Breastfeeding and the Risk of Infant Illness in Asia: A Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Breastfeeding and Specific Infections
3.1.1. Measles
3.1.2. Zika Virus
3.1.3. HIV Infection
3.1.4. Hepatitis B
3.1.5. Hepatitis C
3.1.6. Human T Cell Leukemia Virus (HTLV)
3.1.7. Helicobacter Pylori
3.1.8. Malaria
3.1.9. Neonatal Sepsis
3.1.10. Breastfeeding and Infant Vaccination
3.1.11. Maternal Vaccination
3.1.12. Infection from Infant Formula
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Luca, L.M.; Norum, K.R. Scurvy and cloudberries: A chapter in the history of nutritional sciences. J. Nutr. 2011, 141, 2101–2105. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. State of the World’s Children 2017; Comunications Division UNICEF: New York, NY, USA, 2017. [Google Scholar]
- Binns, C.; Lee, M.K.; Low, W.Y.; Zerfas, A. The Role of Public Health Nutrition in Achieving the Sustainable Development Goals in the Asia Pacific Region. Asia Pac. J. Public Health Asia Pac. Acad. Consort. Public Health 2017, 29, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Scrimshaw, N.S.; Taylor, C.E.; Gordon, J.E. Interactions of nutrition and infection. Monogr. Ser. World Health Organ. 1968, 57, 3–329. [Google Scholar] [PubMed]
- Scrimshaw, N.S. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J. Nutr. 2003, 133, 316S–321S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, J.E.; Ascoli, W.; Mata, L.J.; Guzman, M.A.; Scrimshaw, N.S. Nutrition and infection field study in Guatemalan villages, 1959–1964. VI. Acute diarrheal disease and nutritional disorders in general disease incidence. Arch. Environ. Health 1968, 16, 424–437. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.K.; Kagawa, M. Ethical Challenges in Infant Feeding Research. Nutrients 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, M.S.; Chalmers, B.; Hodnett, E.D.; Sevkovskaya, Z.; Dzikovich, I.; Shapiro, S.; Collet, J.P.; Vanilovich, I.; Mezen, I.; Ducruet, T.; et al. Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA 2001, 285, 413–420. [Google Scholar] [CrossRef]
- Mathias, J.G.; Zhang, H.; Soto-Ramirez, N.; Karmaus, W. The association of infant feeding patterns with food allergy symptoms and food allergy in early childhood. Int. Breastfeed J. 2019, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Oken, E.; Bogdanovich, N.; Matush, L.; Sevkovskaya, Z.; Chalmers, B.; Hodnett, E.D.; Vilchuck, K.; Kramer, M.S.; Martin, R.M. Cohort profile: The promotion of breastfeeding intervention trial (PROBIT). Int. J. Epidemiol. 2014, 43, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Martens, P.J. What do Kramer’s Baby-Friendly Hospital Initiative PROBIT studies tell us? A review of a decade of research. J. Hum. Lact. 2012, 28, 335–342. [Google Scholar] [CrossRef]
- Duijts, L.; Jaddoe, V.W.V.; Hofman, A.; Moll, H.A. Prolonged and Exclusive Breastfeeding Reduces the Risk of Infectious Diseases in Infancy. Pediatrics 2010, 126, E18–E25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Available online: http://www.prisma-statement.org/ (accessed on 11 November 2017).
- Launer, L.J.; Habicht, J.P.; Kardjati, S. Breast-feeding protects infants in Indonesia against illness and weight-loss due to illness. Am. J. Epidemiol. 1990, 131, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Yorifuji, T.; Kato, T.; Inoue, S.; Tokinobu, A.; Tsuda, T.; Doi, H. Long-Term Effects of Breastfeeding on Children’s Hospitalization for Respiratory Tract Infections and Diarrhea in Early Childhood in Japan. Matern. Child Health J. 2015, 19, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Guo, Z.Q.; Bai, Z.J.; MacDonald, N.E. A 4 year prospective study to determine risk factors for severe community acquired pneumonia in children in southern China. Pediatric Pulmonol. 2013, 48, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Gothankar, J.; Doke, P.; Dhumale, G.; Pore, P.; Lalwani, S.; Quraishi, S.; Murarkar, S.; Patil, R.; Waghachavare, V.; Dhobale, R.; et al. Reported incidence and risk factors of childhood pneumonia in India: A community-based cross-sectional study. BMC Public Health 2018, 18, 1111. [Google Scholar] [CrossRef] [PubMed]
- Perera, B.J.C.; Ganesan, S.; Jayarasa, J.; Ranaweera, S. The impact of breastfeeding practices on respiratory and diarrhoeal disease in infancy: A study from Sri Lanka. J. Trop. Pediatrics 1999, 45, 115–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taksande, A.M.; Yeole, M. Risk factors of Acute Respiratory Infection (ARI) in under-fives in a rural hospital of Central India. J. Pediatric Neonatal Individ. Med. 2016, 5, e050105. [Google Scholar] [CrossRef]
- Yu, C.; Binns, C.W.; Lee, A.H. Comparison of breastfeeding rates and health outcomes for infants receiving care from hospital outpatient clinic and community health centres in China. J. Child Health Care 2016, 20, 286–293. [Google Scholar] [CrossRef]
- Li, S.S.; Yue, A.; Abbey, C.; Medina, A.; Shi, Y.J. Breastfeeding and the Risk of Illness among Young Children in Rural China. Int. J. Environ. Res. Public Health 2019, 16, 136. [Google Scholar] [CrossRef] [Green Version]
- Yoon, P.W.; Black, R.E.; Moulton, L.H.; Becker, S. Effect of not breastfeeding on the risk of diarrheal and respiratory mortality in children under 2 years of age in Metro Cebu, The Philippines. Am. J. Epidemiol. 1996, 143, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Raheem, R.A.; Binns, C.W.; Chih, H.J. Protective effects of breastfeeding against acute respiratory tract infections and diarrhoea: Findings of a cohort study. J. Paediatr. Child Health 2017, 53, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Yu, P.; Zhang, Y.M.; Yang, X.G.; Li, W.J.; Wang, P.Y. Effect of feeding pattern on infant illness in Chinese cities. Public Health Nutr. 2016, 19, 1252–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanieh, S.; Ha, T.T.; Simpson, J.A.; Thuy, T.T.; Khuong, N.C.; Thoang, D.D.; Tran, T.D.; Tuan, T.; Fisher, J.; Biggs, B.A. Exclusive breast feeding in early infancy reduces the risk of inpatient admission for diarrhea and suspected pneumonia in rural Vietnam: A prospective cohort study. BMC Public Health 2015, 15, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengstermann, S.; Mantaring, J.B.V.; Sobel, H.L.; Borja, V.E.; Basilio, J.; Iellamo, A.D.; Nyunt-U, S. Formula Feeding Is Associated with Increased Hospital Admissions Due to Infections Among Infants Younger Than 6 Months in Manila, Philippines. J. Hum. Lact. 2010, 26, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Arifeen, S.; Black, R.E.; Antelman, G.; Baqui, A.; Caulfield, L.; Becker, S. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics 2001, 108, E67. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.M.; Speizer, I.S.; Singh, K.; Angeles, G.; Twum-Danso, N.A.Y.; Barker, P. Does postnatal care have a role in improving newborn feeding? A study in 15 sub-Saharan African countries. J. Glob. Health 2017, 7, 020506. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Islam, M.M. Effect of exclusive breastfeeding on selected adverse health and nutritional outcomes: A nationally representative study. BMC Public Health 2017, 17, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihrshahi, S.; Ichikawa, N.; Shuaib, M.; Oddy, W.; Ampon, R.; Dibley, M.J.; Kabir, A.K.; Peat, J.K. Prevalence of exclusive breastfeeding in Bangladesh and its association with diarrhoea and acute respiratory infection: Results of the multiple indicator cluster survey 2003. J. Health Popul. Nutr. 2007, 25, 195–204. [Google Scholar] [PubMed]
- Mihrshahi, S.; Oddy, W.H.; Peat, J.K.; Kabir, I. Association between infant feeding patterns and diarrhoeal and respiratory illness: A cohort study in Chittagong, Bangladesh. Int. Breastfeed. J. 2008, 3, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.; Deb, A.K.; Chawla-Sarkar, M.; Ramamurthy, T.; Ganguly, S.; Pradhan, P.; Chakraborty, A.; Desai, S.; Gupte, M.D.; Dhere, R. Factors associated with diarrhoea in young children and incidence of symptomatic rotavirus infection in rural West Bengal, India. Epidemiol. Infect. 2014, 142, 1848–1858. [Google Scholar] [CrossRef]
- Richard, S.A.; McCormick, B.J.J.; Seidman, J.C.; Rasmussen, Z.; Kosek, M.N.; Rogawski, E.T.; Petri, W.; Bose, A.; Mduma, E.; Maciel, B.L.L.; et al. Relationships among Common Illness Symptoms and the Protective Effect of Breastfeeding in Early Childhood in MAL-ED: An Eight-Country Cohort Study. Am. J. Trop. Med. Hyg. 2018, 98, 904–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. The World Health Organization’s Infant Feeding Recommendation. 2002. Available online: http://www.who.int/nutrition/topics/infantfeeding_recommendation/en/ (accessed on 10 November 2016).
- Tuon, F.F.; Gondolfo, R.B.; Cerchiari, N. Human-to-human transmission of Brucella—A systematic review. Trop. Med. Int. Health 2017, 22, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Acceptable Medical Reasons for Use of Breastmilk Substitutes. Available online: https://www.who.int/nutrition/publications/infantfeeding/WHO_NMH_NHD_09.01/en/ (accessed on 29 November 2019).
- Pasquier, A.; Alais, S.; Roux, L.; Thoulouze, M.I.; Alvarez, K.; Journo, C.; Dutartre, H.; Mahieux, R. How to Control HTLV-1-Associated Diseases: Preventing de Novo Cellular Infection Using Antiviral Therapy. Front. Microbiol. 2018, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Measles Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/measles (accessed on 20 November 2019).
- World Health Organisation. Measles and Rubella Surveillance Data. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en/ (accessed on 1 December 2019).
- Government of Samoa. Measles Outbreak Update November 20 (Travellers Health Alert). Available online: www.health.gov.au (accessed on 1 December 2019).
- Penman, B.S.; Gupta, S.; Shanks, G.D. Rapid mortality transition of Pacific Islands in the 19th century. Epidemiol. Infect. 2017, 145, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Science, M.; Savage, R.; Severini, A.; McLachlan, E.; Hughes, S.L.; Arnold, C.; Richardson, S.; Crowcroft, N.; Deeks, S.; Halperin, S.; et al. Measles Antibody Levels in Young Infants. Pediatrics 2019, 144. [Google Scholar] [CrossRef] [PubMed]
- Silfverdal, S.A.; Ehlin, A.; Montgomery, S.M. Breast-feeding and a subsequent diagnosis of measles. Acta Paediatr. 2009, 98, 715–719. [Google Scholar] [CrossRef]
- Dorea, J.G. Breastfeeding is an essential complement to vaccination. Acta Paediatr. 2009, 98, 1244–1250. [Google Scholar] [CrossRef]
- Mina, M.J.; Metcalf, C.J.E.; de Swart, R.L.; Osterhaus, A.; Grenfell, B.T. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 2015, 348, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Government of Canada. Measles Vaccine: Canadian Immunization Guide. Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-12-measles-vaccine.html#p4c11a5 (accessed on 30 November 2019).
- Binns, C.; Low, W.Y. Zika: Where Are You? Asia Pac. J. Public Health Asia Pac. Acad. Consort. Public Health 2019, 31, 272–274. [Google Scholar] [CrossRef] [Green Version]
- Sampieri, C.L.; Montero, H. Breastfeeding in the time of Zika: A systematic literature review. PeerJ 2019, 7, e6452. [Google Scholar] [CrossRef]
- World Health Organisation. Infant Feeding in Areas of Zika Virus Transmission. Available online: https://apps.who.int/iris/bitstream/handle/10665/204473/WHO_ZIKV_MOC_16.5_eng.pdf?sequence=1 (accessed on 29 November 2019).
- Moller, A.B.; Petzold, M.; Chou, D.; Say, L. Early antenatal care visit: A systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. Lancet Glob. Health 2017, 5, e977–e983. [Google Scholar] [CrossRef] [Green Version]
- Fowler, M.G.; Flynn, P.; Aizire, J. What is new in perinatal HIV prevention? Curr. Opin. Pediatr. 2018, 30, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.T.T.; Anh, N.M.; Bao, N.H.; Tuan, P.L.; Caridha, R.; Gaseitsiwe, S.; Hien, N.T.; Cam, P.D.; Ehrnst, A. HIV-1 mother-to-child transmission, post-test counselling, and antiretroviral prophylaxis in Northern Viet Nam: A prospective observational study. Scand. J. Infect. Dis. 2012, 44, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Binns, C.; Low, W.Y. Hepatitis Requires Public Health Action. Asia Pac. J. Public Health Asia Pac. Acad. Consort. Public Health 2017, 29, 348–350. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Binns, C.W.; Zhao, Y.; Zhang, K.; Xie, X. Hepatitis B and breastfeeding in Hangzhou, Zhejiang Province, People’s Republic of China. Breastfeed. Med. 2010, 5, 109–112. [Google Scholar] [CrossRef]
- Chen, X.R.; Chen, J.; Wen, J.; Xu, C.Y.; Zhang, S.; Zhou, Y.H.; Hu, Y.L. Breastfeeding Is Not a Risk Factor for Mother-to-Child Transmission of Hepatitis B Virus. PLoS ONE 2013, 8, e55303. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 15 December 2019).
- Hanafiah, K.M.; Groeger, J.; Flaxman, A.D.; Wiersma, S.T. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013, 57, 1333–1342. [Google Scholar] [CrossRef]
- Bernstein, H.B.; Dunkelberg, J.C.; Leslie, K.K. Hepatitis C in Pregnancy in the Era of Direct-acting Antiviral Treatment: Potential Benefits of Universal Screening and Antepartum Therapy. Clin. Obstet. Gynecol. 2018, 61, 146–156. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Hepatitis C. Available online: https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/maternal-or-infant-illnesses/hepatitis.html (accessed on 1 December 2019).
- Einsiedel, L.J.; Pham, H.; Woodman, R.J.; Pepperill, C.; Taylor, K.A. The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community. Med. J. Aust. 2016, 205, 305–309. [Google Scholar] [CrossRef]
- Yamada, T.; Togashi, T.; Tsutsumi, H.; Imamura, M.; Okubo, H.; Okabe, M.; Takamuro, N.; Tashiro, K.; Yano, K.; Yamamoto, N.; et al. Prevalence of human T-lymphotropic virus type 1 carriers among pregnant women in Hokkaido, Japan. Microbiol. Immunol. 2014, 58, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Condon, J.R.; Armstrong, B.K.; Barnes, T.; Zhao, Y.J. Cancer incidence and survival for indigenous Australians in the northern territory. Aust. N. Z. J. Public Health 2005, 29, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.R.R.; Walley, R.; Pham, H.; Schinke, S.; Woodman, R.; Wilson, K.; Sajiv, C.; Einsiedel, L. Higher human T-cell leukaemia virus type 1 (HTLV-1) proviral load is associated with end-stage kidney disease in Indigenous Australians: Results of a case-control study in central Australia. J. Med. Virol. 2019, 91, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Blecker, U. Helicobacter pylori infection in children. Acta Paediatr. 1998, 87, 1105–1112. [Google Scholar] [CrossRef]
- Leja, M.; Grinberga-Derica, I.; Bilgilier, C.; Steininger, C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter 2019, 24, e12635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azami, M.; Nasirkandy, M.P.; Mansouri, A.; Darvishi, Z.; Rahmati, S.; Abangah, G.; Dehghan, H.R.; Borji, M.; Abbasalizadeh, S. Global Prevalence of Helicobacter pylori Infection in Pregnant Women: A Systematic Review and Meta-analysis Study. Int. J. Womens Health Reprod. Sci. 2017, 5, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, M.; Natori, M.; Katoh, M.; Sugimoto, K.; Omi, H.; Akiyama, Y.; Sago, H. Maternal transmission of Helicobacter pylori in the perinatal period. J. Obstet. Gynaecol. Res. 2001, 27, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Yucel, O. Prevention of Helicobacter pylori infection in childhood. World J. Gastroenterol. 2014, 20, 10348–10354. [Google Scholar] [CrossRef] [PubMed]
- Asoba, G.N.; Sumbele, I.U.N.; Anchang-Kimbi, J.K.; Metuge, S.; Teh, R.N. Influence of infant feeding practices on the occurrence of malnutrition, malaria and anaemia in children ≤5 years in the Mount Cameroon area: A cross sectional study. 2019, 14, e0219386. [Google Scholar] [CrossRef] [Green Version]
- Brazeau, N.F.; Tabala, M.; Kiketa, L.; Kayembe, D.; Chalachala, J.L.; Kawende, B.; Lapika, B.; Meshnick, S.R.; Yotebieng, M. Exclusive Breastfeeding and Clinical Malaria Risk in 6-Month-Old Infants: A Cross-Sectional Study from Kinshasa, Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2016, 95, 827–830. [Google Scholar] [CrossRef] [Green Version]
- Yooseph, S.; Kirkness, E.F.; Tran, T.M.; Harkins, D.M.; Jones, M.B.; Torralba, M.G.; O’Connell, E.; Nutman, T.B.; Doumbo, S.; Doumbo, O.K.; et al. Stool microbiota composition is associated with the prospective risk of Plasmodium falciparum infection. BMC Genom. 2015, 16. [Google Scholar] [CrossRef]
- Al-Taiar, A.; Hammoud, M.S.; Liu, C.Q.; Lee, J.K.F.; Lui, K.M.; Nakwan, N.; Isaacs, D. Neonatal infections in China, Malaysia, Hong Kong and Thailand. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F249–F255. [Google Scholar] [CrossRef] [Green Version]
- Raihana, S.; Dibley, M.J.; Rahman, M.M.; Tahsina, T.; Siddique, M.A.; Rahman, Q.S.; Islam, S.; Alam, A.; Kelly, P.J.; El Arifeen, S.; et al. Early initiation of breastfeeding and severe illness in the early newborn period: An observational study in rural Bangladesh. PLoS Med. 2019, 16, e1002904. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Vesel, L.; Bahl, R.; Martines, J.C. Timing of Breastfeeding Initiation and Exclusivity of Breastfeeding During the First Month of Life: Effects on Neonatal Mortality and Morbidity-A Systematic Review and Meta-analysis. Matern. Child Health J. 2015, 19, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, D.C.; Garcia, S.S.; Renau, M.I.; Iglesias-Platas, I. Availability of Donor Milk for Very Preterm Infants Decreased the Risk of Necrotizing Enterocolitis without Adversely Impacting Growth or Rates of Breastfeeding. Nutrients 2019, 11, 1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2018, 6, CD002971. [Google Scholar] [CrossRef]
- Silano, M.; Milani, G.P.; Fattore, G.; Agostoni, C. Donor human milk and risk of surgical necrotizing enterocolitis: A meta-analysis. Clin. Nutr. 2019, 38, 1061–1066. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Ten Great Public Health Achievements—United States, 1900–1999. Morb. Mortal. Wkly. Rep. 1999, 48, 241–243. [Google Scholar]
- Grassly, N.C.; Kang, G.; Kannpmann, B. Biological challenges to effective vaccines in the developing world. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Gad, R.F.; Dowling, D.A.; Abusaad, F.E.; Bassiouny, M.R.; Abd El Aziz, M.A. Oral Sucrose Versus Breastfeeding in Managing Infants’ Immunization-Related Pain A Randomized Controlled Trial. MCN Am. J. Matern. Child Nurs. 2019, 44, 108–114. [Google Scholar] [CrossRef]
- Johnston, C.; Campbell-Yeo, M.; Disher, T.; Benoit, B.; Fernandes, A.; Streiner, D.; Inglis, D.; Zee, R. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst. Rev. 2017, 2, CD008435. [Google Scholar] [CrossRef]
- Bergin, N.; Murtagh, J.; Philip, R.K. Maternal Vaccination as an Essential Component of Life-Course Immunization and Its Contribution to Preventive Neonatology. Int. J. Environ. Res. Public Health 2018, 15, 847. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.H.; Liu, Y.; Yu, J.Q.; Li, L.J.; Shi, W.; Shen, Y.J.; Li, L.; Zhan, S.N.; Yang, F.; Wang, Y.J.; et al. Seroprevalence of Maternal and Cord Antibodies Specific for Diphtheria, Tetanus, Pertussis, Measles, Mumps and Rubella in Shunyi, Beijing. Sci. Rep. 2018, 8, 13021. [Google Scholar] [CrossRef] [PubMed]
- Maertens, K.; De Schutter, S.; Braeckman, T.; Baerts, L.; Van Damme, P.; De Meester, I.; Leuridan, E. Breastfeeding after maternal immunisation during pregnancy: Providing immunological protection to the newborn: A review. Vaccine 2014, 32, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Verhasselt, V. Is infant immunization by breastfeeding possible? Philos. Trans. R. Soc. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, S.J.; Mirza, S.A.; Vonglokham, P.; Khanthamaly, V.; Chitry, B.; Pholsena, V.; Chitranonh, V.; Omer, S.B.; Moen, A.; Bresee, J.S.; et al. The Effect of Influenza Vaccination on Birth Outcomes in a Cohort of Pregnant Women in Lao PDR, 2014-2015. Clin. Infect. Dis. 2016, 63, 487–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleary, B.J.; Rice, U.; Eogan, M.; Metwally, N.; McAuliffe, F. 2009 A/H1N1 influenza vaccination in pregnancy: Uptake and pregnancy outcomes—A historical cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 178, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Sadarangani, M.; Garand, M.; Dauby, N.; Verhasselt, V.; Pereira, L.; Bjornson, G.; Jones, C.E.; Halperin, S.A.; Edwards, K.M.; et al. Maternal immunisation: Collaborating with mother nature. Lancet Infect. Dis. 2017, 17, E197–E208. [Google Scholar] [CrossRef]
- Schofield, F.D.; Tucker, V.M.; Westbrook, G.R. Neonatal tetanus in New Guinea. Effect of active immunization in pregnancy. Br. Med. J. 1961, 2, 785–789. [Google Scholar] [CrossRef]
- Datta, S.S.; Barnabas, R.; Sitther, A.; Guarenti, L.; Toikilik, S.; Kariwiga, G.; Sui, G.P. Three cases of neonatal tetanus in Papua New Guinea lead to development of national action plan for maternal and neonatal tetanus elimination. West. Pac. Surveill. Response J. 2013, 4, 40–43. [Google Scholar] [CrossRef]
- Thwaites, C.L.; Beeching, N.J.; Newton, C.R. Maternal and neonatal tetanus. Lancet 2015, 385, 362–370. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding; Department of Health and Human Services Office of the Surgeon General: Washington, DC, USA, 2011.
- Henry, M.; Fouladkhah, A. Outbreak History, Biofilm Formation, and Preventive Measures for Control of Cronobacter sakazakii in Infant Formula and Infant Care Settings. Microorganisms 2019, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Teramoto, S.; Tanabe, Y.; Okano, E.; Nagashima, T.; Kobayashi, M.; Etoh, Y. A first fatal neonatal case of Enterobacter sakazakii infection in Japan. Pediatrics Int. 2010, 52, 312–313. [Google Scholar] [CrossRef] [PubMed]
- United Nations Children’s Fund (UNICEF). Every Child Alive: The Urgent Need to End All Newborn Deaths; UNICEF: Geneva, Switzerland, 2018. [Google Scholar]
- Dey, S.K.; Chisti, M.J.; Das, S.K.; Shaha, C.K.; Ferdous, F.; Farzana, F.D.; Ahmed, S.; Malek, M.A.; Faruque, A.S.; Ahmed, T.; et al. Characteristics of diarrheal illnesses in non-breast fed infants attending a large urban diarrheal disease hospital in Bangladesh. PLoS ONE 2013, 8, e58228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horta, B.; Victora, C. The Short Term Effects of Breastfeeding: A Systematic Review. Available online: http://www.who.int/maternal_child_adolescent/documents/breastfeeding_short_term_effects/en/ (accessed on 1 February 2018).
- United Nations Department of Economic and Social Affairs Population Division. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423); UN DESA: New York, NY, USA, 2019. [Google Scholar]
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Morley, D. Paediatric Priorities in the Developing World; Butterworth: London, UK, 1976. [Google Scholar]
- Ebrahim, G.J. Infant feeding in the Third World. Postgrad Med. J. 1986, 62, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Boix-Amoros, A.; Collado, M.C.; Van’t Land, B.; Calvert, A.; Le Doare, K.; Garssen, J.; Hanna, H.; Khaleva, E.; Peroni, D.G.; Geddes, D.T.; et al. Reviewing the evidence on breast milk composition and immunological outcomes. Nutr. Rev. 2019, 77, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filen, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune Markers in Breast Milk and Fetal and Maternal Body Fluids: A Systematic Review of Perinatal Concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition Nutrients and Bioactive Factors. Pediatric Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Gay, M.C.L.; Koleva, P.T.; Slupsky, C.M.; du Toit, E.; Eggesbo, M.; Johnson, C.C.; Wegienka, G.; Shimojo, N.; Campbell, D.E.; Prescott, S.L.; et al. Worldwide Variation in Human Milk Metabolome: Indicators of Breast Physiology and Maternal Lifestyle? Nutrients 2018, 10, 1151. [Google Scholar] [CrossRef] [Green Version]
- Binns, C.W.; Lee, M.K. Exclusive breastfeeding for six months: The WHO six months recommendation in the Asia Pacific Region. Asia Pac. J. Clin. Nutr. 2014, 23, 344–350. [Google Scholar] [CrossRef]
- Binns, C.; Lee, M.K.; Kagawa, M.; Low, W.Y.; Scott, J.; Lee, A.; Zerfas, A.; Maycock, B.; Qiu, L.; Yusuff, A.; et al. Infant Feeding Guidelines for the Asia Pacific Region. Asia Pac. J. Public Health 2018. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Infant Feeding Guidelines for Health Workers. Available online: www.nhmrc.gov.au (accessed on 23 September 2019).
- Troeger, C.; Colombara, D.V.; Rao, P.C.; Khalil, I.A.; Brown, A.; Brewer, T.G.; Guerrant, R.L.; Houpt, E.R.; Kotloff, K.L.; Misra, K.; et al. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob. Health 2018, 6, E255–E269. [Google Scholar] [CrossRef] [Green Version]
- Gaufin, T.; Tobin, N.H.; Aldrovandi, G.M. The importance of the microbiome in pediatrics and pediatric infectious diseases. Curr. Opin. Pediatrics 2018, 30, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Gupta, A. Influence of Early Life, Diet, and the Environment on the Microbiome. Clin. Gastroenterol. Hepatol. 2019, 17, 231–242. [Google Scholar] [CrossRef]
- Olofin, I.; McDonald, C.M.; Ezzati, M.; Flaxman, S.; Black, R.E.; Fawzi, W.W.; Caulfield, L.E.; Danaei, G.; Nutrition Impact Model Study. Associations of Suboptimal Growth with All-Cause and Cause-Specific Mortality in Children under Five Years: A Pooled Analysis of Ten Prospective Studies. PLoS ONE 2013, 8, e64636. [Google Scholar] [CrossRef] [Green Version]
- Hetzner, N.M.; Razza, R.A.; Malone, L.M.; Brooks-Gunn, J. Associations among feeding behaviors during infancy and child illness at two years. Matern. Child Health J. 2009, 13, 795–805. [Google Scholar] [CrossRef]
- Bass, J.L.; Gartley, T.; Kleinman, R. World Health Organization Baby-Friendly Hospital Initiative Guideline and 2018 Implementation Guidance. JAMA Pediatrics 2019, 173, 93–94. [Google Scholar] [CrossRef]
- Wouk, K.; Tully, K.P.; Labbok, M.H. Systematic Review of Evidence for Baby-Friendly Hospital Initiative Step 3. J. Hum. Lact. 2017, 33, 50–82. [Google Scholar] [CrossRef]
- UNICEF. Mother-Infant Early Skin-to-Skin Contact Implementing Baby Friendly Standards Resources. Available online: https://www.unicef.org.uk/babyfriendly/baby-friendly-resources/implementing-standards-resources/skin-to-skin-contact/ (accessed on 21 November 2019).
- Abdulghani, N.; Edvardsson, K.; Amir, L.H. Worldwide prevalence of mother-infant skin-to-skin contact after vaginal birth: A systematic review. PLoS ONE 2018, 13, e0205696. [Google Scholar] [CrossRef] [PubMed]
- Karall, D.; Ndayisaba, J.P.; Heichlinger, A.; Kiechl-Kohlendorfer, U.; Stojakovic, S.; Leitner, H.; Scholl-Burgi, S. Breast-feeding Duration: Early Weaning-Do We Sufficiently Consider the Risk Factors? J. Pediatric Gastroenterol. Nutr. 2015, 61, 577–582. [Google Scholar] [CrossRef] [PubMed]
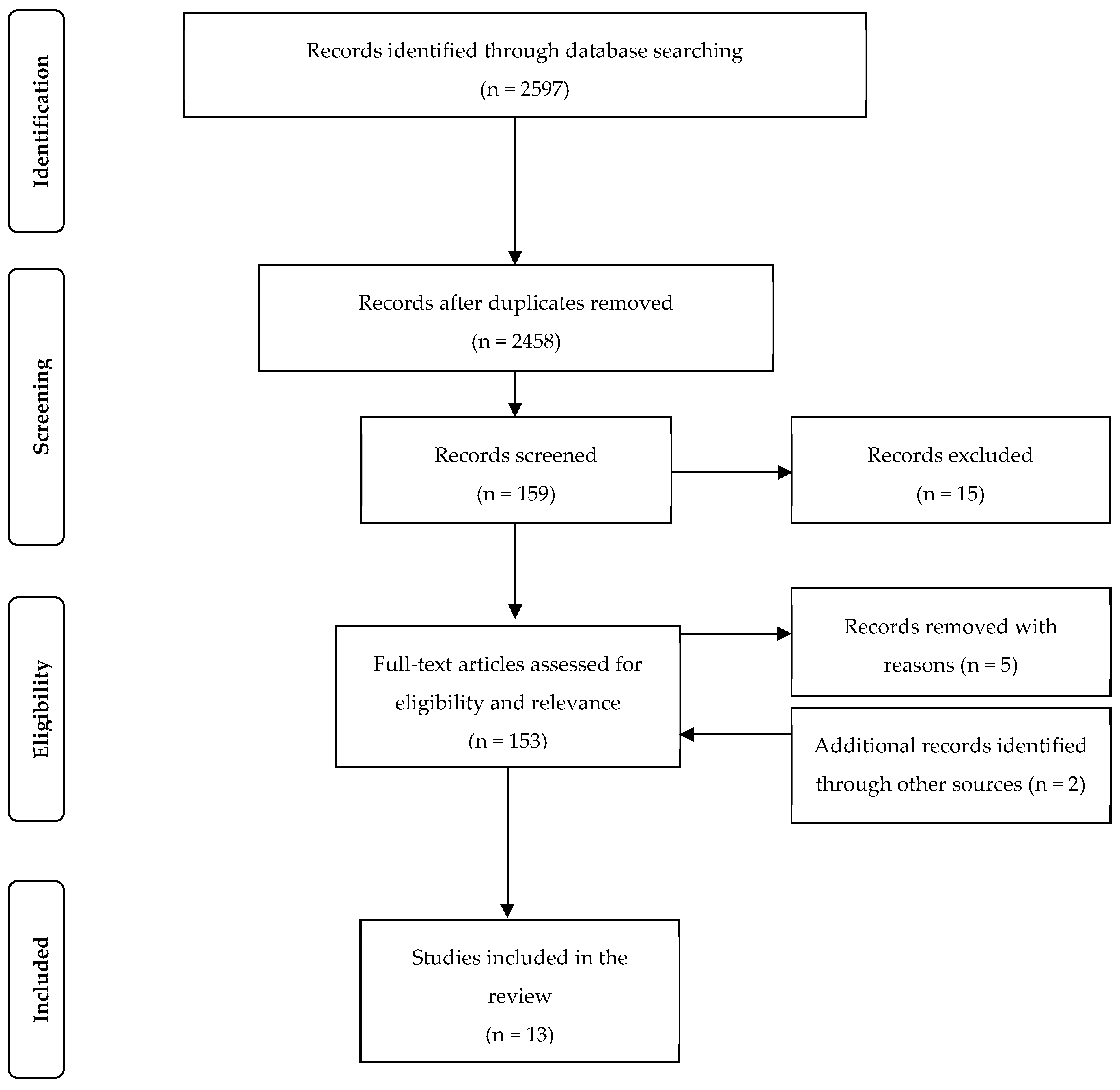
| Country | Author | Study Size | Design | Age Months | Breastfeeding Classification | Outcome Measure | Results |
|---|---|---|---|---|---|---|---|
| Eight Countries incl Nepal India, Bangladesh Pakistan | Richard MALED 2018 | 1731 | Cohort | 0–24 | EBF compared to ABF | aRR diarr 0–2M | 0.58 0.44, 0.76 |
| aRR Resp | NS | ||||||
| aRR diarr 3–5M | 0.83 0.75, 0.93 | ||||||
| aRR Resp | 0.81 0.68, 0.98 | ||||||
| Maldives | Raheem 2017 | 458 | Cohort | 0–6 | Predominant BF 6/12 Y = 153, N = 305 | ARTI aOR | 0.45 (0.24–0.84) |
| Diarrhoea aOR | 0.31 (0.10–0.90) | ||||||
| China (urban) | Yu [20] 2016 | 682 | Cohort | 0–6 | Any BF 1/12 (Y = 607 N = 75) | aOR LRTI (<6/12) | 0.479(0.263-0.872) |
| Vietnam (rural) | Hanieh 2015 | 1049 | Cohort | 0–6 | Exclusive BF at 6 weeks (32.8%) | Diarrhoea OR | 0.37 (0.15 to 0.88) |
| Pneumonia OR | 0.39 (0.20,0.75) | ||||||
| India Rural | Panda 2014 | 696 | Cohort | 0–6 | EBF compared to ABF | aOR diarr | 0.49 (0.27, 0.90) |
| Bangladesh Rural | Mihrshahi 2008 | 351 | Cohort | 0–6 | EBF compared to Partial BF | aOR diarr | 0.29 (0.12, 0.68 |
| aOR ARI | 0.4 (0.21, 0.75) | ||||||
| Bangladesh Urban | Arifeen 2001 | 1677 | Cohort | 0–12 | Predominant breastfeeding compared to partial or none | All deaths aHR | 0.45 (0.29, 0.69) |
| ARI deaths | 0.42(0.20, 0.88) | ||||||
| Diarrhoea | 0.25 (0.09,0.68) | ||||||
| Philippines | Yoon 1996 | 9942 | Cohort | 0–12 | Not BF compared to Breastfed Death rates 0–5 months | aRR diarr | 0.10 (0.25,0.04) |
| aRR ALRI | NS | ||||||
| aRR ALRI | 0.17 (0.56–0.05) | ||||||
| Philippines | Hengstermann 2010 | 399 | Case control | 0–6 | Risk of hospitalisation Exclusive breastfeeding & Formula fed | Any Infection aOR | 0.29 (0.17,0.48) |
| Diarrhoea aOR | 0.05 (0.02,0.15) | ||||||
| Pneumonia aOR | 0.36 (0.19,0.66) | ||||||
| China (rural) | Li 2019 [21] | 1802 | Cross sect | 6–12 | Any BF = 1049 Not BF = 753) Illness in past month | Diarrhoea p < 0.01 | BF 33%, No BF 42% |
| Cough p = 0.03 | BF 43% NoBF49% | ||||||
| Bangladesh national | Khan 2017 | 1918 | DHS cross sectional | 0–6 | EBF T 0–2 M | aOR diarr | 0.20 (0.10, 0.32) |
| aOR ARI | 0.42 (0.31, 0.79) | ||||||
| EBF T 2–4 M | aOR diarr | 0.32 (0.20, 0.47 | |||||
| aOR ARI | 0.71 (0.57, 0.90) | ||||||
| EBF T 4–6 M | aOR diarr | 0.43 (0.31, 0.53) | |||||
| aOR ARI | 0.84 (0.64, 0.96) | ||||||
| China (urban) | Cai 2016 | 1654 | Cross sect | 0–12 | Exclusive BF Mixed Exc Formula | Hospitalisation EBF compared to Exc Formula | Respiratory illness |
| OR 0.69 (0.50, 0.96) | |||||||
| Bangladesh Rural | Mihrshahi 2007 | 1633 DHS | cross section | 0–3 | EBF 0–3 M compared other | aOR diarr | 0.69 (0.49–0.98) |
| aOR ARI | 0.69 (0.54–0.88) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.K.; Binns, C. Breastfeeding and the Risk of Infant Illness in Asia: A Review. Int. J. Environ. Res. Public Health 2020, 17, 186. https://doi.org/10.3390/ijerph17010186
Lee MK, Binns C. Breastfeeding and the Risk of Infant Illness in Asia: A Review. International Journal of Environmental Research and Public Health. 2020; 17(1):186. https://doi.org/10.3390/ijerph17010186
Chicago/Turabian StyleLee, Mi Kyung, and Colin Binns. 2020. "Breastfeeding and the Risk of Infant Illness in Asia: A Review" International Journal of Environmental Research and Public Health 17, no. 1: 186. https://doi.org/10.3390/ijerph17010186
APA StyleLee, M. K., & Binns, C. (2020). Breastfeeding and the Risk of Infant Illness in Asia: A Review. International Journal of Environmental Research and Public Health, 17(1), 186. https://doi.org/10.3390/ijerph17010186






