Estimation of the Allergenic Potential of Urban Trees and Urban Parks: Towards the Healthy Design of Urban Green Spaces of the Future
Abstract
1. Introduction
2. Materials and Methods
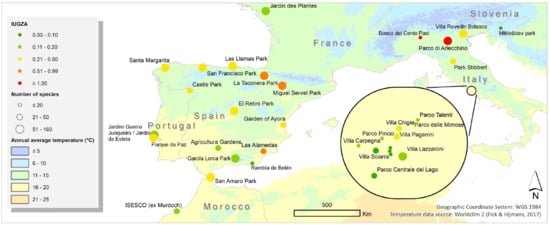
2.1. Selection of Urban Parks and Inventory of Vegetation
2.2. Allergenic Risk Assessment
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiesura, A. The role of urban parks for the sustainable city. Landsc. Urban Plan. 2004, 68, 129–138. [Google Scholar] [CrossRef]
- Andreucci, M.B. Progettare Green Infrastructure Tecnologie, Valori e Strumenti per la Resilienza Urbana; Wolters Kluwer: Milano, Italy, 2017. [Google Scholar]
- Sadeghian, M.M.; Vardanyan, Z. The benefits of Urban Parks, a review of urban research. J. Nov. Appl. Sci. 2013, 2, 231–237. [Google Scholar]
- Mexia, T.; Vieira, J.; Príncipe, A.; Anjos, A.; Silva, P.; Lopes, N.; Freitas, C.; Santos-Reis, M.; Correia, O.; Branquinho, C.; et al. Ecosystem services of urban parks under the magnifying glass. Environ. Res. 2018, 24, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Bardelli, T.; Giovannini, G.; Pecchioli, L. Air quality impact of an urban park over time. Procedia Environ. Sci. 2011, 4, 10–16. [Google Scholar] [CrossRef]
- Vieira, J.; Matos, P.; Mexia, T.; Silva, P.; Lopes, N.; Freitas, C.; Correia, O.; Branquinho, C.; Pinho, P. Green spaces are not all the same for the provision of ecosystem services: The case of air purification and climate regulation. Environ. Res. 2018, 160, 306–313. [Google Scholar] [CrossRef]
- Liu, C.; Li, X. Carbon storage and sequestration by urban forests in Shenyang, China. Urban For. Urban Green. 2012, 11, 121–128. [Google Scholar] [CrossRef]
- Bowler, D.E.; Buyung, A.L.; Knight, T.M.; Pullin, A.S. Urban greening to cool towns and cities: A systematic review of the empirical evidence. Landsc. Urban Plan. 2010, 97, 147–155. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Li, Y.; Wu, S. Water-related ecosystem services provided by urban green space: A case study in Yixing City (China). Landsc. Urban Plan. 2015, 136, 40–51. [Google Scholar] [CrossRef]
- Vilhar, U. Water Regulation and Purification. In The Urban Forest: Cultivating Green Infrastructure for People and the Environment; Pearlmutter, D., Calfapietra, C., Samson, R., O’Brien, L., Krajter Ostoic, S., Sanesi, G., Alonso del, A., Eds.; Springer: Basel, Switzerland, 2017. [Google Scholar]
- Sanesi, G.; Lafortezza, R.; Bonnes, M.; Carrus, G. Comparison of two different approaches for assessing the psychological and social dimensión of Green spaces. Urban For. Urban Green. 2006, 5, 121–129. [Google Scholar] [CrossRef]
- Grahn, P.; Stigsdotter, U.A. Landscape planning and stress. Urban For. Urban Green. 2003, 2, 1–18. [Google Scholar] [CrossRef]
- Adinolfi, C.; Suárez-Cáceres, G.P.; Cariñanos, P. Relation between visitors’ behaviour and characteristics of Green spaces in the city of Granada, south-eastern Spain. Urban For. Urban Green. 2014, 13, 534–542. [Google Scholar] [CrossRef]
- Richardson, E.A.; Mitchell, R. Gender differences in relationships between urban green space and health in the United Kingdom. Soc. Sci. Med. 2010, 71, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, N.; Haase, D.; Annerstedt van den Bosch, M. Adding natural areas to social indicators of intra-urban health inequalities among children: A case study from Berlin, Germany. Int. J. Environ. Res. Public Health 2016, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Andrada, R.; Pierskalla, C. Visitors’ and residents’ perception of urban forests for leisure in Washington DC. Urban For. Urban Green. 2007, 28, 1–11. [Google Scholar] [CrossRef]
- Weber, D.; Anderson, D. Contact with nature: Recreation experiences preference in Australian parks. Ann. Leis. Res. 2010, 13, 46–69. [Google Scholar] [CrossRef]
- Calaza-Martínez, P. Infraestructura Verde. Sistema Natural de Salud Pública; Mundi Prensa: Madrid, Spain, 2017. [Google Scholar]
- Hansen, W.D. Generalizable principles for ecosystem stewardship-based management of social-ecological systems: Lessons learned from Alaska. Ecol. Soc. 2014, 19, 13. [Google Scholar] [CrossRef]
- Lyytimäki, J.; Sipilä, M. Hopping on one leg-The challenge of ecosystem disservices for urban green management. Urban For. Urban Green. 2009, 8, 309–315. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Cariñanos, P.; Calaza-Martínez, P.; O’Brien, L.; Calfapietra, C. The Cost of greening: Disservices of Urban trees. In The Urban Forest: Cultivating Green Infrastructure for People and the Environment; Pearlmutter, D., Calfapietra, C., Samson, R., O’Brien, L., Krajter Ostoic, S., Sanesi, G., Alonso del, A., Eds.; Springer: Basel, Switzerland, 2017. [Google Scholar]
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; van Cauwenberge, P. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef]
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2004, 7, 55. [Google Scholar] [CrossRef]
- Zuberbier, T.; Lötvall, J.; Simoens, S.; Subramaniam, S.V.; Church, M.K. Economic burden of inadequate management of allergic diseases in the European Union: A GA2LEN review. Allergy 2014, 69, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Lake, I.R.; Jones, N.R.; Agnew, M.; Goodess, C.M.; Giorgi, F.; Hmaoui-Lagnel, L.; Semenov, M.A.; Solomon, F.; Storkey, J.; Vantard, R.; et al. Climate Change and Future Pollen Allergy in Europe. Environ. Health Perspect. 2017, 125, 385–391. [Google Scholar] [CrossRef]
- Cariñanos, P.; Adinolfi, C.; Díaz de la Guardia, C.; De Linares, C.; Casares-Porcel, M. Characterization of allergen emission sources in urban áreas. J. Environ. Qual. 2016, 45, 244–252. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G. Environmental urban factors (air pollution and allergens) and the rising trends in allergy respiratory diseases. Allergy 2002, 57, 30–33. [Google Scholar] [CrossRef]
- Jianan, X.; Zhiyun, O.; Hua, Z.; Xiaoke, W.; Hong, M. Allergenic pollen plants and their influential factors in urban areas. Acta Ecol. Sin. 2007, 27, 3820–3827. [Google Scholar] [CrossRef]
- Smith, M.; Jäger, S.; Berger, U.; Sikoparija, B.; Hallsdottir, M.; Sauliene, I.; Bergmann, K.C.; Pashley, C.H.; de Werger, L.; Majkowska-Wojciechowska, B.; et al. Geographic and temporal variations in pollen exposure across Europe. Allergy 2014, 69, 913–923. [Google Scholar] [CrossRef]
- Buters, J. Pollen allergens and geographical factors. In Global Atlas of Allergy; Akdis, C.A., Agache, I., Eds.; European Academy of Allergy and Clinical Immunology: Zurich, Switzerland, 2014. [Google Scholar]
- García-Mozo, H. Poaceae pollen as the leading aeroallergen worldwide: A review. Allergy 2017, 72, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G. Pollen allergy in the Mediterranean area. Rev. Fr. d’Allergol. d’Immunol. Clin. 1998, 38, 5160–5162. [Google Scholar] [CrossRef]
- Maeda, Y.; Akiyama, K.; Shida, T. A clinical study of Japanese cedar (Cryptomeria japonica) pollen-induced asthma. Allergol. Int. 2008, 57, 413–417. [Google Scholar] [CrossRef]
- Asam, C.; Hofer, H.; Wolf, M.; Agla, L.; Wallner, M. Tree pollen allergens—An update from a molecular perspective. Allergy 2015, 70, 1201–1211. [Google Scholar] [CrossRef]
- Dales, R.E.; Cakmak, S.; Judek, S.; Coates, F. Tree pollen and Hospitalization for asthma in urban Canada. Int. Arch. Allergy Immunol. 2008, 146, 241–247. [Google Scholar] [CrossRef]
- Harbele, S.G.; Bowman, D.M.J.S.; Newham, R.M.; Johnston, F.H.; Beggs, P.J.; Buters, J.; Campbell, B.; Erbas, B.; Godwin, I.; Green, B.J.; et al. The macroecology of airborne pollen in Australia and New Zealand urban areas. PLoS ONE 2014, 9, e97925. [Google Scholar]
- McMichael, A.J.; Woodruff, R.E.; Hales, S. Climate change and human health: Present and future risks. The Lancet 367:11-17 Médeil F, Quézel P. 1999. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 2006, 13, 1510–1513. [Google Scholar]
- Oteros, J.A.; García-Mozo, H.; Alcázar, P.; Belmonte, J.; Bermejo, D.; Boi, M.; Cariñanos, P.; Díaz de la Guardia, C.; Fernández-González, D.; Gozález-Minero, F.J.; et al. A new method for determining the sources of airborne particles. J. Environ. Manag. 2015, 155, 212–218. [Google Scholar] [CrossRef]
- Rieux, C.; Personnaz, M.B.; Thibaudon, M. Spatial variation of airborne pollen over south-east France: Characterization and implications for monitoring network management. Aerobiologia 2008, 24, 43–52. [Google Scholar] [CrossRef]
- Hruska, K. Assessment of urban allergophytes using an allergen index. Aerobiologia 2003, 19, 107–111. [Google Scholar] [CrossRef]
- Ogren, T.L.O. Allergy-Free Gardening, the Revolutionary Guide to Healthy Landscaping; Ten Speed Press: Berkeley, CA, USA; Toronto, ON, Canada, 2000. [Google Scholar]
- Ogren, P.; Corp, A.; Shoreview, M.N. Trees, shrubs and urban allergies. Wis. Urban Community For. 2003, 11, 1–2. [Google Scholar]
- Cariñanos, P.; Casares-Porcel, M. Urban Green Zones and related pollen allergy: A review. Guidelines for designing spaces of low allergy impact. Landsc. Urban Plan. 2011, 101, 205–214. [Google Scholar] [CrossRef]
- Branquinho, C.; Cvejic, R.; Eler, P.; Gonzales, P.; Haase, D.; Hansen, R.; Kabish, N.; Rall, L.; Niemela, J.; Pauleit, S.; et al. A Typology of Urban Green Spaces, Ecosystem Services, Provisioning Services and Demands; Report D3.; Green Surge Project: Copenhagen, Denmark, 2015. [Google Scholar]
- Salbitano, F.; Borelli, S.; Conigliaro, M.; Chen, Y. Guidelines on Urban and Periurban Forestry; FAO Forestry Papers N° 178; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Wakter, S.M.; Webb, D.A. (Eds.) Flora Europaea; Cambridge University Press: Cambridge, UK, 1964. [Google Scholar]
- Mitchell, A.; More, D. Trees of Spain and Europe; Blume: Barcelona, Spain, 1987. [Google Scholar]
- Owen, J.; More, D. Drevesa: Najpopolnejši Vodnik za Prepoznavanje Naravnih in Gojenih Dreves v Evropi; Založba Narava: Kranj, Slovenia, 2010; p. 464. [Google Scholar]
- Flora of North America Editorial Committee (Ed.) Flora of North America North of Mexico; Oxford University Press: New York, NY, USA; Oxford, UK, 1993+; 20+ vols. [Google Scholar]
- Daly, C.; Widrlecher, M.P.; Halbeib, M.D.; Smith, J.I.; Gibson, W.P. Development of a new USDA plant hardiness zone map for the United States. J. Appl. Meteorol. Climatol. 2012, 51, 242–264. [Google Scholar] [CrossRef]
- Cariñanos, P.; Casares-Porcel, M.; Quesada-Rubio, J.M. Estimating the allergenic potential of urban Green spaces: A case-study in Granada, Spain. Landsc. Urban Plan. 2014, 123, 134–144. [Google Scholar] [CrossRef]
- Cariñanos, P.; Casares-Porcel, M.; Díaz de la Guardia, C.; Aira, M.J.; Belmonte, J.; Boi, M.; Elvira-Rendueles, B.; De Linares, C.; Fernández-Rodriguez, S.; Maya-Manzano, J.M.; et al. Assessing allergenicity i urban parks: A nature-based solution to reduce the impacto n public health. Environ. Res. 2017, 155, 219–225. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Cwik, A.; Ksprzyk, I.; Wójcik, T.; Borycka, K.; Cariñanos, P. Attractiveness of urban parks for visitors versus their potential allergenic hazard: A case study in Rzeszów, Poland. Urban For. Urban Green. 2018, 35, 221–229. [Google Scholar] [CrossRef]
- Dominguez, E.; Galán, C.; Guerra, F.; Villamandos, F.; Infante, F.; Mediavilla, A. Spring pollen and related allergies in Southern Spain. J. Invest. Allergol. Clin. Immunol. 1993, 3, 271–275. [Google Scholar]
- Charpin, D.; Hughes, B.; Mallea, M.; Sutra, J.P.; Balansard, G.; Vervloet, D. Seasonal allergic symptoms and their relation to pollen exposure in south-east France. Clin. Exp. Allergy 1993, 22, 435–439. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Werner, P. The ecology of urban areas and their functions for species diversity. Landsc. Ecol. Eng. 2011, 7, 231–240. [Google Scholar] [CrossRef]
- Capotorti, G.; Del Vico, E.; Lattanzi, E.; Tilia, A.; Celesti-Grapow, L. Exploring biodiversity in a metropolitan área in the Mediterranean region: The urban and suburban flora of Rome (Italy). Plant Byosyst. 2013, 147, 174–185. [Google Scholar] [CrossRef]
- Sjöman, H.; Östberg, J.; Bühler, O. Diversity and distribution of the urban tree population in ten major Nordic cities. Urban For. Urban Green. 2012, 11, 31–39. [Google Scholar] [CrossRef]
- Nielssen, A.B.; van des Bosch, M.; Maruthaveeran, S.; Konijnendijk van den Bosch, C. Species richness in urban parks and its drivers: A review of empirical evidence. Urban Ecosyst. 2014, 17, 305–327. [Google Scholar] [CrossRef]
- Tommassi, P.; Miro, A.; Higo, H.A.; Winston, M. Bee diversity and abundance in a urban setting. Can. Enthomol. 2004, 136, 851–869. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Barton, D.N. Classifying and valuing ecosystem services for urban planning. Ecol. Econ. 2013, 86, 235–245. [Google Scholar] [CrossRef]
- Tormo, R.; Muñoz, A.; Silva-Palacios, I.; Gallardo, F. Pollen production in anemophilous tres. Grana 1999, 35, 38–46. [Google Scholar]
- Charpin, D.; Calleja, M.; Pichot, C.; Penel, V.; Highes, B.; Poncet, P. Cypress pollen allergy. Rev. Mal. Respir. 2013, 30, 868–878. [Google Scholar] [CrossRef]
- Alcázar, P.; Cariñanos, P.; De Castro, C.; Guerra, F.; Moreno, C.; Dominguez-Vilches, E.; Galán, C. Airborne plane tree (Platanus hispanica) pollen distribution in the city of Cordoba, southwestern Spain, and posible implications on pollen allergy. J. Investig. Allergol. Clin. Immunol. 2004, 14, 238–243. [Google Scholar] [PubMed]
- Attorre, F.; Bruno, M.; Francesconi, F.; Valenti, R.; Bruno, F. Landscape changes of Rome through tree-lined roads. Landsc. Urban Plan. 2000, 49, 115–128. [Google Scholar] [CrossRef]
- Staffolani, L.; Velasco-Jiménez, M.J.; Galán, C.; Hruska, K. Allergenicity of the ornamental urban flora: Ecological and aerobiological analysis in Córdoba (Spain) and Ascoli Piceno (Italy). Aerobiologia 2011, 27, 239–246. [Google Scholar] [CrossRef]
- Pauleit, S.; Jones, N.; García-Martín, G.; García-Valdecasas, J.L.; Riviére, L.M.; Vidal-Baudet, L.; Bodson, M.; Randrup, T.B. Tree stablishment practice in towns and cities. Results from a European survey. Urban For. Urban Green. 2002, 1, 83–96. [Google Scholar] [CrossRef]
- Saebo, A.; Benedikz, T.; Randrup, T.B. Selection of trees for urban forestry in Nordic countries. Urban For. Urban Green. 2003, 2, 101–114. [Google Scholar] [CrossRef]
- Iglesias, I.; Rodriguez-Rajo, F.J.; Méndez, J. Behaviour of Platanus hispanica pollen, an important spring aeroallergen in Northwest Spain. J. Investig. Allergol. Clin. Immunol. 2007, 19, 145–156. [Google Scholar]
- Fernández-González, D.; González-Parrado, Z.; Vega-Maray, A.M.; Valencia-Barrera, R.M.; Camazón-Izquierdo De Nuntiis, P.; Mandrioli, P. Platanus pollen allergen PL a 1: Quantification in the atmosphere and influence on asensitizing population. Clin. Exp. Allergy 2010, 40, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Aboulaich, N.; Bouziane, H.; El Kadiri, M.; Riadi, H. Male phenology and pollen production of Cupressus sempervirens in Tetouan (Morocco). Grana 2008, 47, 130–138. [Google Scholar] [CrossRef][Green Version]
- Díaz de la Guardia, C.; Alba, F.; De Linares, C.; Nieto-Lugilde, D.; López-Caballero, J. Aerobiological and allergenic analysis of Cupressaceae pollen in Granada (Southern Spain). J. Investig. Allergol. Clin. Immunol. 2006, 16, 24–33. [Google Scholar] [PubMed]
- Charpin, D.; Pichot, C.; Belmonte, J.; Sutra, J.P.; Zidkova, J.; Chavez, P.; Shahali, Y.; Senéchal, H.; Poncet, P. Cypress pollinosis: From tree to clinic. Chin. Rev. Allergy Immunol. 2017, 54. [Google Scholar] [CrossRef] [PubMed]
- Verdú, M. Physiological and reproductive differences between hermaphrodites and males in the androdioecious plant Fraxinus ornus. Oikos 2004, 105, 239–246. [Google Scholar] [CrossRef]
- Cariñanos, P.; Alcázar, P.; Galán, C.; Dominguez, E. Privet pollen (Ligustrum sp.) as potential cause of polinosis in the city of Córdoba, southwest Spain. Allergy 2002, 57, 92–97. [Google Scholar] [CrossRef]
- Wheeler, A.W. Hypersensitivity to the allergens of the pollen from the olive tree (Olea europaea). Clin. Exp. Allergy 1992, 22, 1052–1057. [Google Scholar] [CrossRef]
- Rodriguez, R.; Villalba, M.; Monsalve, R.I.; Batanero, E. The spectrum of olive pollen allergens. Int. Arch. Allergy Immunol. 2001, 125, 185–195. [Google Scholar] [CrossRef]
- Marcos, C.; Rodriguez, F.J.; Luna, I.; Jato, V.; González, R. Pinus pollen aerobiology and clinical sensitization in northwest Spain. Ann. Allergy Asthma Immunol. 2001, 87, 39–42. [Google Scholar] [CrossRef]
- Fuentes Aparicio, V.; Zapatero-Remón, L.; Martínez-Molero, M.I.; Alonso Labreros, E.; Beitia Mazuecos, J.M.; Bartolomé-Zavala, B. Allergy t opine processionary Caterpillar (Thaumetopoea pityocampa) in children. Allergol. Immunopathol. 2006, 34, 59–63. [Google Scholar] [CrossRef]
- Paolucci, I.; Gaudet, M.; Jorge, V.; Beritognolo, I.; Terzoli, S.; Kuzminsky, E.; Muleo, R.; Scarascia Mugnozza, G.; Sabatti, M. Genetic linkage maps of Populus alba L. and comparative mapping analysis of sex determination across Populus species. Tree Genet. Genomes 2010, 6, 863–875. [Google Scholar] [CrossRef]
- Renner, S.S.; Beenken, L.; Grimm, G.W.; Rocyan, A.; Ricklefs, R.E. The evolution of dioecy, heterodichogamy and labile sex expression in Acer. Evol. Int. J. Org. Evol. 2007, 61, 2701–2719. [Google Scholar] [CrossRef] [PubMed]
- Vanhaelen, M.; Duchoteau, J.; Vanhaelen-Fastré, R.; Jaziri, M. Taxanes in Taxus baccata pollen: Cardiotoxicity and/or Allergenicity? Planta Med. 2007, 68, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Mur, P.; Feo Brito, F.; Lombardero, M.; Barber, D.; Galindo, P.A.; Gómez, E.; Borja, J. Allergy to linden pollen (Tilia cordata). Allergy 2002, 56, 457–458. [Google Scholar] [CrossRef]
- Stewart, I.D.; Oke, T.R. Local climate zones for urban temperature studies. Am. Meteorol. Soc. 2012, 93, 1879–1900. [Google Scholar] [CrossRef]
- Moore, L.M.; Laurenroth, W.K. Differential effects of temperature and precipitation on early—Vs. late- flowering species. Ecosphere 2017, 85, e01819. [Google Scholar] [CrossRef]
- Vázquez, L.M.; Galán, C.; Dominguez, E. Influence of meteorological parameters on olea pollen concentrations in Cordoba (Southwestern Spain). Int. J. Biometeorol. 2003, 48, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodriguez, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with wáter stress in the field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Hellener, A.; Vilkénas, J. A Search for a Sustainable Alternatives to Lawns-Connecting Research with Landscape Design. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2014. [Google Scholar]
- Gautrin, D.; Vandenplas, O.; DeWite, J.D.; L’Archevéque, J.; Leblanc, C. Allergenic exposure IgE mediated sensitization and related symptoms in lawn cutters. J. Allergy Clin. Immunol. 1994, 93, 437–445. [Google Scholar] [CrossRef]
- Mathiesen, F.; Schumacher, M.; Lowenstein, H. Characterization of the major allergen of Cynodon dactylon (Bermuda grass) pollen Cyn d I. J. Allergy Clin. Immunol. 1991, 85, 765–774. [Google Scholar] [CrossRef]
- Potter, P.C.; Mather, S.; Lockey, P.; Aiinslie, G.; Cadwen, A. IgE specific immune responses to an African grass (Kikuyu, Pennisetum clandestinum). Clin. Exp. Allergy 1993, 23, 581–586. [Google Scholar] [CrossRef]
- Pares Franzi, M.; Saurí-Pujol, D.; Domene, E. Evaluating the environmental performance of urban parks in Mediterranean cities: An example from the Barcelona Metropolitan Region. Environ. Manag. 2006, 38, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S. Weed dynamics in the Mediterranean urban ecosystem: Ecology, biodiversity and management. Weed Res. 2004, 44, 341–354. [Google Scholar] [CrossRef]
- Chen, W.Y.; Jim, C.V. Assessment and Valuation of the Ecosystem Services provided by urban forests. In Ecology, Planning and Management of Urban Forests; Carreiro, M.M., Song, Y.C., Wu, J., Eds.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Fraser, E.D.G.; Kenney, W.A. Cultural background and landscape history as factors affecting perception of the urban forest. J. Arboric. 2000, 26, 106–113. [Google Scholar]
- Parés, M.; March, H.; Saurí, D. Atlantic gardens in Mediterranean climates: Understanding the production of suburban natures in Barcelona. Int. J. Urban Reg. Res. 2013, 37, 328–347. [Google Scholar] [CrossRef]




| SPECIES RICHNESS | 355 taxa (including species, sub-species, cultivars, hybrids) |
| N° of FAMILIES | 83 |
| FAMILIES WITH THE LARGEST NUMBER OF GENERA | |
| Fabaceae | 14 (Bauhinia, Ceratonia, Cladrastis, Cytisus, Erythrina, Gleditsia, Gymnocladus, Maackia, Parkinsonia, Robinia, Sophora, Tipuana, Vachellia, Wisteria) |
| Cupressaceae | 11 (Calocedrus, Chamaecyparis, Cupressus, Cupressocyparis, Hesperocyparis, Juniperus, Metasequoia, Platycladus, Sequoiadendron, Tetraclinis, Thuja) |
| Rosaceae | 10 (Cercocarpus, Cotoneaster, Crataegus, Cydonia, Eriobotria, Malus, Photinia, Prunus, Pyrus, Sorbus) |
| Fagaceae | 5 (Castanea, Castanopsis, Fagus, Lithocarpus, Quercus) |
| Betulaceae | 5 (Alnus, Betula, Carpinus, Corylus, Ostrya) |
| Pinaceae | 4 (Abies, Cedrus, Pinus, Pseudotsuga) |
| Oleaceae | 4 (Fraxinus, Ligustrum, Olea, Syringa) |
| GENERA WITH THE LARGEST NUMBER OF SPECIES | |
| Acer | 20 spp. |
| Quercus | 16 spp. |
| Betula | 12 spp. |
| Pinus | 11 spp. |
| Magnolia | 9 spp. |
| Fraxinus | 8 spp. |
| Populus | 8 spp. |
| Prunus | 5 spp. |
| Tilia | 5 spp. |
| LOCATION OF REPRODUCTIVE ORGANS | M.—On the same plant (Monoecious): 33.6% (119) D.—On different plants (Dioecious): 18.8% (67) H.—Having both sexes (Hermaphrodite): 28.6% (102) O.—Others (Polygamous, sub-dioecious, Parthenogenesis): 19.0% (67) |
| POLLINATION STRATEGY | I.—Insect-pollinated: 46.7% (166) W.—Wind-pollinated: 42.3% (150) A.—Ambiphillous: 11.0% (39) |
| PERMANENCE OF THE LEAVES | DE.—Deciduous: 65.0% (231) SD.—Semi-deciduous: 1.9% (7) EV.—Evergreen: 33.1% (117) |
| Species | Attributes | Origin | Allergenicity Level | Hardiness Zone |
|---|---|---|---|---|
| Acer campestre | D-I-DE | Eur.As.Afr. | Moderate | 6a–6b |
| Acer pseudoplatanus | H-I-DE | Eur.As.Afr. | Moderate | 4b |
| Acer negundo | D-W-DE | N-Am. | High | 4b |
| Celtis australis | M-W-DE | Eur (Med). | Moderate | 5b |
| Cupressus sempervirens | M-W-EV | Eur (Med). | Very High | 8b |
| Fraxinus excelsior | D-W-DE | Eur (Med). | High | 4b–5a |
| Fraxinus angustifolia | D-W-DE | Eur (Med). | Moderate | 6b–7b |
| Ligustrum lucidum | H-A-EV | China | Moderate | 8a–11b |
| Magnolia spp. | H-I-EV | N-Am.China | Low | 6b–8a |
| Pinus halepensis | M-W-EV | Eur (Med). | Low | 8a–9b |
| Pinus pinea | M-W-EV | Eur (Med). | Low | 6b |
| Pinus pinaster | M-W-EV | Eur (Med). | Low | 8a |
| Platanus x hispanica | M-W-DE | Eur. | Very High | 6b |
| Populus alba | D-W-DE | Eur.As.Afr. | High | 4b |
| Quercus ilex | M-W-EV | Eur (Med). | Moderate | 5b–7b |
| Robinia pseudoacacia | H-I-DE | N-Am. | Low | 4b–5a |
| Taxus baccata | D-W-EV | Eur. | High | 5a–5b |
| Tilia cordata | H-A-DE | Eur. | Low | 5a |
| Tilia platyphyllos | H-A-DE | Eur. As. | Low | 5a |
| Ulmus minor | D-W-DE | Eur. N-Am. As. | High | 6b |
| Variable | R |
|---|---|
| Tree density (ha.) | 0.70 ** |
| Number of trees | 0.65 ** |
| Number of species | 0.54 ** |
| Shannon index | 0.45 ** |
| Precipitation of May | 0.43 * |
| Precipitation of July | 0.38 * |
| BIO 18 (Precipitation of the warmest quarter) | 0.35 * |
| Temperature of August | −0.34 * |
| BIO 1 (Annual mean temperature) | −0.35 * |
| BIO 9 (Mean temperature of the driest quarter) | −0.38 * |
| Effect | Estimate | Standard | Wald | Lower CL | Upper CL | p |
|---|---|---|---|---|---|---|
| Intercept | 0.541676 | 0.180138 | 9.0421 | 0.188612 | 0.894741 | 0.002638 |
| Tree density(ha) | 0.001736 | 0.000171 | 102.6181 | 0.001400 | 0.002072 | 0.000000 |
| BIO1 | −0.033637 | 0.011622 | 8.3761 | −0.056416 | −0.010857 | 0.003802 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cariñanos, P.; Grilo, F.; Pinho, P.; Casares-Porcel, M.; Branquinho, C.; Acil, N.; Andreucci, M.B.; Anjos, A.; Bianco, P.M.; Brini, S.; et al. Estimation of the Allergenic Potential of Urban Trees and Urban Parks: Towards the Healthy Design of Urban Green Spaces of the Future. Int. J. Environ. Res. Public Health 2019, 16, 1357. https://doi.org/10.3390/ijerph16081357
Cariñanos P, Grilo F, Pinho P, Casares-Porcel M, Branquinho C, Acil N, Andreucci MB, Anjos A, Bianco PM, Brini S, et al. Estimation of the Allergenic Potential of Urban Trees and Urban Parks: Towards the Healthy Design of Urban Green Spaces of the Future. International Journal of Environmental Research and Public Health. 2019; 16(8):1357. https://doi.org/10.3390/ijerph16081357
Chicago/Turabian StyleCariñanos, Paloma, Filipa Grilo, Pedro Pinho, Manuel Casares-Porcel, Cristina Branquinho, Nezha Acil, Maria Beatrice Andreucci, Andreia Anjos, Pietro Massimiliano Bianco, Silvia Brini, and et al. 2019. "Estimation of the Allergenic Potential of Urban Trees and Urban Parks: Towards the Healthy Design of Urban Green Spaces of the Future" International Journal of Environmental Research and Public Health 16, no. 8: 1357. https://doi.org/10.3390/ijerph16081357
APA StyleCariñanos, P., Grilo, F., Pinho, P., Casares-Porcel, M., Branquinho, C., Acil, N., Andreucci, M. B., Anjos, A., Bianco, P. M., Brini, S., Calaza-Martínez, P., Calvo, E., Carrari, E., Castro, J., Chiesura, A., Correia, O., Gonçalves, A., Gonçalves, P., Mexia, T., ... Vilhar, U. (2019). Estimation of the Allergenic Potential of Urban Trees and Urban Parks: Towards the Healthy Design of Urban Green Spaces of the Future. International Journal of Environmental Research and Public Health, 16(8), 1357. https://doi.org/10.3390/ijerph16081357