; Ho, S.H.; Huang, X. Magnetic nanoscale zero-valent iron assisted biochar: Interfacial chemical behaviors and heavy metals remediation performance. ACS Sustain. Chem. Eng. 2017, 5, 9673–9682. [Google Scholar] [CrossRef]
- Cho, D.W.; Yoon, K.; Kwon, E.E. Fabrication of magnetic biochar as a treatment medium for As(V) via pyrolysis of FeCl3-pretreated spent coffee ground. Environ. Pollut. 2017, 229, 942–949. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, G.; Liu, S. Nano-Fe0 encapsulated in microcarbon spheres: Synthesis, characterization, and environmental applications. ACS App. Mater. Int. 2012, 4, 6235–6241. [Google Scholar] [CrossRef]
- Theydan, S.K.; Ahmed, M.J. Adsorption of methylene blue onto biomass-based activated carbon by FeCl3 activation: Equilibrium, kinetics, and thermodynamic studies. J. Anal. Appl. Pyrol. 2012, 97, 116–122. [Google Scholar] [CrossRef]
- Márcio, C.P.; Flávia, S.C.; Clésia, C.N. Use of activated carbon as a reactive support to produce highly active-regenerable Fe-based reduction system for environmental remediation. Chemosphere 2010, 81, 7–12. [Google Scholar]
- Duarte, F.M.; Maldonado-Hódar, F.J.; Madeira, L.M. Influence of the iron precursor in the preparation of heterogeneous Fe/activated carbon Fenton-like catalysts. Appl. Catal. A 2013, 458, 39–47. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Phan, N.H.; Do, M.H.; Ngo, K.T. Magnetic Fe2MO4 (M:Fe, Mn) activated carbons: Fabrication, characterization and heterogeneous Fenton oxidation of methyl orange. J. Hazard. Mater. 2011, 185, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, L.; Yang, J.; Yu, X.; Jiang, Y.; Zhou, M. Nanoscale zero-valent iron/AC as heterogeneous Fenton catalysts in three-dimensional electrode system. Environ. Sci. Pollut. Res. 2014, 21, 8398–8405. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, M.; Yu, F.; Chen, J. Easy solid-phase synthesis of pH-insensitive heterogeneous CNTs/FeS Fenton-like catalyst for the removal of antibiotics from aqueous solution. J. Colloid Interface Sci. 2015, 444, 24–32. [Google Scholar] [CrossRef]
- Wan, Z.; Hu, J.; Wang, J. Removal of sulfamethazine antibiotics using CeFe-graphene nanocomposite as catalyst by Fenton-like process. J. Environ. Manag. 2016, 182, 284–291. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Kakavandi, B.; Takdastan, A.; Kalantary, R.R.; Azizi, M.; Jorfi, S. Powder activated carbon/Fe3O4 hybrid composite as a highly efficient heterogeneous catalyst for Fenton oxidation of tetracycline: Degradation mechanism and kinetic. RSC Adv. 2015, 5, 84718–84728. [Google Scholar] [CrossRef]
- Shi, T.; Peng, J.; Chen, J.; Sun, C.; He, H. Heterogeneous photo-fenton degradation of norfloxacin with Fe3O4-multiwalled carbon nanotubes in aqueous solution. Catal. Lett. 2017, 147, 1598–1607. [Google Scholar] [CrossRef]
- Zhou, G.; Fang, F.; Chen, Z.; He, Y.; Sun, H.; Shi, H. Facile synthesis of paper mill sludge-derived heterogeneous catalyst for the Fenton-like degradation of methylene blue. Catal. Commun. 2015, 62, 71–74. [Google Scholar] [CrossRef]
- Yuan, S.J.; Dai, X.H. Facile synthesis of sewage sludge-derived mesoporous material as an efficient and stable heterogeneous catalyst for photo-Fenton reaction. Appl. Catal. B 2014, 154–155, 252–258. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Guo, Y.; Yu, J.C. Graphene oxide—Fe2O3 hybrid material as highly efficient heterogeneous catalyst for degradation of organic contaminants. Carbon 2013, 60, 437–444. [Google Scholar] [CrossRef]
- Xu, X.; Pliego, G.; Garcia-Costa, A.L.; Zazo, J.A.; Liu, S.; Casas, J.A.; Rodriguez, J.J. Cyclohexanoic acid breakdown by two-step persulfate and heterogeneous Fenton-like oxidation. Appl. Catal. B 2018, 232, 429–435. [Google Scholar] [CrossRef]
- Li, B.; Ma, J.; Zhou, L.; Qiu, Y. Magnetic microsphere to remove tetracycline from water: Adsorption, H2O2 oxidation and regeneration. Chem. Eng. J. 2017, 330, 191–201. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Moreno-Castilla, C.; Costa, C.A.; Madeira, L.M. Azo-dye Orange II degradation by heterogeneous Fenton-like reaction using carbon-Fe catalysts. Appl. Catal. B 2007, 75, 312–323. [Google Scholar] [CrossRef]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S.; et al. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, L.; Yu, G.; Xie, S.; Li, C.; Lai, D.; Li, Z.; You, F.; Wang, Y. The synthesis of heterogeneous Fenton-like catalyst using sewage sludge biochar and its application for ciprofloxacin degradation. Sci. Total Environ. 2019, 654, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Coll. Int. Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Bai, B.; Guan, W.; Wang, H.; Suo, Y. Degradation of tetracycline hydrochloride by heterogeneous Fenton-like reaction using Fe@Bacillus subtilis. RSC Adv. 2016, 6, 4101–4107. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Fang, Z.; Tsang, E.P. Ceria accelerated nanoscale zerovalent iron assisted heterogenous Fenton oxidation of tetracycline. Chem. Eng. J. 2019, 369, 588–599. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Karpinska, J.; Sokol, A.; Koldys, J.; Ratkiewicz, A. Studies on the Kinetics of Doxazosin Degradation in Simulated Environmental Conditions and Selected Advanced Oxidation Processes. Water 2019, 11, 1001. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Cao, Y.; Zang, J.; Huang, Q.; Wang, L.; Zhang, Y.; Fan, S.; Tang, J.; Xie, Z. Preparation of Iron-Loaded Granular Activated Carbon Catalyst and Its Application in Tetracycline Antibiotic Removal from Aqueous Solution. Int. J. Environ. Res. Public Health 2019, 16, 2270. https://doi.org/10.3390/ijerph16132270
Pan L, Cao Y, Zang J, Huang Q, Wang L, Zhang Y, Fan S, Tang J, Xie Z. Preparation of Iron-Loaded Granular Activated Carbon Catalyst and Its Application in Tetracycline Antibiotic Removal from Aqueous Solution. International Journal of Environmental Research and Public Health. 2019; 16(13):2270. https://doi.org/10.3390/ijerph16132270
Chicago/Turabian StylePan, Ling, Yanzhi Cao, Ji Zang, Qinqing Huang, Lin Wang, Yingsheng Zhang, Shisuo Fan, Jun Tang, and Zhengxin Xie. 2019. "Preparation of Iron-Loaded Granular Activated Carbon Catalyst and Its Application in Tetracycline Antibiotic Removal from Aqueous Solution" International Journal of Environmental Research and Public Health 16, no. 13: 2270. https://doi.org/10.3390/ijerph16132270



