Characteristics of Authigenic Minerals around the Sulfate-Methane Transition Zone in the Methane-Rich Sediments of the Northern South China Sea: Inorganic Geochemical Evidence
Abstract
:1. Introduction
2. Materials and Methods
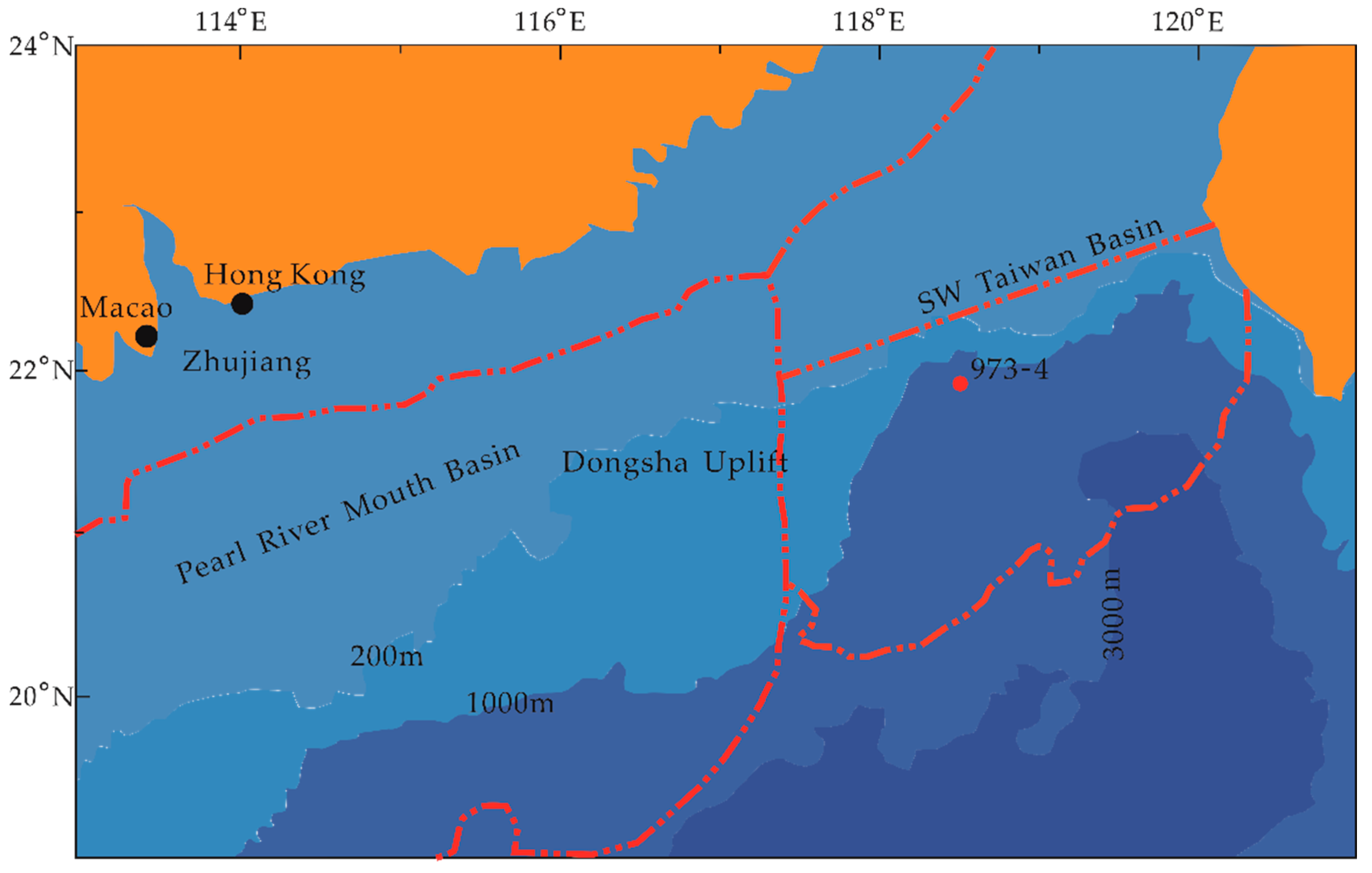
2.1. Geological Setting
2.2. Materials and Methods
3. Results
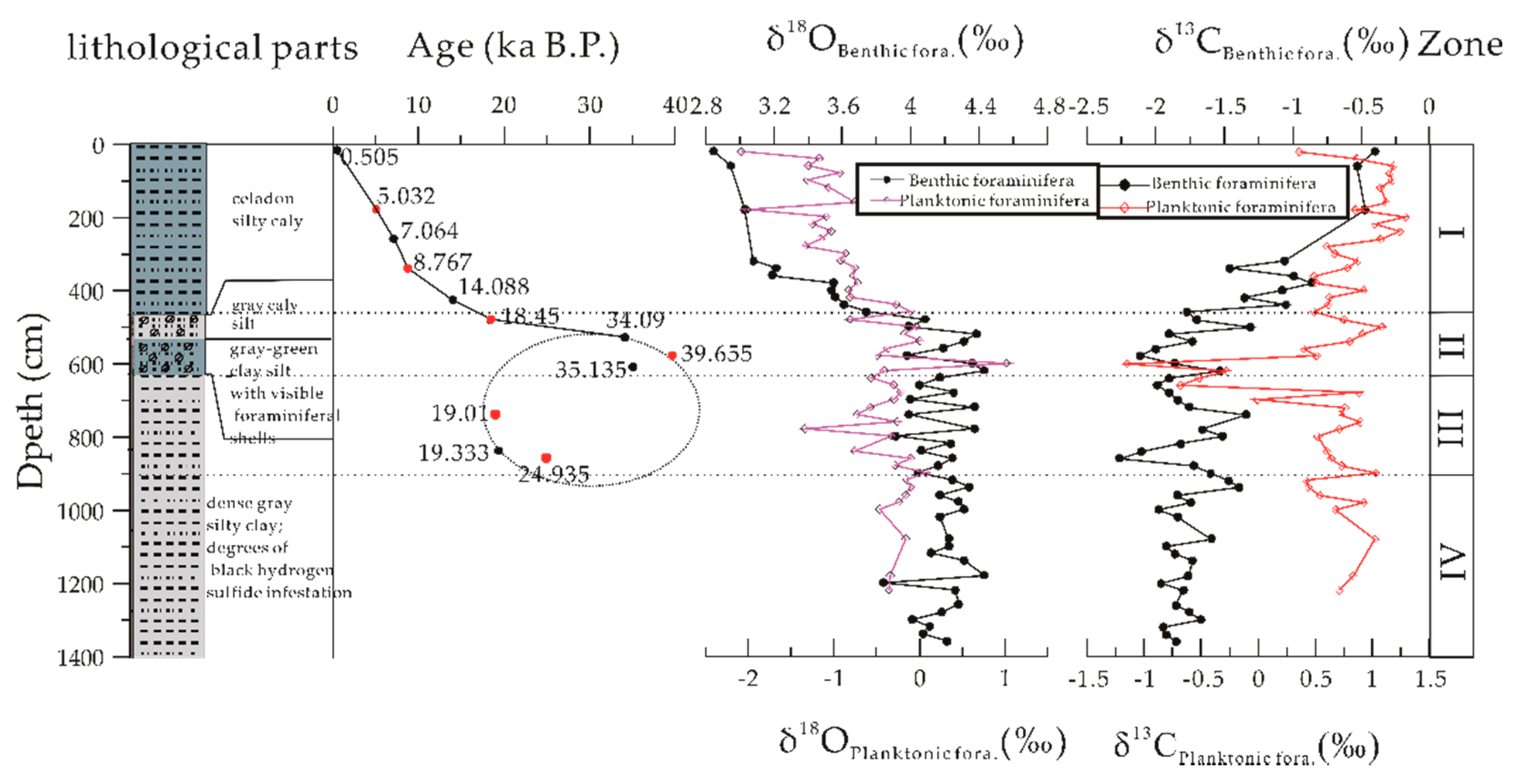
3.1. Core Description and Radiocarbon Dating
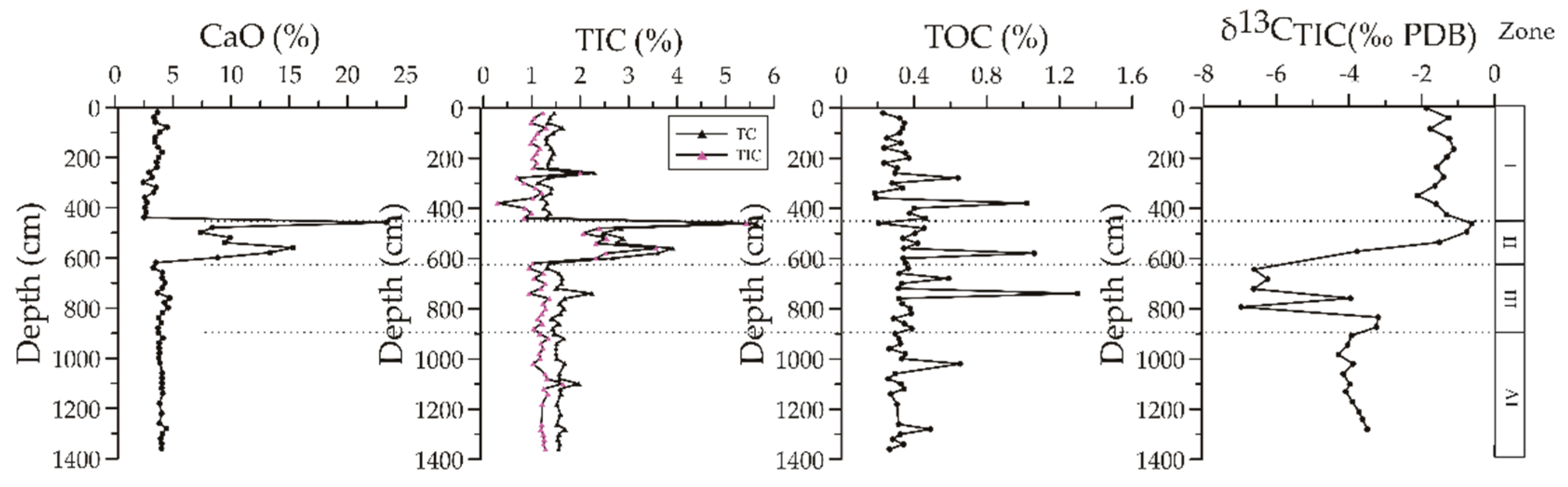
3.2. Relevant Data of Carbonates in Bulk Sediments
3.3. Chromium Reducible Sulfur and δ34SCRS
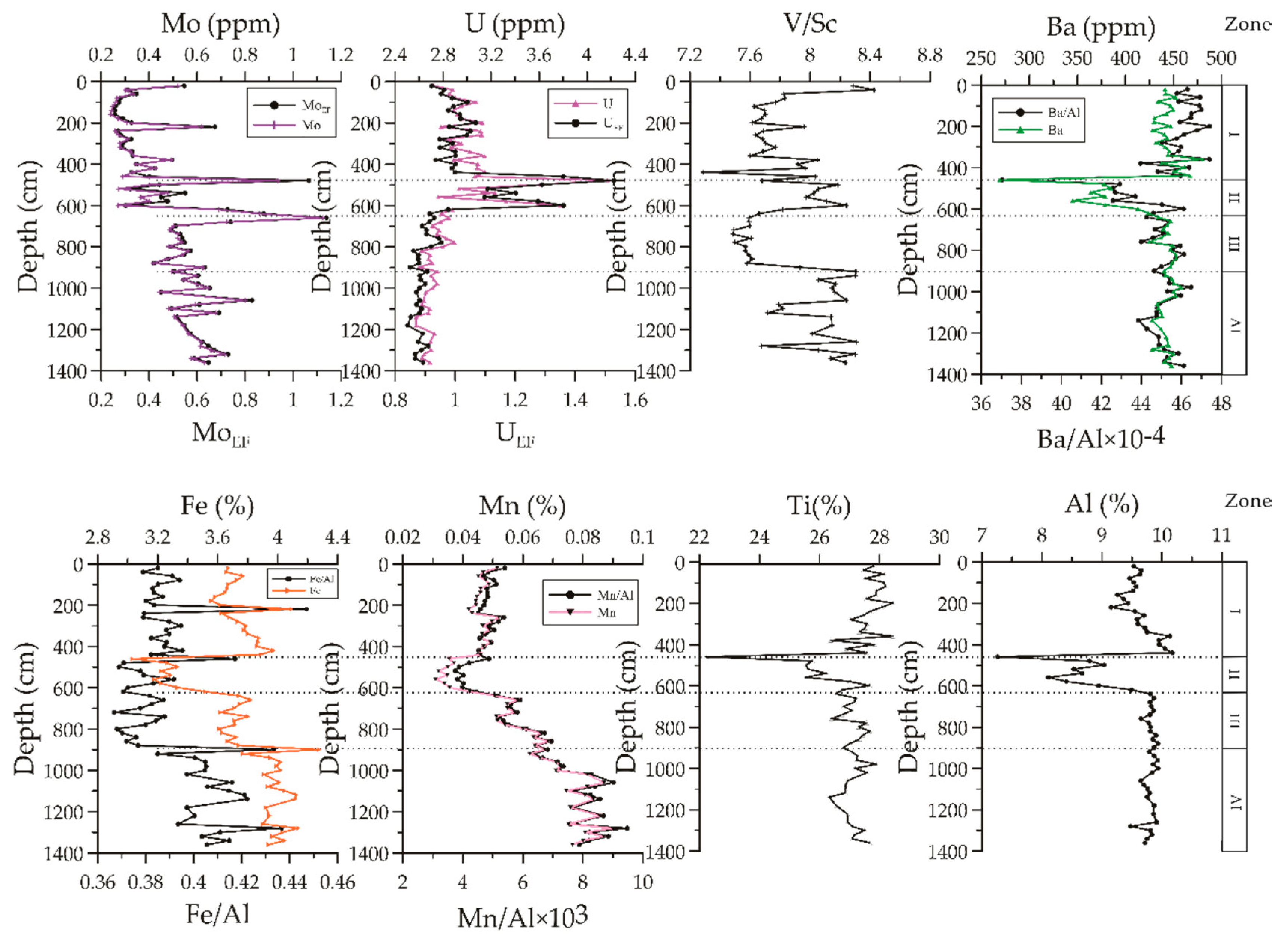
3.4. Concentration Profiles of Major and Trace Elements
4. Discussion
4.1. Evidence of Anaerobic Oxidation of Methane in Sediments
4.1.1. CRS and δ34SCRS
4.1.2. Authigenic Carbonates Precipitation
4.2. Source Origin of Methane-Rich Fluids: Constraint of the Stable C–O–S Isotope
4.3. Conditions for Mo Enrichments in the Methane Seep Environments
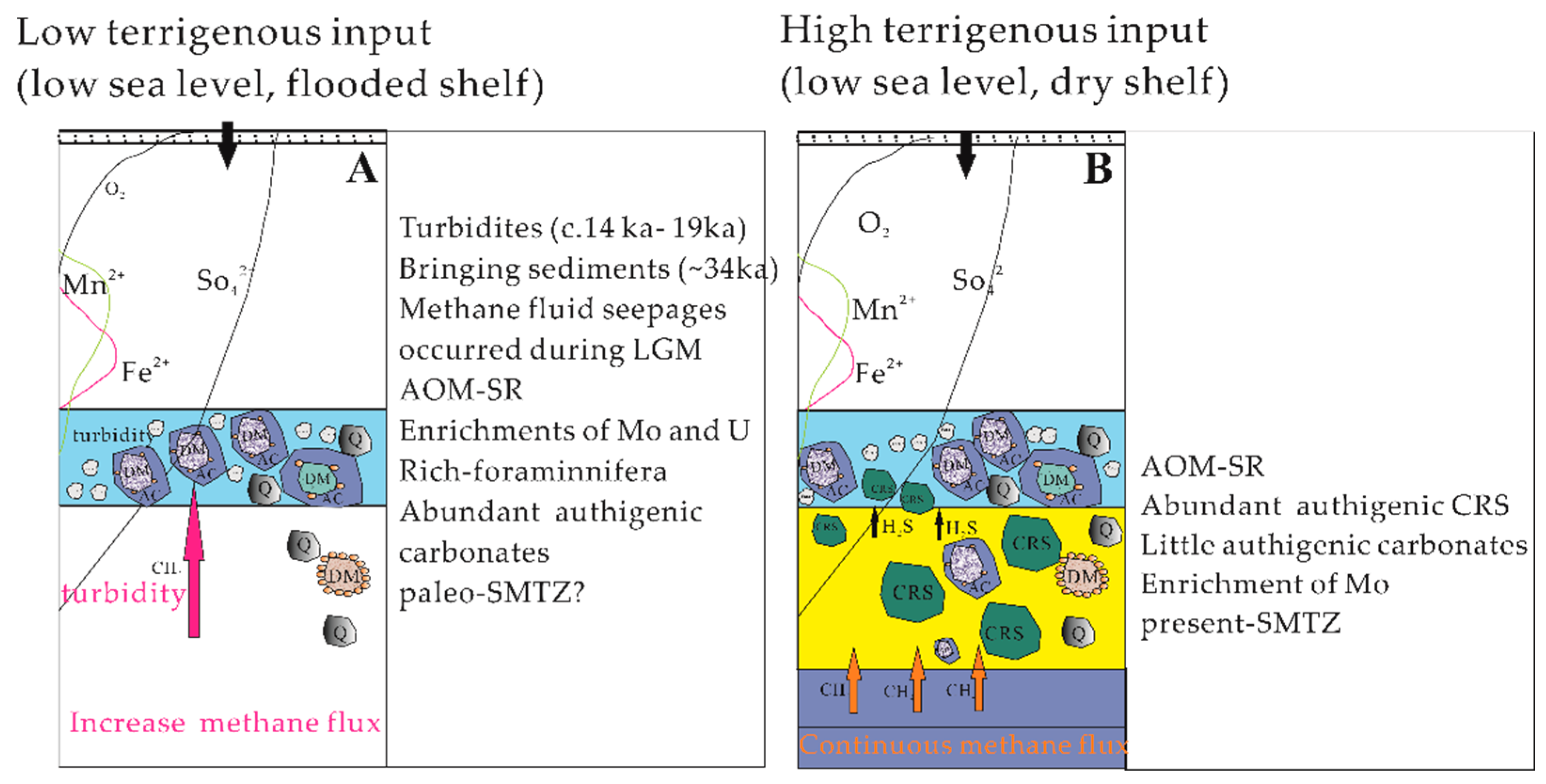
4.4. Implications for the Dynamics of Past Methane Seepages
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Supporting Information
| Depth (cm) | CRS (wt.%) | δ34 S‰VCDT | Extraction Rate | Depth (cm) | CRS (wt.%) | δ34S ‰VCDT | Extraction Rate | Depth (cm) | CRS (wt.%) | δ34S ‰VCDT | Extraction Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 63 | - | −14.68 | 88.89% | 483 | 0.350 | −42.74 | 89.92% | 783 | 0.181 | 4.02 | 88.03% |
| 103 | - | −19.01 | 503 | 0.150 | −42.45 | 803 | 0.148 | 1.42 | |||
| 143 | - | −20.16 | 523 | 0.123 | −43.00 | 823 | 0.129 | 8.25 | |||
| 183 | - | −27.03 | 543 | 0.264 | - | 843 | 1.080 | 17.42 | |||
| 263 | 0.140 | −31.58 | 563 | 0.315 | - | 863 | 1.110 | 22.05 | |||
| 283 | 0.072 | −31.98 | 92.08% | 583 | 0.383 | - | 88.13% | 883 | 0.309 | 23.56 | 89.11% |
| 303 | 0.076 | −28.64 | 603 | 0.365 | - | 903 | 0.230 | 15.10 | |||
| 323 | 0.072 | −36.34 | 623 | 1.385 | - | 963 | 0.163 | 8.38 | |||
| 343 | 0.103 | −40.26 | 643 | 0.406 | 2.55 | 1003 | 0.130 | 8.07 | |||
| 363 | 0.139 | −42.18 | 663 | 1.114 | −3.89 | 1103 | 0.183 | 5.94 | |||
| 383 | 0.114 | −41.90 | 91.40% | 683 | 1.211 | 20.13 | 91.89% | 1163 | 0.101 | 10.41 | 88.75% |
| 403 | 0.133 | −41.08 | 703 | 0.254 | −5.89 | 1203 | 0.293 | 6.01 | |||
| 423 | 0.170 | −42.16 | 723 | 0.874 | - | 1223 | 0.023 | 3.10 | |||
| 443 | 0.187 | −44.12 | 743 | 0.247 | 18.24 | ||||||
| 463 | 0.241 | −39.57 | 763 | 0.961 | 17.36 |
| Depth (cm) | Mo ppm | U ppm | V ppm | Fe (%) | Mn (%) | Depth (cm) | Mo ppm | U ppm | V ppm | Fe (%) | Mn (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 0.52 | 118.23 | 40.39 | 3.67 | 0.051 | 679 | 0.73 | 118.30 | 44.54 | 3.75 | 0.055 |
| 40 | 0.30 | 124.71 | 41.00 | 3.66 | 0.047 | 699 | 0.50 | 116.66 | 45.24 | 3.71 | 0.055 |
| 59 | 0.33 | 114.99 | 40.67 | 3.77 | 0.045 | 719 | 0.48 | 117.65 | 45.49 | 3.62 | 0.057 |
| 79 | 0.27 | 110.45 | 39.24 | 3.73 | 0.048 | 739 | 0.52 | 115.90 | 44.96 | 3.80 | 0.051 |
| 99 | 0.26 | 114.94 | 40.73 | 3.67 | 0.049 | 759 | 0.52 | 112.76 | 44.25 | 3.71 | 0.052 |
| 119 | 0.25 | 112.64 | 41.44 | 3.66 | 0.046 | 779 | 0.54 | 120.46 | 46.31 | 3.72 | 0.054 |
| 139 | 0.24 | 110.87 | 40.09 | 3.65 | 0.046 | 799 | 0.48 | 117.85 | 45.46 | 3.61 | 0.060 |
| 159 | 0.24 | 107.69 | 39.77 | 3.58 | 0.045 | 819 | 0.56 | 118.53 | 46.69 | 3.63 | 0.066 |
| 179 | 0.27 | 107.32 | 39.22 | 3.56 | 0.044 | 839 | 0.54 | 120.35 | 46.63 | 3.72 | 0.064 |
| 199 | 0.31 | 114.03 | 41.72 | 3.62 | 0.044 | 859 | 0.47 | 119.52 | 44.18 | 3.66 | 0.068 |
| 219 | 0.62 | 110.50 | 39.88 | 4.09 | 0.042 | 879 | 0.42 | 120.28 | 46.11 | 3.74 | 0.064 |
| 239 | 0.26 | 111.42 | 41.08 | 3.62 | 0.043 | 899 | 0.62 | 131.48 | 46.32 | 4.28 | 0.067 |
| 259 | 0.27 | 113.28 | 43.10 | 3.68 | 0.052 | 919 | 0.49 | 132.06 | 45.47 | 3.77 | 0.062 |
| 279 | 0.31 | 111.56 | 41.22 | 3.74 | 0.050 | 939 | 0.60 | 134.03 | 46.26 | 3.95 | 0.066 |
| 299 | 0.28 | 114.31 | 44.56 | 3.79 | 0.047 | 959 | 0.54 | 129.89 | 45.67 | 4.02 | 0.071 |
| 319 | 0.28 | 113.74 | 43.25 | 3.78 | 0.049 | 979 | 0.60 | 132.57 | 45.43 | 3.99 | 0.072 |
| 339 | 0.32 | 115.56 | 43.92 | 3.80 | 0.046 | 999 | 0.65 | 131.06 | 44.86 | 4.02 | 0.071 |
| 359 | 0.34 | 122.71 | 46.28 | 3.87 | 0.046 | 1019 | 0.44 | 129.81 | 45.49 | 3.91 | 0.081 |
| 379 | 0.49 | 123.80 | 45.87 | 3.87 | 0.049 | 1059 | 0.80 | 125.71 | 43.82 | 4.01 | 0.087 |
| 399 | 0.35 | 129.75 | 47.58 | 3.86 | 0.047 | 1079 | 0.59 | 119.12 | 43.71 | 3.93 | 0.081 |
| 419 | 0.43 | 128.41 | 47.13 | 3.97 | 0.046 | 1099 | 0.48 | 120.02 | 44.07 | 4.05 | 0.074 |
| 439 | 0.33 | 119.39 | 47.19 | 3.89 | 0.046 | 1119 | 0.68 | 121.50 | 44.35 | 4.12 | 0.081 |
| 459 | 0.29 | 73.02 | 26.38 | 3.03 | 0.035 | 1139 | 0.51 | 124.06 | 43.41 | 4.12 | 0.084 |
| 479 | 0.94 | 96.97 | 39.15 | 3.26 | 0.037 | 1180 | 0.54 | 126.77 | 44.25 | 3.92 | 0.076 |
| 499 | 0.40 | 104.41 | 36.80 | 3.33 | 0.035 | 1200 | 0.49 | 128.89 | 44.27 | 3.95 | 0.086 |
| 519 | 0.27 | 95.96 | 33.04 | 3.22 | 0.032 | 1220 | 0.56 | 127.57 | 45.12 | 3.90 | 0.075 |
| 539 | 0.48 | 97.87 | 34.90 | 3.29 | 0.035 | 1260 | 0.62 | 131.88 | 45.95 | 4.14 | 0.090 |
| 559 | 0.36 | 86.34 | 31.46 | 3.18 | 0.031 | 1280 | 0.61 | 114.87 | 42.85 | 4.03 | 0.081 |
| 579 | 0.40 | 94.41 | 32.39 | 3.22 | 0.034 | 1300 | 0.66 | 127.67 | 44.81 | 3.97 | 0.087 |
| 599 | 0.27 | 101.67 | 34.97 | 3.33 | 0.036 | 1320 | 0.72 | 131.27 | 45.06 | 4.05 | 0.080 |
| 619 | 0.69 | 110.83 | 41.84 | 3.52 | 0.042 | 1340 | 0.57 | 127.80 | 45.24 | 3.94 | 0.077 |
| 639 | 0.86 | 115.18 | 43.81 | 3.74 | 0.051 | 1360 | 0.63 | 126.95 | 45.51 | 3.75 | 0.055 |
| 659 | 1.12 | 116.55 | 45.43 | 3.82 | 0.058 |
References
- Bohrmann, G.; Torres, M.E. Gas Hydrates in Marine Sediments; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Dickens, G.R.; Rea, D.K.; Owen, R.M. Dissociation of oceanic methane hydrate as a cause of the carbon isotope excursion at the end of the. Paleoceanography 1995, 10, 965–971. [Google Scholar] [CrossRef]
- Kasten, S.; Zabel, M.; Heuer, V.; Hensen, C. Processes and Signals of Nonsteady-State Diagenesis in Deep-Sea Sediments and their Pore Waters. In The South Atlantic in the Late Quaternary: Reconstruction of Material Budgets and Current Systems; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Zimov, S.A.; Zimov, N. Late Quaternary Atmospheric CH4 Isotope Record Suggests Permafrost was a Source of CH4 and CO2; American Geophysical Union Fall Meeting: San Francisco, CA, USA, 2009. [Google Scholar]
- Wellsbury, P.; Mather, I.; Parkes, R.J. Geomicrobiology of deep, low organic carbon sediments in the Woodlark Basin, Pacific Ocean. FEMS Microbiol. Ecol. 2002, 42, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Feng, D.; Chen, L.; Wang, H.; Chen, D. Using sediment geochemistry to infer temporal variation of methane flux at a cold seep in the South China Sea. Mar. Pet. Geol. 2016, 77, 835–845. [Google Scholar] [CrossRef]
- Bo, T. Bacterial Manganese and Iron Reduction in Aquatic Sediments. Adv. Microb. Ecol. 2000, 16, 41–84. [Google Scholar]
- Wegener, G.; Krukenberg, V.; Riedel, D.; Tegetmeyer, H.E.; Boetius, A. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 2015, 526, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Haqqmisra, J.D.; Domagalgoldman, S.D.; Kasting, P.J.; Kasting, J.F. A revised, hazy methane greenhouse for the Archean Earth. Astrobiology 2008, 8, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Suess, E. Marine cold seeps and their manifestations: Geological control, biogeochemical criteria and environmental conditions. Int. J. Earth Sci. 2014, 103, 1889–1916. [Google Scholar] [CrossRef]
- Bayon, G.; Dupré, S.; Ponzevera, E.; Etoubleau, J.; Chéron, S.; Pierre, C.; Mascle, J.; Boetius, A.; Lange, G.J.D. Formation of carbonate chimneys in the Mediterranean Sea linked to deep-water oxygen depletion. Nat. Geosci. 2013, 6, 755–760. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Örgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623. [Google Scholar] [CrossRef]
- Schwartz, W. R. A. Berner, Early Diagenesis, A Theoretical Approach. XII + 241 S., 17 Tab., 60 Abb. Princeton, N.Y. 1980. Princeton University Press. $ 31.50 (Cloth), $ 13.00 (Paperb.). J. Basic Microbiol. 1981, 21, 765. [Google Scholar]
- Haese, R.R.; Meile, C.; Van Cappellen, P.; De Lange, G.J. Carbon geochemistry of cold seeps: Methane fluxes and transformation in sediments from Kazan mud volcano, eastern Mediterranean Sea. Earth Planet. Sci. Lett. 2003, 212, 361–375. [Google Scholar] [CrossRef]
- Yoshinaga, M.Y.; Holler, T.; Goldhammer, T.; Wegener, G.; Pohlman, J.W.; Brunner, B.; Kuypers, M.M.M.; Hinrichs, K.U.; Elvert, M. Carbon isotope equilibration during sulphate-limited anaerobic oxidation of methane. Nat. GeoSci. 2014, 7, 190–194. [Google Scholar] [CrossRef]
- Jørgensen, P.R.; Urup, J.; Helstrup, T.; Jensen, M.B.; Eiland, F.; Vinther, F.P. Transport and reduction of nitrate in clayey till underneath forest and arable land. J. Contam. Hydrol. 2004, 73, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Reeburgh, W.S. Oceanic Methane Biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, K.G.; Macquaker, J.H.S. Iron in marine sediments: Minerals as records of chemical environments. J. Reprod. Fertil. Suppl. 2011, 7, 113–118. [Google Scholar] [CrossRef]
- David Rickard, A.; Luther, G.W. Chemistry of Iron Sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Borowski, W.S.; Rodriguez, N.M.; Paull, C.K.; Iii, W.U. Are 34 S-enriched authigenic sulfide minerals a proxy for elevated methane flux and gas hydrates in the geologic record? Mar. Pet. Geol. 2013, 43, 381–395. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [PubMed]
- Peketi, A.; Joshi, R.K.; Patil, D.J.; Srinivas, P.L.; Dayal, A.M. Tracing the Paleo sulfate-methane transition zones and H2S seepage events in marine sediments: An application of C-S-Mo systematics. Geochem. Geophys. Geosyst. 2012, 13. [Google Scholar] [CrossRef]
- Lim, Y.C.; Lin, S.; Yang, T.F.; Chen, Y.G.; Liu, C.S. Variations of methane induced pyrite formation in the accretionary wedge sediments offshore Southwestern Taiwan. Mar. Pet. Geol. 2011, 28, 1829–1837. [Google Scholar] [CrossRef]
- Chen, F.; Hu, Y.; Feng, D.; Zhang, X.; Cheng, S.; Cao, J.; Lu, H.; Chen, D. Evidence of intense methane seepages from molybdenum enrichments in gas hydrate-bearing sediments of the northern South China Sea. Chem Geol. 2016, 443, 173–181. [Google Scholar] [CrossRef]
- Tribovillard, N.; Bout-Roumazeilles, V.; Algeo, T.; Lyons, T.; Sionneau, T.; Montero-Serrano, J.; Riboulleau, A.; Baudin, F. Paleodepositional conditions in the Orca Basin as inferred from organic matter and trace metal contents. Mar. Geol. 2008, 254, 62–72. [Google Scholar] [CrossRef]
- Argentino, C.; Lugli, F.; Cipriani, A.; Conti, S.; Fontana, D. A deep fluid source of radiogenic Sr and highly dynamic seepage conditions recorded in Miocene seep carbonates of the northern Apennines (Italy). Chem. Geol. 2019, 522, 135–147. [Google Scholar] [CrossRef]
- Mutti, D.O.; Mitchell, G.L.; Jones, L.A.; Friedman, N.E.; Frane, S.L.; Lin, W.K.; Moeschberger, M.L.; Zadnik, K. Accommodation, Acuity, and their Relationship to Emmetropization in Infants. Optom. Vis. Sci. 2009, 86, 666–676. [Google Scholar] [CrossRef]
- Piper, D.J.W.; Normark, W.R. Processes That Initiate Turbidity Currents and Their Influence on Turbidites: A Marine Geology Perspective. J. Sediment. Res. 2009, 79, 347–362. [Google Scholar] [CrossRef]
- Feng, D.; Roberts, H.H.; Cheng, H.; Peckmann, J.; Bohrmann, G.; Edwards, R.L.; Chen, D. U/Th dating of cold-seep carbonates: An initial comparison. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 2055–2060. [Google Scholar] [CrossRef]
- Bayon, G.; Henderson, G.M.; Etoubleau, J.; Caprais, J.C.; Ruffine, L.; Marsset, T.; Dennielou, B.; Cauquil, E.; Voisset, M.; Sultan, N. U-Th isotope constraints on gas hydrate and pockmark dynamics at the Niger delta margin. Mar. Geol. 2015, 370, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.L.; Wang, Y.P.; Wang, J.Z.; Jian, P.; Wang, Y.H.; Cheng, D.W.; Ye, L.I. Geochemical Characters of Ree in the Seafloor Sediment in Northern Continental Slope of the South China Sea and Analysis of Source of Material and Diagenesis Environment. Mar. Geol. Quat. Geol. 2004, 24, 17–23. [Google Scholar]
- Luan, L.; Lippman, T.M.; Hicks, C.W.; Bert, J.A.; Auslaender, O.M.; Chu, J.H.; Analytis, J.G.; Fisher, I.R.; Moler, K.A. Local measurement of the superfluid density in the pnictide superconductor Ba(Fe(1−x)Co(x))(2)As(2) across the superconducting dome. Phys. Rev. Lett. 2011, 106, 067001. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, Y.; Xu, H. Gas Hydrate on the Passive Continental Margin and Its Pool-formation Process. Geol. Rev. 2003, 2, 181–186. [Google Scholar]
- Sha, Z.B.; Wang, H.B.; Yang, M.Z.; Liang, J.Q.; Zhang, G.X.; Liu, X.W.; Gong, Y.H. Study on Recognizing Technology of Gas Hydrates Zone. GeoScience 2008, 22, 438–446. [Google Scholar]
- Wu, N.; Yang, S.X.; Wang, H.B.; Liang, J.Q.; Gong, Y.H.; Lu, Z.Q.; Wu, D.; Guan, H.X. Gas-bearing fluid influx sub-system for gas hydrate geological system in Shenhu Area, Northern South China Sea. Chin. J. Geophys. 2009, 6, 1641–1650. [Google Scholar]
- Zhang, A.; Al, E. Effect of Acid Stress on Growth of and Ca and Mg Uptake by Maize of Different Genotypes. Chin. J. Appl. Environ. Biol. 2007, 6, 794–798. [Google Scholar]
- Zhang, G.; Liang, J.; Lu, J.; Yang, S.; Zhang, M.; Su, X.; Xu, H.; Fu, S.; Kuang, Z. Characteristics of natural gas hydrate reservoirs on the northeastern slope of the South China Sea. Nat. Gas Ind. 2014, 11, 1–10. [Google Scholar]
- Zhong, C.; Yan, W.; Chen, M.; Muhong, C. Discovery of seep authigenic carbonate nodules on northern continental slope of South China Sea: New evidence of gas hydrate. J. Trop. Oceanogr. 2006, 51, 1065–1072. [Google Scholar]
- Daidai, W.U.; Nengyou, W.U.; Shaoying, F.U.; Liang, J.; Guan, H. Geochemical Characteristics of Shallow Sediments in the Gas Hydrate Distribution Area of Dongsha, the Northern South China Sea. Mar. Geol. Quat. Geol. 2010, 30, 41–51. [Google Scholar]
- Lu, H.; Liu, J.; Chen, F.; Liao, Z.L.; Sun, X.M.; Xin, S.U. Mineralogy and stable isotopic composition of authigenic carbonates in bottom sediments in the offshore area of southwest Taiwan, South China Sea: Evidence for gas hydrates occurrence. Earth Sci. Front. 2005, 17, 981–1007. [Google Scholar]
- He, F.; Chen, Y.C.; Liu, A.B. The Characteristics of Geochemical and Hydrocarbon-Generation Potential Evaluation in Hydrocarbon Source Rock of Shanxi Group of Sulige Area. Comput. Tech. Geophys. GeoChem. Explor. 2009, 618–633. [Google Scholar] [CrossRef]
- Pan, M.; Wu, D.; Yang, F.; Sun, T.; Wu, N.; Liu, L. Geochemical sedimentary evidence from core 973-2 for methane activity near the Jiulong Methane Reef in the northern South China Sea. Interpretation 2017, 6, 1–39. [Google Scholar] [CrossRef]
- Canfield, P.; Forbes, L. Supression of drain conductance transients, drain current oscillations, and low-frequency generation—Recombination noise in GaAs FET’s using buried channels. IEEE Trans. Electron Devices 1986, 33, 925–928. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, X.; Zou, J.; Cheng, Z.; Wang, K.; Ge, S.; Shi, F. Benthic foraminiferal δ 13 C minimum events in the southeastern Okhotsk Sea over the last 180 ka. Chin. Sci. Bull. 2014, 59, 3066–3074. [Google Scholar] [CrossRef]
- Gibbs, A.K. The Continental Crust: Its Composition and Evolution. J. Geol. 1985, 4, 632–633. [Google Scholar]
- Ying, Q. Response of Cold Seep Benthic Foraminifera and Methane Eruption in Northern Slope of the South China Sea. Master’s Thesis, China University of GeoSciences, Wuhan, Beijing, 2013. [Google Scholar]
- Lin, Q.; Wang, J.; Fu, S.; Lu, H.; Bu, Q.; Lin, R.; Sun, F. Elemental sulfur in northern South China Sea sediments and its significance. Sci. China Earth Sci. 2015, 58, 2271–2278. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, M.; Wu, D.; Wu, N. Distribution and isotopic composition of foraminifera at cold-seep Site 973-4 in the Dongsha area, northeastern South China Sea. J. Asian Earth Sci. 2018, 168, 145–154. [Google Scholar] [CrossRef]
- Liu, J.; Izon, G.; Wang, J.; Antler, G.; Wang, Z.; Zhao, J.; Egger, M. Vivianite formation in methane-rich deep-sea sediments from the South China Sea. Biogeoscience 2018, 15, 6329–6348. [Google Scholar] [CrossRef] [Green Version]
- Thiel, V.; Peckmann, J.; Richnow, H.H.; Luth, U.; Reitner, J.; Michaelis, W. Molecular signals for anaerobic methane oxidation in Black Sea seep carbonates and a microbial mat. Mar. Chem. 2001, 73, 97–112. [Google Scholar] [CrossRef]
- Peckmann, J.; Thiel, V.; Reitner, J.; Taviani, M.; Aharon, P.; Michaelis, W. A Microbial Mat of a Large Sulfur Bacterium Preserved in a Miocene Methane-Seep Limestone. GeoMicrobiol. J. 2004, 21, 247–255. [Google Scholar] [CrossRef]
- Formolo, M.J.; Lyons, T.W. Sulfur biogeochemistry of cold seeps in the Green Canyon region of the Gulf of Mexico. Geochim. Cosmochim. Acta 2013, 119, 264–285. [Google Scholar] [CrossRef]
- Berner, R.A.; Scott, M.R.; Thomlinson, C. Carbonate Alkalinity in the Pore Waters of Anoxic Marine Sediments. Limnol. Oceanogr. 1970, 15, 544–549. [Google Scholar] [CrossRef]
- Morse, J.W.; Berner, R.A. What determines sedimentary C S ratios? Geochim. Cosmochim. Acta 1995, 59, 1073–1077. [Google Scholar] [CrossRef]
- Habicht, K.; Canfield, D.E. The Effect of Sulfate Concentration on the Sulfur Isotope Fractionation During Sulfate Reduction by Sulfate-reducing Bacteria. In Proceedings of the Eleventh Annual VM Goldschmidt Conference, Hot Springs, VA, USA, 20–24 May 2001. [Google Scholar]
- Jørgensen, B.B.; Findlay, A.J.; Pellerin, A. The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 2019, 10, 1–27. [Google Scholar] [CrossRef]
- Sugita, A.; Sugii, A.; Sato, K.; Zhang, X.Y.; Dai, A.L.; Taguchi, G.; Shimosaka, M. Cloning and Characterization of a Gene Coding for a Major Extracellular Chitosanase from the Koji Mold Aspergillus oryzae. BioSci. Biotechnol. Biochem. 2012, 76, 193–195. [Google Scholar] [CrossRef]
- Aharon, P.; Schwarcz, H.P.; Roberts, H.H. Radiometric dating of submarine hydrocarbon seeps in the Gulf of Mexico. Geol. Soc. Am. Bull. 1997, 109, 568–579. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nakai, S.I.; Hiruta, A.; Matsumoto, R.; Yoshida, K. U–Th dating of carbonate nodules from methane seeps off Joetsu, Eastern Margin of Japan Sea. Earth Planet. Sci. Lett. 2008, 272, 89–96. [Google Scholar] [CrossRef]
- Pan, M. Evidence from Foraminifera and Geochemical Characteristics of Sediments for Methane Seeps in the Northern Slope of the South China Sea; Chinese Academy of Sciences: Beijing, China, 2017; pp. 31–32. [Google Scholar]
- Labeyrie, L.D.; Duplessy, J.C.; Blanc, P.L. Variations in mode of formation and temperature of oceanic deep waters over the past 125,000 years. Nature 1987, 327, 477–482. [Google Scholar] [CrossRef]
- Peckmann, J.; Reimer, A.; Luth, U.; Luth, C.; Hansen, B.T.; Heinicke, C.; Hoefs, J.; Reitner, J. Methane-derived carbonates and authigenic pyrite from the northwestern Black Sea. Mar. Geol. 2001, 177, 129–150. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S.; Zhang, Z.; Lan, X.; Gu, Z.; Zhang, X. Paleo-fluvial sedimentation on the outer shelf of the East China Sea during the last glacial maximum. Chin. J. Oceanol. Limnol. 2013, 31, 886–894. [Google Scholar] [CrossRef]
- Liu, J.P.; Xu, K.H.; Li, A.C.; Milliman, J.D.; Velozzi, D.M.; Xiao, S.B.; Yang, Z.S. Flux and fate of Yangtze River sediment delivered to the East China Sea. Geomorphology 2007, 85, 208–224. [Google Scholar] [CrossRef]
- Dou, Y.; Yang, S.; Liu, Z.; Clift, P.D.; Shi, X.; Hua, Y.; Berne, S. Provenance discrimination of siliciclastic sediments in the middle Okinawa Trough since 30 ka: Constraints from rare earth element compositions. Mar. Geol. 2010, 275, 212–220. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Colin, C.; Xie, X.; Wu, Q. Turbidite deposition in the southern South China Sea during the last glacial: Evidence from grain-size and major elements records. Chin. Sci. Bull. 2011, 56, 3558–3565. [Google Scholar] [CrossRef] [Green Version]
- Gulin, S.B.; Polikarpov, G.G.; Egorov, V.N. The age of microbial carbonate structures grown at methane seeps in the Black Sea with an implication of dating of the seeping methane. Mar. Chem. 2003, 84, 67–72. [Google Scholar] [CrossRef]
- Tong, H.; Feng, D.; Cheng, H.; Yang, S.; Wang, H.; Min, A.G.; Edwards, R.L.; Chen, Z. Authigenic carbonates from seeps on the northern continental slope of the South China Sea: New insights into fluid sources and geochronology. Mar. Pet. Geol. 2013, 43, 260–271. [Google Scholar] [CrossRef]
- Han, X.; Suess, E.; Liebetrau, V.; Eisenhauer, A.; Huang, Y. Past methane release events and environmental conditions at the upper continental slope of the South China Sea: Constraints by seep carbonates. Int. J. Earth Sci. 2014, 103, 1873–1887. [Google Scholar] [CrossRef]
- Jian, Z.; Wang, L.; Kienast, M.; Sarnthein, M.; Kuhnt, W. Benthic foraminiferal paleoceanography of the South China Sea over the last 40,000 years. Mar. Geol. 1999, 156, 159–186. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Schwieger, W.; Unger, A. Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion-exchange method. Appl. Catal. B Environ. 2008, 84, 277–288. [Google Scholar] [CrossRef]
- Fang, C. Characteristics of Turbidity Current Deposits of Core SA14-34 in Deep Sea Basin of the Western South China Sea. Geol. Res. South China Sea 2007, 1, 31–39. [Google Scholar]
- Scholz, A.; Lang, V.; Henschler, R.; Czabanka, M.; Vajkoczy, P.; Chavakis, E.; Drynski, J.; Harter, P.N.; Mittelbronn, M.; Dumont, D.J. Angiopoietin-2 promotes myeloid cell infiltration in a β2-integrin–dependent manner. Blood 2011, 118, 5050–5059. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Anschutz, P.; Jorissen, F.J.; Chaillou, G.; Abu-Zied, R.; Fontanier, C. Recent turbidite deposition in the eastern Atlantic: Early diagenesis and biotic recovery. J. Mar. Res. 2002, 60, 835–854. [Google Scholar] [CrossRef]
- Sato, H.; Hayashi, K.I.; Ogawa, Y.; Kawamura, K. Geochemistry of deep sea sediments at cold seep sites in the Nankai Trough: Insights into the effect of anaerobic oxidation of methane. Mar. Geol. 2012, 323, 47–55. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, D.; Peckmann, J.; Roberts, H.; Chen, D. New insights into cerium anomalies and mechanisms of trace metal enrichment in authigenic carbonate from hydrocarbon seeps. Chem. Geol. 2014, 381, 55–66. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, L.; Feng, D.; Liang, Q.; Xia, Z.; Chen, D. Geochemical record of methane seepage in authigenic carbonates and surrounding host sediments: A case study from the South China Sea. J. Asian Earth Sci. 2017, 138, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.-L.; Solomon, E.A.; Torres, M.E. A kinetic-model approach to quantify the effect of mass transport deposits on pore water profiles in the Krishna–Godavari Basin, Bay of Bengal. Mar. Pet. Geol. 2014, 58, 223–232. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Sun, T.; Xie, R.; Pan, M.; Chen, X.; Ye, Y.; Liu, L.; Wu, N. Characteristics of Authigenic Minerals around the Sulfate-Methane Transition Zone in the Methane-Rich Sediments of the Northern South China Sea: Inorganic Geochemical Evidence. Int. J. Environ. Res. Public Health 2019, 16, 2299. https://doi.org/10.3390/ijerph16132299
Wu D, Sun T, Xie R, Pan M, Chen X, Ye Y, Liu L, Wu N. Characteristics of Authigenic Minerals around the Sulfate-Methane Transition Zone in the Methane-Rich Sediments of the Northern South China Sea: Inorganic Geochemical Evidence. International Journal of Environmental Research and Public Health. 2019; 16(13):2299. https://doi.org/10.3390/ijerph16132299
Chicago/Turabian StyleWu, Daidai, Tiantian Sun, Rui Xie, Mengdi Pan, Xuegang Chen, Ying Ye, Lihua Liu, and Nengyou Wu. 2019. "Characteristics of Authigenic Minerals around the Sulfate-Methane Transition Zone in the Methane-Rich Sediments of the Northern South China Sea: Inorganic Geochemical Evidence" International Journal of Environmental Research and Public Health 16, no. 13: 2299. https://doi.org/10.3390/ijerph16132299






