1. Introduction
Statistics from Taiwan’s National Health Insurance Administration show that as of 2016, the incidence and prevalence rates of kidney disease in Taiwan had become the highest in the world, with 78,000 patients on hemodialysis (HD) or peritoneal dialysis, indicating a prevalence rate of 3177.8 per million people [
1]. Although the incidence rate of kidney disease has been gradually decreasing in Taiwan, the prevalence rate of HD has risen steadily, owing to the following reasons: (1) population aging and an increase in patients with chronic diseases such as hypertension, hyperlipidemia, and hyperglycemia; (2) provision of full insurance coverage for dialysis by the National Health Insurance and lowering of the coverage threshold for such treatments; (3) excellent dialysis treatment quality; and (4) a low kidney transplant rate [
2].
Patients on HD must receive treatment 3 times per week and for 4 hours per treatment session, in order to maintain the basic kidney function necessary for survival [
3]. HD is a common alternative treatment in Taiwan, and medical personnel are well-practiced at intravenous insertion; however, venous needle dislodgement (VND) still occurs occasionally, which can lead to blood leakage. VND is most commonly caused by improper arm movement in the patients, and is particularly common among agitated patients. When this occurs with patients of advanced age or in a state of disturbed consciousness, these patients may barely notice that the needle has been dislodged and they have blood leakage. Therefore, for such patients, constant inspection by medical personnel and caregivers is indispensable. Furthermore, although dialysis machines usually have a built-in mechanism for detecting blood leakage, the presence of sediment in the system can sometimes cause false alarms. Moreover, when blood leakage occurs from a VND, the machines usually require 3−5 minutes to detect it, and sometimes they even fail to detect it at all because the puncture is small and the leakage is slow. As a consequence, the patients may sustain varying degrees of injury. For this reason, care should be taken during dialysis to prevent the needle from being covered by blankets or clothing, to make the detection of VND easier for medical personnel, caregivers, and the patients themselves.
The 2016 annual report of the Taiwan Patient Safety Reporting System listed the dislodgement of needles, catheters, and similar devices as third among the 13 most common incident types that threaten patient safety (the top two being drug- and fall-related incidents). In addition, it was reported that HD-related device dislodgement alone accounted for 357 incidents in 2016 [
4]. A survey by Axley et al. [
5] reported that up to 76.6% of respondents had witnessed VND during HD in the previous 5 years, 57.9% indicated that they were concerned about VND very often or often, and 85.3% considered education material beneficial for the reduction of VND risk. Two studies published in 2005 warned that although VND is not an extremely common occurrence, it could be life-threatening or even cause death [
6,
7]. In 2012, the American Nephrology Nurses Association established a team to examine the occurrence and consequences of VND, as well as to propose measures for medical personnel, patients with kidney disease, and patients’ families to prevent VND. All of these studies suggested that although the likelihood of VND directly causing death is extremely small, it can, nevertheless, have fatal consequences. This is particularly true for patients who are in an agitated state, have impaired cognitive abilities, or are receiving HD alone.
Numerous factors impose massive pressure on nursing staff and affect their retention rate and personal health; these include a sense of responsibility to medical services and to life, as well as clinical situations such as workload, nurse–patient relationships, irregular work schedules, role-specific conflicts and requirements, administrative work, personnel turnover, work environment, interpersonal relationships, and lack of professional knowledge and skills [
8,
9,
10,
11,
12,
13]. Due to the unique roles and importance of clinical nursing staff, excessive pressure not only affects their personal health, but also the quality of the clinical services they provide. Hence, medical institutions worldwide strive to improve working conditions for nursing staff and mitigate their stress without sacrificing nursing quality.
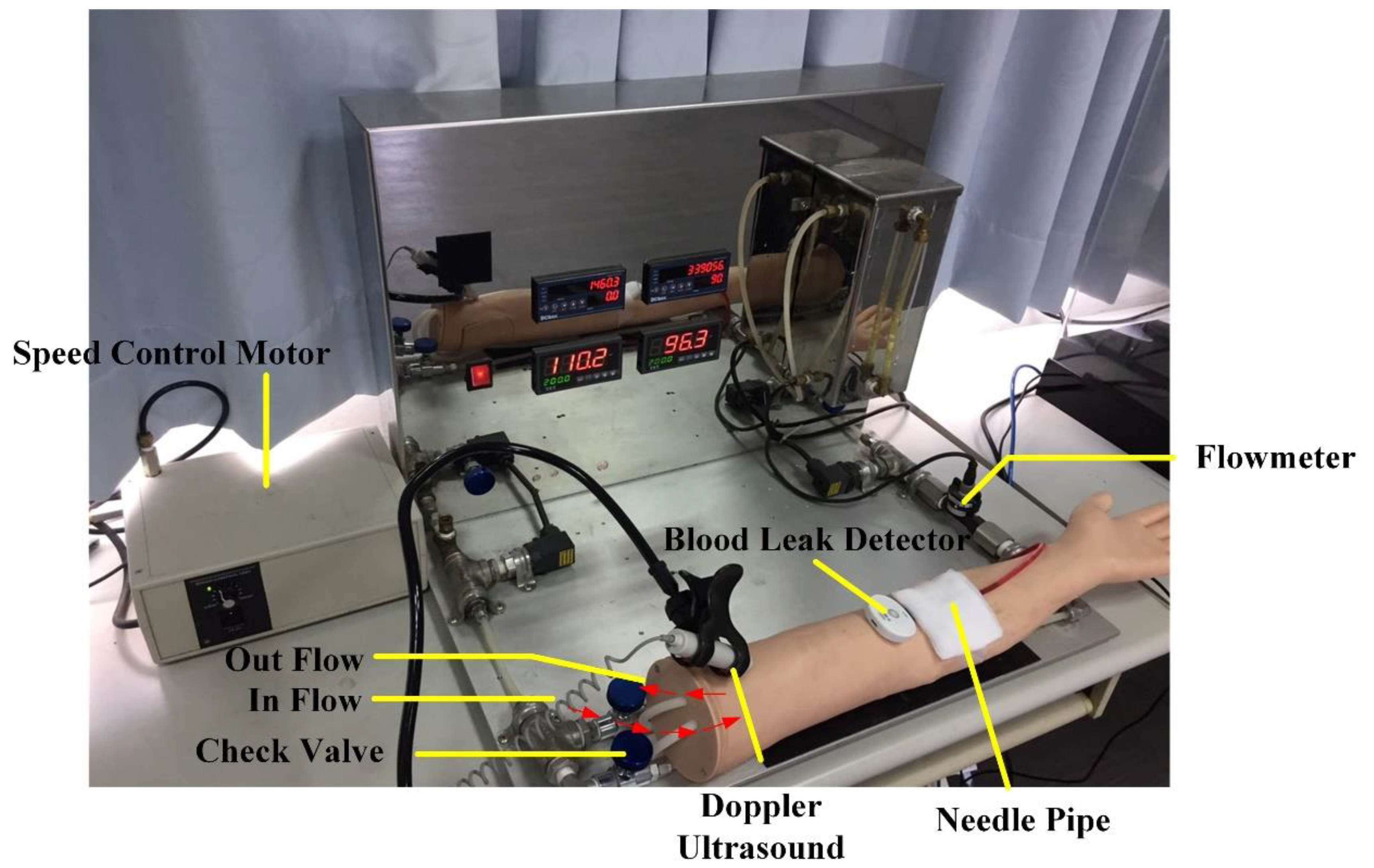
Thus, in order to provide an additional layer of protection for patients undergoing HD, as well as to alleviate the clinical pressure of nursing staff and facilitate positive nurse–patient interactions, a blood leakage detection device has been developed. The present study describes device testing in both a simulated and clinical setting, and users’ responses from nursing staff and patients.
4. Discussion
This study evaluated a blood leakage detection device in clinical tests and found its accuracy rate to be 98.9%. The device failed to detect blood leakage 6 times; the reason for this was that a tiny gap existed between the tape and the puncture, and hence, when an extremely small amount of leakage occurred, the blood was quickly absorbed by the gauze before it was able to trigger the alarm.
HD is a prolonged undertaking that requires patients to undergo treatment on a regular basis, during which they are subjected to extensive physiological, psychological, and social stress [
15]. Moreover, the discomfort involved in the process can often cause patients to develop an aversion to the treatment [
16]. Because of the importance of patient’s mood in managing disease and maintaining well-being, the design of the device intends to improve the HD experience for the patient. The device was found to alleviate the mental stress of patients on HD, allowing them to undergo the treatment in a relatively calm, relaxed, and stable state. Such a quality is considered beneficial to both patients’ conditions and the effectiveness of their treatment.
Nursing staff are subjected to high emotional labor and stress, which not only affects their physical and mental health, but also reduces their morale and work efficiency. When patients receive HD, nursing staff are required to check the patients on a regular basis to prevent VND. Taiwan has seen numerous cases of nurses being convicted because of VND [
17], and such precedents are a form of work pressure for nurses. In severe cases, the pressure can even affect their work performance and lead to errors. The proposed blood leakage device was found to be reliable and capable of facilitating a positive nurse–patient relationship. Therefore, from a medical quality perspective, the device is beneficial for patient safety, nurse–patient relationships, and quality of care.
In Taiwan, most HD patients undergo hemodialysis during the daytime, and many fall asleep during the 3–4 hour long session, during which the occasional motions and movements of the body or limbs is a great risk for VND. Furthermore, patients at high risk for being agitated or restless—such as elderly patients with dementia—require constant attention. There has even been a case of fatal hemorrhaging from VND where a long-term HD patient suffering from major depression pulled out his own needle. Reports such as these put considerable stress on HD clinical care personnel in Taiwan. Although the HD staff-to-patient ratio is 1:4, the nursing staff may need to temporarily leave for other clinical duties and not be able to remain at the patient’s side throughout the entire HD session. Therefore, this device can provide significant help to improve patient safety.
One major purpose of product design was to provide patients undergoing HD with further safety and convenience in their lives. In the medical industry, the development of assistive medical devices aims to improve the safety and convenience of medical services for both patients and medical personnel. The device proposed in this study uses flexible array sensing technology, and is made up of a green halogen-free and lead-free printed circuit board (PCB) and medical-grade plastic approved by the US Food and Drug Administration. Therefore, it not only conforms to standing medical regulations and EU standards, but also has excellent potential for extended clinical applications. Specifically, in addition to HD, use of this device may be expanded for the safety of patients to prevent the dislodgement of peripheral venous catheters.