Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
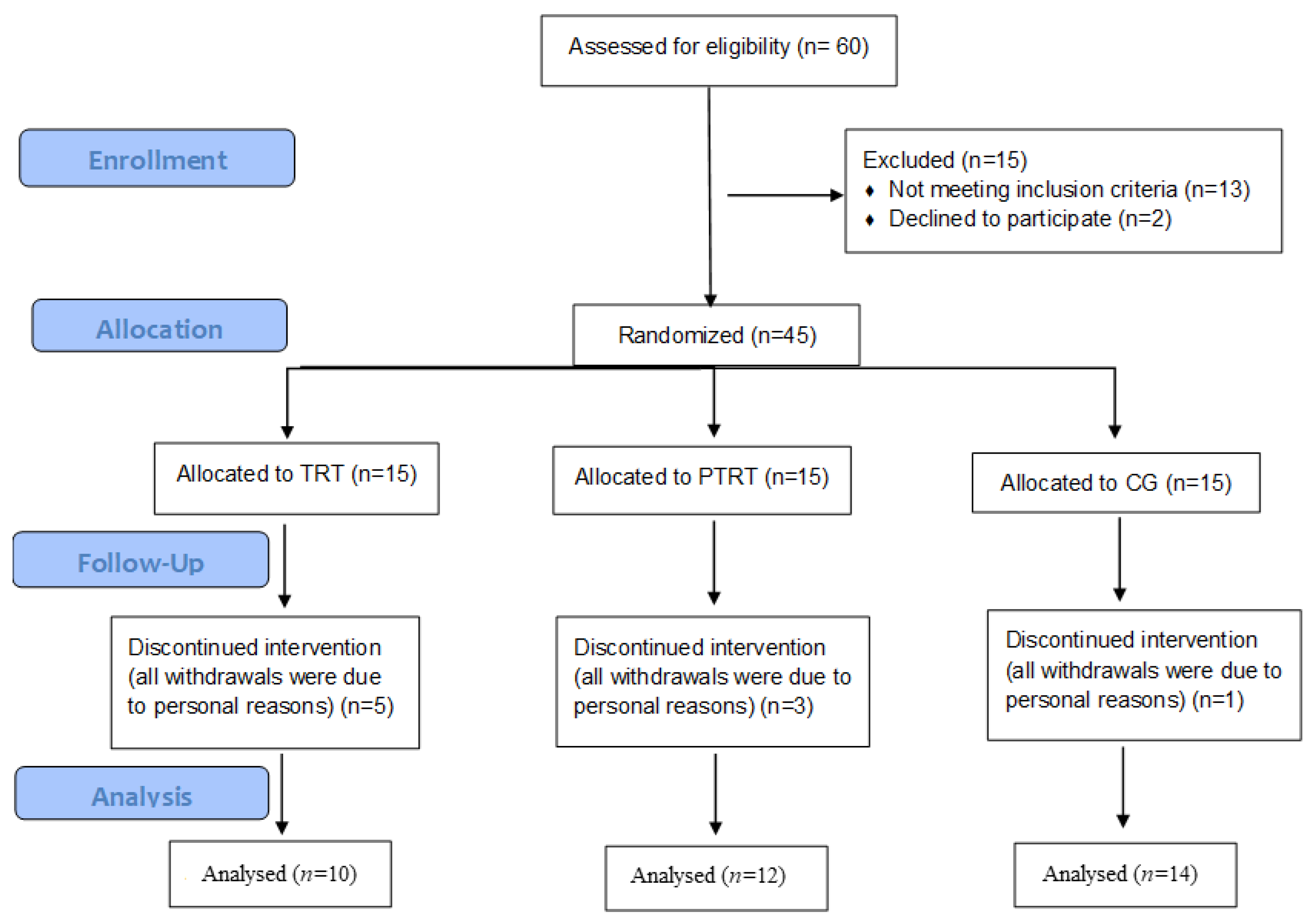
2.1. Study Design and Participants
2.2. Outcomes
2.2.1. Cognitive Function
Mini-Mental State Examination
TUG with a Cognitive Task
Picture Memory Test
2.2.2. Secondary Outcomes
2.3. Resistance Training Program
2.4. Control Group
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Effects of TRT and PTRT on Cognitive Function
3.3. Time-Course Effects of TRT and PTRT on Cognitive Function
3.4. Effects of TRT and PTRT on Depressive Symptoms
3.5. Effects of TRT and PTRT on BDNF Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- APA Dictionary of Psychology. Available online: https://dictionary.apa.org/cognition (accessed on 12 May 2020).
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, L.; Gahbauer, E.A.; Allore, H.; Gill, T.M. Joint Trajectories of Cognition and Frailty and Associated Burden of Patient-Reported Outcomes. J. Am. Med Dir. Assoc. 2018, 19, 304–309.e2. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Belli, L.; Giudice, T.L.; Lorenzo, F.; Sancesario, G.; Sorge, R.; Bernardini, S.; Martorana, A. Frailty Among Alzheimer’s Disease Patients. CNS Neurol. Disord. Drug Targets 2013, 12, 507–511. [Google Scholar] [CrossRef]
- Kidd, P.M. Alzheimer’s disease, amnestic mild cognitive impairment, and age-associated memory impairment: Current understanding and progress toward integrative prevention. Altern. Med. Rev. J. Clin. Ther. 2008, 13, 85–115. [Google Scholar]
- Pliatsikas, C.; Veríssimo, J.; Babcock, L.; Pullman, M.Y.; Glei, D.A.; Weinstein, M.; Goldman, N.; Ullman, M. Working memory in older adults declines with age, but is modulated by sex and education. Q. J. Exp. Psychol. 2018, 72, 1308–1327. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Kamal, F.; Taler, V. The influence of working memory performance on event-related potentials in young and older adults. Cogn. Neurosci. 2019, 10, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar]
- Åhman, H.B.; Giedraitis, V.; Cedervall, Y.; Lennhed, B.; Berglund, L.; McKee, K.; Kilander, L.; Rosendahl, E.; Ingelsson, M.; Åberg, A.C. Dual-Task Performance and Neurodegeneration: Correlations Between Timed Up-and-Go Dual-Task Test Outcomes and Alzheimer’s Disease Cerebrospinal Fluid Biomarkers. J. Alzheimer’s Dis. 2019, 71, S75–S83. [Google Scholar] [CrossRef]
- Ehsani, H.; Mohler, M.J.; O’Connor, K.; Zamrini, E.; Tirambulo, C.V.; Toosizadeh, N. The association between cognition and dual-tasking among older adults: The effect of motor function type and cognition task difficulty. Clin. Interv. Aging 2019, 14, 659–669. [Google Scholar] [CrossRef]
- Lundin-Olsson, L.; Nyberg, L.; Gustafson, Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet 1997, 349, 617. [Google Scholar] [CrossRef]
- Borges, S.D.M.; Radanovic, M.; Forlenza, O.V. Functional Mobility in a Divided Attention Task in Older Adults with Cognitive Impairment. J. Mot. Behav. 2015, 47, 1–8. [Google Scholar] [CrossRef]
- Beekman, A.T.F.; Copeland, J.; Prince, M.J. Review of community prevalence of depression in later life. Br. J. Psychiatry 1999, 174, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Kok, R.; Reynolds, C.F. Management of Depression in Older Adults. JAMA 2017, 317, 2114. [Google Scholar] [CrossRef] [PubMed]
- Noel, P.; Williams, J.J.; Unützer, J.; Worchel, J.; Lee, S.; Cornell, J.; Katon, W.; Harpole, L.H.; Hunkeler, E. Depression and Comorbid Illness in Elderly Primary Care Patients: Impact on Multiple Domains of Health Status and Well-being. Ann. Fam. Med. 2004, 2, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.; LaMonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- André, N.; Ferrand, C.; Albinet, C.; Audiffren, M. Cognitive Strategies and Physical Activity in Older Adults: A Discriminant Analysis. J. Aging Res. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Singh, M.A.F.; Minson, C.T.; Nigg, C.; Salem, G.J.; Skinner, J.S. Exercise and Physical Activity for Older Adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Cassilhas, R.; Viana, V.A.R.; Grassmann, V.; Thomatieli-Santos, R.V.; Santos, R.F.; Tufik, S.; De Mello, M.T. The Impact of Resistance Exercise on the Cognitive Function of the Elderly. Med. Sci. Sports Exerc. 2007, 39, 1401–1407. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Nagamatsu, L.S.; Graf, P.; Beattie, B.L.; Ashe, M.C.; Handy, T.C. Resistance Training and Executive Functions. Arch. Intern. Med. 2010, 170, 170–178. [Google Scholar] [CrossRef]
- Chang, Y.; Pan, C.-Y.; Chen, F.-T.; Tsai, C.-L.; Huang, C.-C. Effect of Resistance-Exercise Training on Cognitive Function in Healthy Older Adults: A Review. J. Aging Phys. Act. 2012, 20, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, X.; Xiang, W.; Han, J.; Li, K. The effect of resistance training on cognitive function in the older adults: A systematic review of randomized clinical trials. Aging Clin. Exp. Res. 2018, 30, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.R.; McDowell, C.; Hallgren, M.; Meyer, J.D.; Lyons, M.; Herring, M.P. Association of Efficacy of Resistance Exercise Training With Depressive Symptoms. JAMA Psychiatry 2018, 75, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-G.; Kim, J.-H.; Jun, T.-W. Effects of 12-Week Resistance Exercise on Electroencephalogram Patterns and Cognitive Function in the Elderly with Mild Cognitive Impairment. Clin. J. Sport Med. 2018, 28, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Obuchi, S.; Arai, T.; Nagasawa, H.; Shiba, Y.; Watanabe, S.; Kojima, M. The influence of short-term strength training on health-related quality of life and executive cognitive function. J. Physiol. Anthr. 2010, 29, 95–101. [Google Scholar] [CrossRef]
- Izquierdo, M.; Ibañez, J.; Gorostiaga, E.; Garrues, M.; Zúñiga, A.; Antón, A.; Larrión, J.L.; Häkkinen, K. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle-aged and older men. Acta Physiol. Scand. 1999, 167, 57–68. [Google Scholar] [CrossRef]
- Izquierdo, M.; Cadore, E.L. Muscle power training in the institutionalized frail: A new approach to counteracting functional declines and very late-life disability. Curr. Med Res. Opin. 2014, 30, 1385–1390. [Google Scholar] [CrossRef]
- Cadore, E.L.; Izquierdo, M. Muscle Power Training: A Hallmark for Muscle Function Retaining in Frail Clinical Setting. J. Am. Med Dir. Assoc. 2018, 19, 190–192. [Google Scholar] [CrossRef]
- Bean, J.F.; Leveille, S.; Kiely, D.K.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. A Comparison of Leg Power and Leg Strength within the InCHIANTI Study: Which Influences Mobility More? J. Gerontol. Ser. A Biol. Sci. Med Sci. 2003, 58, M728–M733. [Google Scholar] [CrossRef]
- Bean, J.F.; Kiely, D.K.; LaRose, S.; O’Neill, E.; Goldstein, R.; Frontera, W.R. Increased velocity exercise specific to task training versus the National Institute on Aging’s strength training program: Changes in limb power and mobility. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2009, 64, 983–991. [Google Scholar] [CrossRef]
- Reid, K.F.; Fielding, R.A. Skeletal Muscle Power. Exerc. Sport Sci. Rev. 2012, 40, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Strassnig, M.; Signorile, J.; Potiaumpai, M.; Romero, M.A.; Gonzalez, C.; Czaja, S.; Harvey, P.D. High velocity circuit resistance training improves cognition, psychiatric symptoms and neuromuscular performance in overweight outpatients with severe mental illness. Psychiatry Res. 2015, 229, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Kang, D.; Kim, H.; Kim, J.-S.; Song, H.S.; Song, W. Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr. Gerontol. Int. 2016, 17, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Cherup, N.; Roberson, K.; Potiaumpai, M.; Widdowson, K.; Jaghab, A.-M.; Chowdhari, S.; Armitage, C.; Seeley, A.; Signorile, J. Improvements in cognition and associations with measures of aerobic fitness and muscular power following structured exercise. Exp. Gerontol. 2018, 112, 76–87. [Google Scholar] [CrossRef]
- van Uffelen, J.; Chin A Paw, M.J.M.; Hopman-Rock, M.; Van Mechelen, W. The Effects of Exercise on Cognition in Older Adults with and without Cognitive Decline: A Systematic Review. Clin. J. Sport Med. 2008, 18, 486–500. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.-Y.; Moon, S.; Park, D.-H.; Kwak, H.-B.; Kang, J.-H. Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflüger Arch. 2019, 471, 491–505. [Google Scholar] [CrossRef]
- Pedersen, L.; Hojman, P. Muscle-to-organ cross talk mediated by myokines. Adipocyte 2012, 1, 164–167. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Picca, A.; Calvani, R.; Uchida, M.C.; Marzetti, E. If my muscle could talk: Myokines as a biomarker of frailty. Exp. Gerontol. 2019, 127, 110715. [Google Scholar] [CrossRef]
- Raschke, S.; Eckardt, K.; Holven, K.B.; Jensen, J.; Eckel, J. Identification and Validation of Novel Contraction-Regulated Myokines Released from Primary Human Skeletal Muscle Cells. PLoS ONE 2013, 8, e62008. [Google Scholar] [CrossRef]
- Rutti, S.; Dusaulcy, R.; Hansen, J.S.; Howald, C.; Dermitzakis, E.T.; Pedersen, B.K.; Pinget, M.; Plomgaard, P.; Bouzakri, K. Angiogenin and Osteoprotegerin are type II muscle specific myokines protecting pancreatic beta-cells against proinflammatory cytokines. Sci. Rep. 2018, 8, 10072. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Looney, D.P. Underlying Mechanisms and Physiology of Muscular Power. Strength Cond. J. 2012, 34, 13–19. [Google Scholar] [CrossRef]
- McKinnon, N.B.; Connelly, D.; Rice, C.L.; Hunter, S.W.; Doherty, T.J. Neuromuscular contributions to the age-related reduction in muscle power: Mechanisms and potential role of high velocity power training. Ageing Res. Rev. 2017, 35, 147–154. [Google Scholar] [CrossRef] [PubMed]
- De Vincenti, A.P.; Ríos, A.S.; Paratcha, G.; Ledda, F. Mechanisms That Modulate and Diversify BDNF Functions: Implications for Hippocampal Synaptic Plasticity. Front. Cell. Neurosci. 2019, 13, 135. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar] [CrossRef]
- De Almeida, A.; Da Silva, S.G.; Lopim, G.; Campos, D.V.; Fernandes, J.; Cabral, F.R.; Arida, R.M. Resistance Exercise Reduces Seizure Occurrence, Attenuates Memory Deficits and Restores BDNF Signaling in Rats with Chronic Epilepsy. Neurochem. Res. 2017, 42, 1230–1239. [Google Scholar] [CrossRef]
- Fragala, M.S.; Beyer, K.S.; Jajtner, A.R.; Townsend, J.R.; Pruna, G.J.; Boone, C.H.; Bohner, J.D.; Fukuda, D.H.; Stout, J.R.; Hoffman, J.R. Resistance Exercise May Improve Spatial Awareness and Visual Reaction in Older Adults. J. Strength Cond. Res. 2014, 28, 2079–2087. [Google Scholar] [CrossRef]
- Prestes, J.; Nascimento, D.D.C.; Tibana, R.A.; Teixeira, T.G.; Vieira, D.C.L.; Tajra, V.; De Farias, D.L.; Silva, A.O.; Funghetto, S.S.; De Souza, V.C.; et al. Understanding the individual responsiveness to resistance training periodization. Age (Dordr.) 2015, 37. [Google Scholar] [CrossRef]
- Silva, B.A.E.; Cassilhas, R.; Attux, C.; Cordeiro, Q.; Gadelha, A.L.; Telles, B.A.; Bressan, R.A.; Ferreira, F.N.; Rodstein, P.H.; Daltio, C.S.; et al. A 20-week program of resistance or concurrent exercise improves symptoms of schizophrenia: Results of a blind, randomized controlled trial. Braz. J. Psychiatry 2015, 37, 271–279. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2014, 60, 56–64. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Rodrigues, B.; Aguiar, S.D.S.; Gonçalves, I.D.O.; Asano, R.Y.; Irigoyen, M.-C.; Feriani, D.J.; Uchida, M.C. Low blood pressure is sustained during subsequent activities of daily living performed after power training in older women. J. Exerc. Rehabil. 2017, 13, 454–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coelho-Júnior, H.J.; Irigoyen, M.C.; da Silva Aguiar, S.; de Oliveira Gonçalves, I.; Câmara, N.O.S.; Cenedeze, M.A.; Asano, R.Y.; Rodrigues, B.; Uchida, M.C. Acute effects of power and resistance exercises on hemodynamic measurements of older women. Clin Interv. Aging 2017, 12, 1103–1114. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Gonçalvez, I.D.O.; Sampaio, R.A.C.; Sampaio, P.Y.S.; Cadore, E.L.; Izquierdo, M.; Marzetti, E.; Uchida, M.C. Periodized and non-periodized resistance training programs on body composition and physical function of older women. Exp. Gerontol. 2019, 121, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, S. Measurement of Functional Activities in Older Adults in the Community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Herrera, E.; Caramelli, P.; Silveira, A.S.B.; Nitrini, R. Epidemiologic Survey of Dementia in a Community-Dwelling Brazilian Population. Alzheimer Dis. Assoc. Disord. 2002, 16, 103–108. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Sheikh, J. 9/Geriatric Depression Scale (GDS). Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Takechi, H.; Dodge, H.H. Scenery Picture Memory Test: A new type of quick and effective screening test to detect early stage Alzheimer’s disease patients. Geriatr. Gerontol. Int. 2010, 10, 183–190. [Google Scholar] [CrossRef]
- Håkansson, K.; Ledreux, A.; Daffner, K.; Terjestam, Y.; Bergman, P.; Carlsson, R.; Kivipelto, M.; Winblad, B.; Granholm, A.-C.; Mohammed, A.K.H. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J. Alzheimer’s Dis. 2016, 55, 645–657. [Google Scholar] [CrossRef]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A New Approach to Monitoring Exercise Training. J. Strength Cond. Res. 2001, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.D.; Winchester, J.B.; Foster, C.; McGuigan, M.R. Using Session RPE to Monitor Different Methods of Resistance Exercise. J. Sports Sci. Med. 2006, 5, 289–295. [Google Scholar]
- Brydges, C.R. Effect Size Guidelines, Sample Size Calculations, and Statistical Power in Gerontology. Innov. Aging 2019, 3, igz036. [Google Scholar] [CrossRef] [PubMed]
- Burback, D.; Molnar, F.J.; John, P.S.; Man-Son-Hing, M. Key Methodological Features of Randomized Controlled Trials of Alzheimer’s Disease Therapy. Dement. Geriatr. Cogn. Disord. 1999, 10, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Lamas, L.; Aoki, M.S.; Ugrinowitsch, C.; Campos, G.E.R.; Regazzini, M.; Moriscot, A.; Tricoli, V. Expression of genes related to muscle plasticity after strength and power training regimens. Scand. J. Med. Sci. Sports 2009, 20, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wallerstein, L.F.; Tricoli, V.; Barroso, R.; Rodacki, A.L.F.; Russo, L.; Aihara, A.Y.; Fernandes, A.D.R.C.; De Mello, M.T.; Ugrinowitsch, C. Effects of Strength and Power Training on Neuromuscular Variables in Older Adults. J. Aging Phys. Act. 2012, 20, 171–185. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Åkerström, T.; Nielsen, A.R.; Fischer, C. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Whitham, M. From cytokine to myokine: The emerging role of interleukin-6 in metabolic regulation. Immunol. Cell Boil. 2014, 92, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Park, H.-K. Connecting Myokines and Metabolism. Endocrinol. Metab. 2015, 30, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal Res. 2017, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2017, 38, 579–593. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Ukropec, J.; Ukropcová, B.; Pai, M.-C. An acute bout of aerobic or strength exercise specifically modifies circulating exerkine levels and neurocognitive functions in elderly individuals with mild cognitive impairment. NeuroImage Clin. 2017, 17, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Maass, A.; Duezel, S.; Brigadski, T.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L.; et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 2016, 131, 142–154. [Google Scholar] [CrossRef]
- Carro, E.; Núñez, A.; Busiguina, S.; Aleman, I.T. Circulating Insulin-Like Growth Factor I Mediates Effects of Exercise on the Brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Trejo, J.L.; Carro, E.; Aleman, I.T. Circulating Insulin-Like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus. J. Neurosci. 2001, 21, 1628–1634. [Google Scholar] [CrossRef]
- Vellas, B.; Andrieu, S.; Sampaio, C.; Wilcock, G. Disease-modifying trials in Alzheimer’s disease: A European task force consensus. Lancet Neurol. 2007, 6, 56–62. [Google Scholar] [CrossRef]
- Lin, J.S.; O’Connor, E.A.; Rossom, R.C.; Perdue, L.A.; Burda, B.U.; Thompson, M.; Eckstrom, E. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force. JAMA 2020, 323, 757–763. [Google Scholar] [CrossRef]
- Falk, N.; Cole, A.; Meredith, T.J. Evaluation of Suspected Dementia. Am. Fam. Physician 2018, 97, 398–405. [Google Scholar]
- Taniguchi, Y.; Kitamura, A.; Murayama, H.; Amano, H.; Shinozaki, T.; Yokota, I.; Seino, S.; Nofuji, Y.; Nishi, M.; Yokoyama, Y.; et al. Mini-Mental State Examination score trajectories and incident disabling dementia among community-dwelling older Japanese adults. Geriatr. Gerontol. Int. 2017, 17, 1928–1935. [Google Scholar] [CrossRef]
- Kimura, N.; Aso, Y.; Yabuuchi, K.; Ishibashi, M.; Hori, D.; Sasaki, Y.; Nakamichi, A.; Uesugi, S.; Fujioka, H.; Iwao, S.; et al. Modifiable Lifestyle Factors and Cognitive Function in Older People: A Cross-Sectional Observational Study. Front. Neurol. 2019, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.E.; Carrier, J.; Postuma, R.B.; Gosselin, N.; Kakinami, L.; Thompson, C.; Chouchou, F.; Dang-Vu, T.T. Association between insomnia disorder and cognitive function in middle-aged and older adults: A cross-sectional analysis of the Canadian Longitudinal Study on Aging. Sleep 2019, 42. [Google Scholar] [CrossRef] [PubMed]
- Gamage, M.K.; Hewage, C.; Pathirana, K.D. Associated factors for cognition of physically independent elderly people living in residential care facilities for the aged in Sri Lanka. BMC Psychiatry 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Grouin, J.-M.; Coste, M.; Lewis, J. Subgroup Analyses in Randomized Clinical Trials: Statistical and Regulatory Issues. J. Biopharm. Stat. 2005, 15, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, J.-F.; Bell, T.; Crowe, M.; Clay, O.J.; Mirman, D. Lifting cognition: A meta-analysis of effects of resistance exercise on cognition. Psychol. Res. 2019. [Google Scholar] [CrossRef] [PubMed]


| Variables | TRT (n = 10) | PTRT (n = 12) | CG (n = 14) | p-Value |
|---|---|---|---|---|
| General Characteristics | ||||
| Age (years) | 67.0 ± 6.2 | 66.7 ± 5.1 | 66.7 ± 4.6 | 0.98 |
| Weight (kg) | 71.7 ± 12.9 | 68.2 ± 12.2 | 69.3 ± 20.4 | 0.61 |
| Height (cm) | 153 ± 0.0 | 158 ± 0.0 | 160 ± 0.1 | 0.18 |
| Body Mass Index (kg/m²) | 30.2 ± 4.1 | 27.8 ± 6.2 | 27.0 ± 7.7 | 0.15 |
| Katz Index (points) | 5.8 ± 0.7 | 5.5 ± 0.6 | 5.2 ± 0.4 | 0.16 |
| Pfeffer Index (points) | 0.4 ± 0.6 | 1.2 ± 1.4 | 2.0 ± 2.6 | 0.11 |
| Schooling (years) | 7.6 ± 4.1 | 7.4 ± 4.4 | 8.5 ± 4.3 | 0.79 |
| GDS (points) | 3.3 ± 2.1 | 3.3 ± 3.2 | 2.2 ± 2.4 | 0.87 |
| Total Workload (kg) * | 172.1 ± 8.6 | 103.2 ± 3.2 | NA | 0.001 |
| Cognitive Domains | ||||
| MMSE (points) | 23.4 ± 4.5 | 23.6 ± 2.4 | 24.8 ± 3.0 | 0.51 |
| TUG-cog (s) | 11.6 ± 2.8 | 14.8 ± 6.5 | 11.9 ± 2.7 | 0.16 |
| TUG-cog (steps) | 17.4 ± 3.5 | 18.7 ± 3.4 | 18.5 ± 4.1 | 0.65 |
| Short-term Memory Test (points) | 8.2 ± 4.4 | 8.4 ± 4.4 | 8.1 ± 2.9 | 0.98 |
| Time Points | TRT (n = 10) | PTRT (n = 12) | CG (n = 14) | p-Value | ||
|---|---|---|---|---|---|---|
| MMSE (points) | Time | Group | Time × Group | |||
| Baseline | 23.4 ± 4.5 (15−30) | 23.6 ± 2.4 (18−27) | 24.8 ± 3.0 (19−30) | |||
| 23rd week | 27.2 ± 4.1 (18−30) b | 27.0 ± 2.5 (23−30) a,b | 23.2 ± 3.1 (18−28) | |||
| ES (classification) | −1.0 (large) | −2.0 (large) | 0.33 (small) | |||
| ∆% | 16.2 | 14.4 | −6.5 | 0.001 | >0.05 | 0.01 |
| TUG-cog (s) | ||||||
| Baseline | 11.6 ± 2.8 (7−18) | 14.8 ± 6.5 (8−30) | 11.9 ± 2.7 (8.6−16.5) | |||
| 23rd week | 6.6 ± 1.2 (5−8.6) a,b | 7.0 ± 1.3 (5.4−9) a,b | 12.3 ± 2.3 (9.0−15.6) | |||
| ES (classification) | 3.16 (large) | 1.62 (large) | −0.50 (medium) | |||
| ∆% | −43.1 | −52.7 | 3.4 | 0.001 | 0.01 | 0.001 |
| TUG-cog (steps) | ||||||
| Baseline | 17.4 ± 3.5 (14−24) | 18.7 ± 3.4 (14−24) | 18.6 ± 3.6 (14−25) | |||
| 23rd week | 12.4 ± 2.1 (9−17) a,b | 12.1 ± 2.8 (5.6−17) a,b | 16.5 ± 3.2 (13−24) | |||
| ES (classification) | 1.96 (large) | 2.35 (large) | 0.66 (medium) | |||
| ∆% | −28.7 | −35.3 | −11.3 | >0.05 | 0.01 | 0.001 |
| Short-term Memory Test (points) | ||||||
| Baseline | 8.2 ± 4.4 (1−14) | 8.4 ± 4.4 (0−14) | 8.1 ± 2.9 (4−12) | |||
| 23rd week | 12.8 ± 5.2 (4−20) a,b | 11.7 ± 6.2 (3−22) b | 7.0 ± 2.7 (2−10) | |||
| ES (classification) | −0.95 (large) | −0.61 (medium) | 0.39 (small) | |||
| ∆% | 56.1 | 39.3 | −13.6 | >0.05 | >0.05 | 0.01 |
| Time Points | TRT (n = 10) | PTRT (n = 12) | CG (n = 14) | p-Value | ||
|---|---|---|---|---|---|---|
| GDS (points) | Time | Group | Time × Group | |||
| Baseline | 3.3 ± 2.1 (1.0−8.0) | 3.3 ± 3.2 (0.0−10.0) | 3.0 ± 3.1 (0.0−9.0) | |||
| 23rd Week | 0.9 ± 1.8 (0.0−6.0) | 1.9 ± 1.8 (0.0−6.0) | 2.5 ± 2.5 (0.0−9.0) | |||
| ES (23rd vs. Baseline) | 1.17 (large) | 0.53 (medium) | 0.17 (small) | |||
| ∆% | −72.2 | −42.4 | −16.6 | 0.01 | >0.05 | >0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho-Júnior, H.J.; Oliveira Gonçalves, I.d.; Sampaio, R.A.C.; Sampaio, P.Y.S.; Lusa Cadore, E.; Calvani, R.; Picca, A.; Izquierdo, M.; Marzetti, E.; Uchida, M.C. Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3435. https://doi.org/10.3390/ijerph17103435
Coelho-Júnior HJ, Oliveira Gonçalves Id, Sampaio RAC, Sampaio PYS, Lusa Cadore E, Calvani R, Picca A, Izquierdo M, Marzetti E, Uchida MC. Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2020; 17(10):3435. https://doi.org/10.3390/ijerph17103435
Chicago/Turabian StyleCoelho-Júnior, Hélio José, Ivan de Oliveira Gonçalves, Ricardo Aurélio Carvalho Sampaio, Priscila Yukari Sewo Sampaio, Eduardo Lusa Cadore, Riccardo Calvani, Anna Picca, Mikel Izquierdo, Emanuele Marzetti, and Marco Carlos Uchida. 2020. "Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 17, no. 10: 3435. https://doi.org/10.3390/ijerph17103435
APA StyleCoelho-Júnior, H. J., Oliveira Gonçalves, I. d., Sampaio, R. A. C., Sampaio, P. Y. S., Lusa Cadore, E., Calvani, R., Picca, A., Izquierdo, M., Marzetti, E., & Uchida, M. C. (2020). Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 17(10), 3435. https://doi.org/10.3390/ijerph17103435












