Seasonal Shift in Physicochemical Factors Revealed the Ecological Variables that Modulate the Density of Acinetobacter Species in Freshwater Resources
Abstract
1. Introduction
2. Methods and Materials
2.1. Description of Sampling Sites
2.2. Sample Collection
2.3. Measurement of Physicochemical Variables of Freshwater Resources
2.4. Isolation of Presumptive Acinetobacter Species
2.5. DNA Extraction and Molecular Identification of Acinetobacter Species
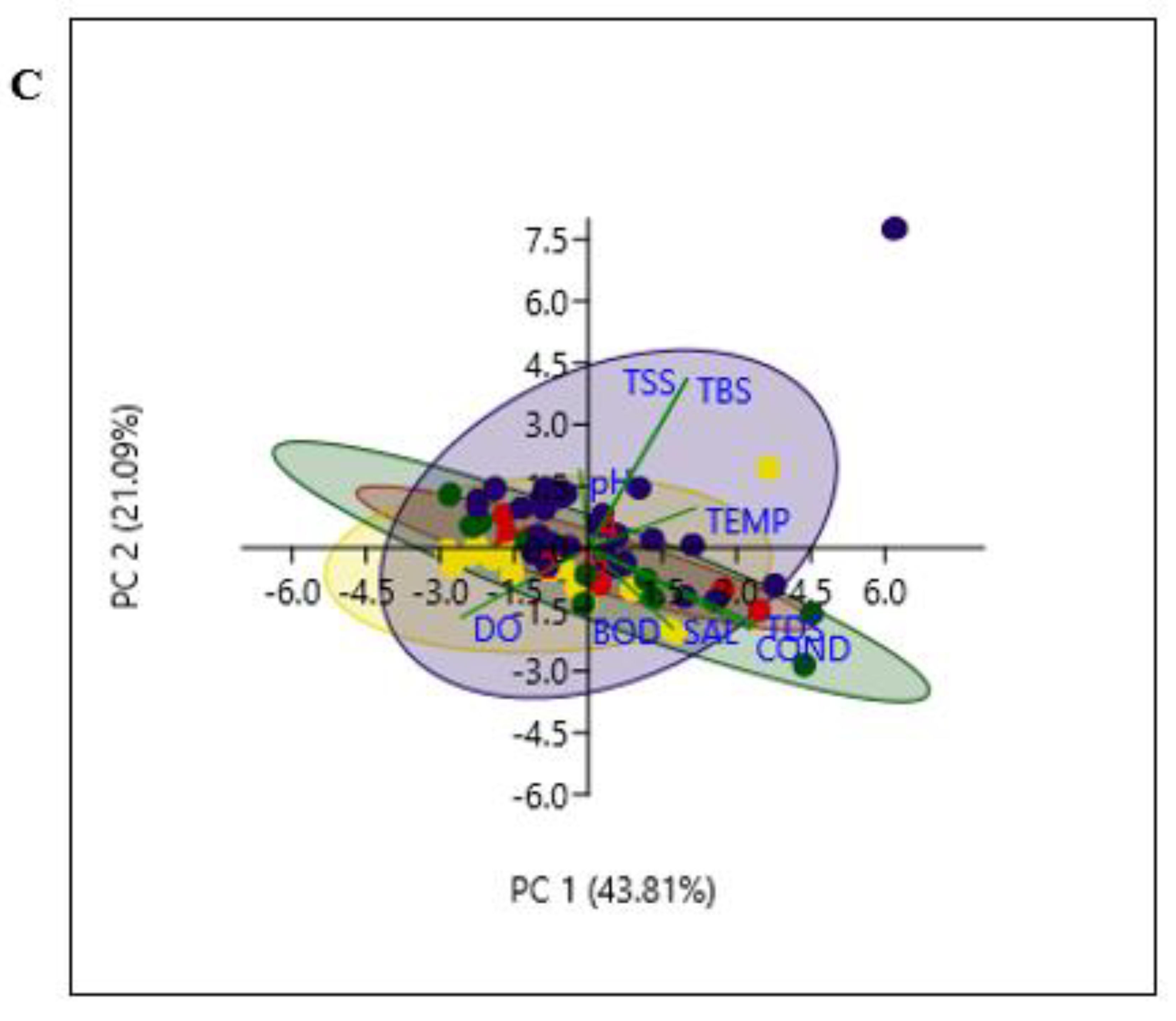
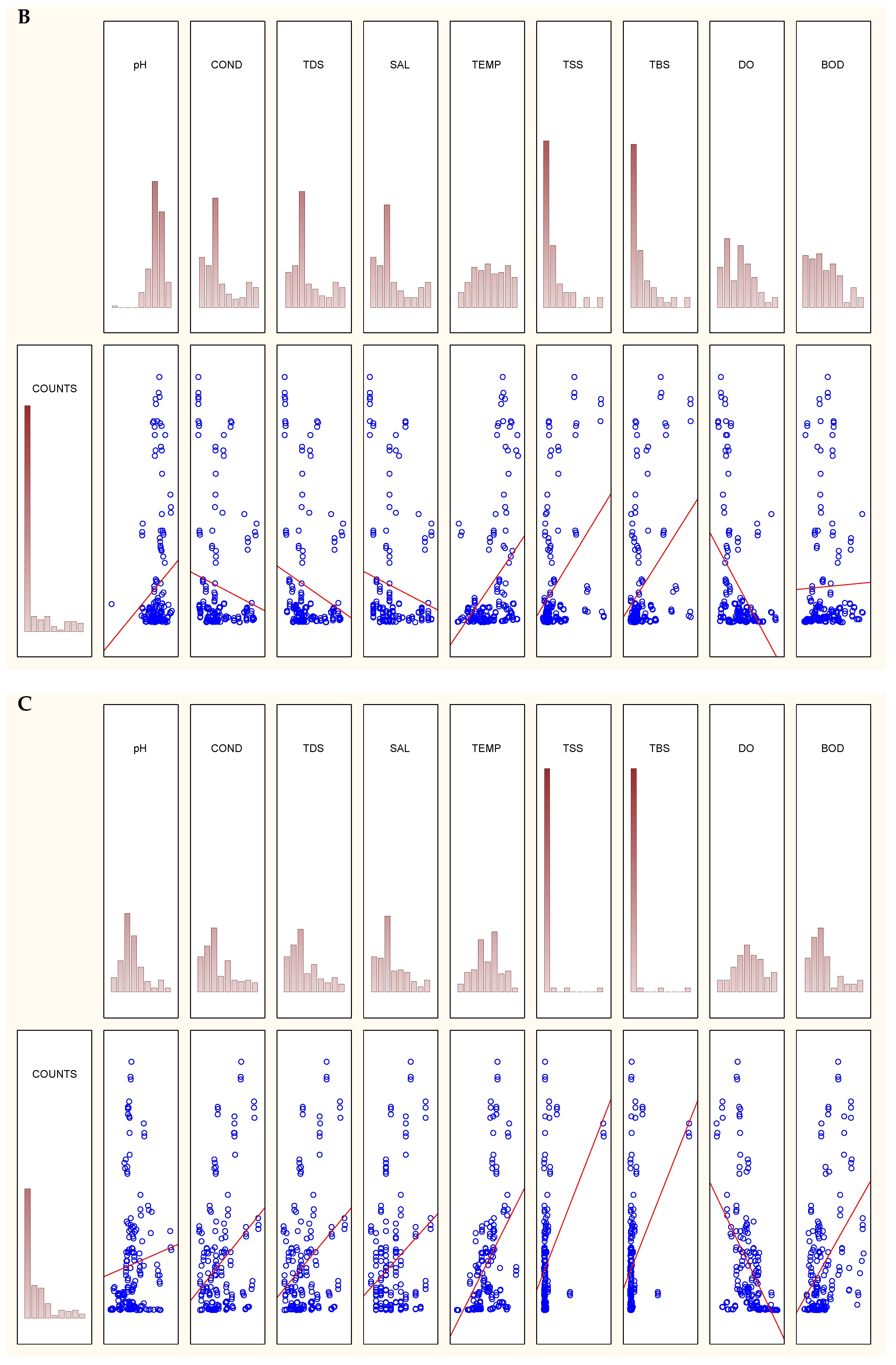
2.6. Statistical Data Analysis
3. Results
3.1. Physicochemical Parameters Vary with Seasons
3.2. Density of Acinetobacter Species in the Freshwater Resources
3.3. Confirmation of Acinetobacter Species from Presumptive Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Tringe, S.G.; von Mering, C.; Kobayashi, A.; Salamov, A.A.; Chen, K.; Chang, H.W.; Podar, M.; Short, J.M.; Mathur, E.J.; Detter, J.C.; et al. Comparative Metagenomics of Microbial Communities. Science 2005, 308, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.K.; Frandsen, R.J.N.; Holm, P.E.; Nybroe, O. Development of pollution-induced community tolerance is linked to structural and functional resilience of a soil bacterial community following a five-year field exposure to copper. Soil Biol. Biochem. 2010, 4, 748–757. [Google Scholar] [CrossRef]
- Tian, Y.; Haibara, K.; Toda, H.; Ding, F.; Liu, Y.; Choi, D. Microbial biomass and activity along a natural pH gradient in forest soils in a Karst region of the upper Yangtze River, China. J. For. Res. 2008, 13, 205–214. [Google Scholar] [CrossRef]
- Konopka, A. What is microbial community ecology? ISME J. 2009, 3, 1223–1230. [Google Scholar] [CrossRef]
- Gupta, N.; Pandey, P.; Hussain, J. Effect of physicochemical and biological parameters on the quality of river water of Narmada, Madhya Pradesh, India. Water Sci. 2017, 31, 11–23. [Google Scholar] [CrossRef]
- Shah, K.A.; Geeta, J.S. Evaluation of water quality index for River Sabarmati, Gujarat, India. Appl. Water Sci. 2015, 7, 1349–1358. [Google Scholar] [CrossRef]
- Bisi-Johnson, M.A.; Adediran, K.O.; Akinola, S.A.; Popoola, E.O.; Okoh, A.I. Comparative Physicochemical and Microbiological Qualities of Source and Stored Household Waters in Some Selected Communities in Southwestern Nigeria. Sustainability 2017, 9, 454. [Google Scholar] [CrossRef]
- Shittu, O.B.; Olaitan, J.O.; Amusa, T.S. Physico-Chemical and Bacteriological Analysis of Water Used for Drinking and Swimming Purpose. Afr. J. Biol. Res. 2008, 11, 285–290. [Google Scholar]
- Sun, Y.; Asante, F.; Birner, R. Opportunities and Challenges of Community-Based Rural Drinking Water Supplies: An Analysis of Water and Sanitation Committees in Ghana; Discussion paper 01026; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2010. [Google Scholar]
- Falagas, M.E.; Vardakas, K.Z.; Kapaskelis, A.; Triarides, N.A.; Roussos, N.S. Tetracyclines for multidrug-resistant Acinetobacter baumannii infections. Int. J. Antimicrob. Agents 2015, 45, 455–460. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Amaya-Villar, R.; Ferrándiz-Millón, C.; Díaz-Martín, A.; López-Sánchez, J.M.; Gutiérrez-Pizarraya, A. Optimum treatment strategies for carbapenem-resistant Acinetobacter baumannii bacteremia. Expert Rev. Anti Infect. Ther. 2015, 13, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Basri, R.; Zueter, A.R.; Mohamed, Z.; Alam, M.K.; Norsáadah, B.; Hasan, S.A.; Hasan, H.; Ahmad, F. Burden of bacterial meningitis: A retrospective review on laboratory parameters and factors associated with death in meningitis, Kelantan Malaysia. Nagoya J. Med. Sci. 2015, 77, 59–68. [Google Scholar] [PubMed]
- Bhattacharya, A.; Gupta, A.; Kaur, A.; Malik, D. Assessment of phenol-degrading ability of Acinetobacter sp. B9 for its application in bioremediation of phenol-contaminated industrial effluents. Chem. Ecol. 2015, 31, 607–621. [Google Scholar] [CrossRef]
- Sihag, S.; Sharma, S.; Pathak, H.; Dave, S.; Jaroli, D.P. Biodegradation of engine oil by Acinetobacter calcoaceticus BD4, isolated from Coastal Area Mumbai. Int. J. Biotechnol. Bioeng. Res. 2013, 4, 235–242. [Google Scholar]
- Ortega-de la Rosa, N.D.; Vázquez-Vázquez, J.L.; Huerta-Ochoa, S.; Gimeno, M.; Gutiérrez-Rojas, M. Stable bioemulsifiers are produced by Acinetobacter bouvetii UAM25 growing in different carbon sources. Bioprocess. Biosyst. Eng. 2018, 41, 859–869. [Google Scholar] [CrossRef]
- Triawan, A.; Ni’Matuzahroh; Supriyanto, A. Effects of the Combination between Bio-surfactant Product Types and Washing Times on the Removal of Crude Oil in Nonwoven Fabric. AIP Conf. Proc. 2017, 1854, 1–7. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant. Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Prittesh, P.; Rushabh, S.; Krunal, M. Isolation and characterization of plant growth promoting the potential of Acinetobacter sp. RSC7 isolated from Saccharum officinarum cultivar co 671. J. Exp. Biol. Agric. Sci. 2017, 5, 483–491. [Google Scholar] [CrossRef]
- Doughari, H.J.; Ndakidemi, P.A.; Human, I.S.; Benade, S. The ecology, biology and pathogenesis of Acinetobacter spp.: An overview. Microbes Environ. 2011, 26, 101–112. [Google Scholar] [CrossRef]
- Kanafani, A.Z.; Kanj, S.S. Ministry of Health, Kingdome of Saudi Arabia. 2014. Available online: http://www.uptodate.com/contents/acinetobacterinfection-treatment-and-prevention (accessed on 6 April 2020).
- Bitton, G. Microbiology of Drinking Water Production and Distribution, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; p. 312. [Google Scholar]
- The American Public Health Association (APHA); The American Water Works Association (AWWA); The Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater; APHA, AWWA, and WEF: Washington, DC, USA, 2005. [Google Scholar]
- Maugeri, T.L.; Carbone, M.; Fera, M.T.; Gugliandolo, C. Detection and differentiation of Vibrio vulnificus in seawater and plankton of a coastal zone of the Mediterranean Sea. Res. Microbiol. 2006, 157, 194–200. [Google Scholar] [CrossRef]
- Chen, T.L.; Lee, Y.T.; Kuo, S.C.; Yang, S.P.; Fung, C.P.; Lee, S.D. Rapid identification of Acinetobacter baumannii, Acinetobacter nosocomialis and Acinetobacter pittii with a multiplex PCR assay. J. Med. Microbiol. 2014, 63, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Development of Water Affair (DWA). Strategy and Guideline Development for National Groundwater Planning Requirements; The Atlantis Water Resource Management Scheme: 30 years of Artificial Groundwater Recharge; Development of Water Affair: Pretoria, South Africa, 2010.
- Mandakovic, D.; Rojas, C.; Maldonado, J.; Latorre, M.; Travisany, D.; Delage, E.; Bihouée, A.; Jean, G.; Díaz, F.P.; Fernández-Gómez, B.; et al. Structure and co-occurrence patterns in microbial communities under acute environmental stress reveal ecological factors fostering resilience. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Bryanskaya, A.V.; Malup, T.K.; Lazareva, E.V.; Taran, O.P.; Rozanov, A.S.; Efimov, V.M.; Peltek, S.E. The role of environmental factors for the composition of microbial communities of saline lakes in the Novosibirsk region (Russia). BMC Microbiol. 2016, 16, 4. [Google Scholar] [CrossRef]
- Aremu, M.O.; Olaofe, O.; Ikokoh, P.P.; Yakubu, M.M. Physicochemical characteristics of stream, well and borehole water sources in Eggon, Nasarawa State, Nigeria. J. Chem. Soc. Niger. 2011, 36, 131–136. [Google Scholar]
- Edimeh, P.O.; Eneji, I.S.; Oketunde, O.F.; Sha’ato, R. Physicochemical parameters and some heavy metals content of rivers Inachalo and Niger in Idah, Kogi State. J. Chem. Soc. Niger. 2011, 36, 95–101. [Google Scholar]
- Department of Water Affairs (DWAF). Revision of General Authorisations in Terms of Section 39 of The National Water Act, 1998 (ACT NO. 36 OF 1998) (THE ACT); South African Government Gazette: Pretoria, South Africa, 2013; Volume 36820, pp. 3–32.
- Ekundayo, T.C.; Okoh, A.I. Modelling the effects of physicochemical variables and anthropogenic activities as ecological drivers of Plesiomonas shigelloides distribution and freshwaters quality. Sci. Total Environ. 2019, 682, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Santosh, M.; Shrihari, A.S. Evaluation of water quality index for drinking purposes for river Netravathi, Mangalore, South India. Environ. Monit. Assess 2008, 143, 279–290. [Google Scholar] [CrossRef]
- Wisplinghoff, H. 181-Pseudomonas spp., Acinetobacter spp. and Miscellaneous Gram-Negative Bacilli. In Infectious Diseases, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 1579–1599.e2. [Google Scholar]
- Almeida, C.; Gonzalez, S.O.; Mallea, M.; Gonzalez, P. A recreational water quality index using chemical, physical and microbiological parameters Environ. Sci. Pollut. Res. Int 2012, 19, 3400–3411. [Google Scholar] [CrossRef] [PubMed]
- Brown & Caldwell. A Guidebook for Local Governments for Developing Regional Watershed Protection Plans; Northeast Georgia Regional Development Centre: Athens, GA, USA, 2001; p. 22. [Google Scholar]
- Verma, P.U.; Chandawat, D.K.; Gupta, U.; Solanki, H.A. Water quality analysis of an organically polluted lake by investigating different physical and chemical parameters. Int. J. Res. Chem. Environ. 2012, 2, 105–111. [Google Scholar]
- Environmental Protection Agency (EPA). Technical Support. Document for the Endangerment and Cause or Contributes Findings for Greenhouse Gases Under Section 202(a) of the Clean Air Act; U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- Momba, M.N.B.; Kaleni, P. Regrowth and survival of indicator microorganisms on the surfaces of household containers used for the storage of drinking water in rural communities of South Africa. Water Res. 2002, 36, 3023–3028. [Google Scholar] [CrossRef]
- De Sousa, D.N.R.; Mozeto, A.A.; Carneiro, R.L.; Fadini, P.S. Electrical conductivity and emerging contaminant as markers of surface freshwater contamination by wastewater. Sci. Total Environ. 2014, 484, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.Y.; Brandes, D.; Kney, A.D. Using electronic conductivity and hardness data for rapid assessment of stream water quality. J. Environ. Manag. 2012, 104, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Chang, H. Spatial analysis of water quality trends in the Han River basin, South Korea. Water Res. 2008, 42, 3285–3304. [Google Scholar] [CrossRef]
- South African Water Quality Guidelines, Aquatic Ecosystems, 1st ed.; Department of Water Affairs and Forestry (DWAF): Pretoria, South Africa, 1996; Volume 7.
- European Parliament v Council of the European Union. Directive 2006/44/EC of the European Parliament and of the Council of 6 September 2006 on the quality of fresh waters needing protection or improvement in order to support fish life. Off. J. Eur. Union. 2006, 264, 20–31. Available online: https://www.eumonitor.eu/9353000/1/j4nvke1fm2yd1u0_j9vvik7m1c3gyxp/vkcweeed4pwa/v=s7z/f=/com(2004)19_en.pdf (accessed on 8 May 2020).
- World Health Organization (WHO). Guidelines for Drinking Water Quality- Geneva, 2nd ed.; World Health Organization: Geneva, Switzerland, 1999; pp. 97–100. [Google Scholar]
- Li, Y.; Li, M.; Kemp, W.M. A budget analysis of bottom-water dissolved oxygen in Chesapeake Bay. Estuar. Coasts 2015, 38, 2132–2148. [Google Scholar] [CrossRef]
- Von Sperling, M.; de Lemos Chernicharo, C.A. Biological Wastewater Treatment in Warm Climate Regions; IWA publishing: London, UK, 2017; p. 857. [Google Scholar]
- AL-Kadmy, I.M.S.; Ali, A.N.M.; Salman, I.M.A.; Khazaal, S.S. Molecular characterization of Acinetobacter baumannii isolated from Iraqi hospital environment. New Microbes New Infect. 2018, 21, 51–57. [Google Scholar] [CrossRef]





| Rivers | Site Codes | Site Name | Major Human Activities | Coordinates |
|---|---|---|---|---|
| Great Fish | GF1 | Craddock upstream 1 | Irrigation farming, fishing | S 32°05.902′ E 025°35.784′ |
| GF2 | Craddock upstream 2 | Fishing, farming, animal rearing | S 32°19.169′ E 025°36.823′ | |
| GF3 | Craddock township 1 | Fishing, swimming, animal rearing, domestic purposes | S 32°10.256′ E 025°36.831′ | |
| GF4 | Craddock township 2 | Downstream of Craddock WWTP, fishing, swimming, animal rearing, refuse dumping | S 32°11.322′ E 025°37.846′ | |
| GF5 | Craddock location | Fishing, swimming, traditional ritual washing, refuse dumping | S 32°11.527′ E 025°39.616′ | |
| Keiskamma | KE1 | Keiskammahoek upstream | Farming, animal rearing | S 32°38.427′ E 027°11.436′ |
| KE2 | Keiskammahoek township 1 | Domestic use, car washing, domestic waste pipe leakages, animal rearing, community runoff | S 32°40.538′ E 027°08.477′ | |
| KE3 | Keiskammahoek township 2 | Refuse dumping, community runoff, water pipe leakages | 32°41.271′ E 027°09.127′ | |
| KE4 | Keiskammahoek downstream | Animal rearing, Farming, downstream of Sandile Dam, | S 32°44.292′ E 027°05.895′ | |
| KE5 | Sandile community | Downstream of Sandile WWTP, animal rearing, farming, vehicles crossing | S 32°45.579′ E 027°04.110′ | |
| Tyhume | TY1 | Tyhume source | Tourism, swimming | S 32°36.683′ E 022°57.612′ |
| TY2 | Kayaletu village | Domestic use, animal rearing, farming, community runoff | S 32°38.374′ E 026°56.163′ | |
| TY3 | Bin field Dam | Fishing, recreational purposes, farming, animal drinking | S 32°40.980′ E 026°54.080′ | |
| TY4 | Melani village | Swimming, domestic use, fishing, animal rearing | S 32°43.223′ E 026°51.646′ | |
| TY5 | Alice town | Fishing, construction purposes, farming, downstream of hospital waste discharge, UFH wastes discharge, animal rearing | S 32°46.920′ E 026°50.796′ |
| Parameter | pH | Temperature (°C) | EC (µS/cm) | TDS (mg/dm3) | SAL (PSU) | TSS (mg/dm3) | Turbidity (NTU) | DO (mg/dm3) | BOD (mg/dm3) |
|---|---|---|---|---|---|---|---|---|---|
| Great Fish River | |||||||||
| Autumn | 8.2 ± 0.3 | 15.8 ± 1.1 | 299 ± 39.4 | 149 ± 19.7 | 0.14 ± 0.01 | 57 ± 49.5 | 168 ± 49.1 | 9.0 ± 0.4 | 3.1 ± 1.6 |
| Winter | 8.0 ± 0.4 | 12.7 ± 1.4 | 369 ± 113.5 | 184 ± 56.8 | 0.18 ± 0.1 | 85.6 ± 24.0 | 96 ± 27.4 | 9.9 ± 0.4 | 4.9 ± 2.5 |
| Spring | 8.2 ± 0.2 | 20.3 ± 0.7 | 339 ± 28.4 | 169 ± 14.1 | 0.16 ± 0.0 | 44.3 ± 8.9 | 48 ± 10.5 | 8.5 ± 0.4 | 3.9 ± 2.1 |
| Summer | 8.0 ± 0.4 | 22.3 ± 2.6 | 274 ± 18.2 | 137 ± 8.9 | 0.13 ± 0.01 | 99.4 ± 43.6 | 214 ± 45.9 | 7.8 ± 0.5 | 3.7 ± 0.6 |
| Keiskemma River | |||||||||
| Autumn | 7.7 ± 0.3 | 14.5 ± 1.9 | 247 ± 101.8 | 123 ± 50.8 | 0.12 ± 0.1 | 31 ± 24.8 | 36 ± 29.1 | 9.1 ± 0.4 | 3.0 ± 2.0 |
| Winter | 7.5 ± 0.4 | 11.0 ± 1.9 | 285 ± 115.3 | 143 ± 57.7 | 0.14 ± 0.1 | 39 ± 59.3 | 42 ± 58.0 | 9.8 ± 0.6 | 3.2 ± 2.2 |
| Spring | 7.7 ± 0.3 | 16.1 ± 1.3 | 216 ± 102.2 | 108 ± 51.4 | 0.10 ± 0.1 | 27 ± 15.1 | 31 ± 17.6 | 9.2 ± 0.7 | 6.0 ± 2.6 |
| Summer | 7.9 ± 0.5 | 21.4 ± 1.9 | 153.2 ± 63.7 | 86 ± 33.0 | 0.07 ± 0.0 | 55.6 ± 48.4 | 61 ± 49.6 | 8.3 ± 0.5 | 4.5 ± 1.9 |
| Tyhume River | |||||||||
| Autumn | 7.5 ± 0.3 | 15.4 ± 2.8 | 141 ± 62.7 | 71 ± 31.3 | 0.07 ± 0.0 | 30 ± 24.2 | 35 ± 27.6 | 8.9 ± 0.6 | 2.4 ± 0.8 |
| Winter | 7.2 ± 0.5 | 11.3 ± 3.0 | 129 ± 56.9 | 65 ± 28.4 | 0.06 ± 0.0 | 51 ± 133.3 | 57 ± 151.8 | 9.8 ± 0.6 | 2.0 ± 0.8 |
| Spring | 7.3 ± 0.5 | 16.8 ± 3.5 | 141 ± 85.6 | 70 ± 42.7 | 0.07 ± 0.0 | 30 ± 29.4 | 35 ± 31.0 | 8.9 ± 0.8 | 4.2 ± 2.6 |
| Summer | 7.7 ± 0.5 | 20.2 ± 4.1 | 125 ± 50.8 | 62 ± 25.6 | 0.06 ± 0.0 | 89.6 ± 243.2 | 96 ± 255.9 | 8.2 ± 0.7 | 3.4 ± 1.7 |
| Regulation [26] | 5.5–9.5 | ≤35 | 7000–15000 | 450 | - | - | <5 | <5 | 3–6 |
| Physicochemical Variables | Great Fish | Keiskamma | Tyhume | |||
|---|---|---|---|---|---|---|
| r-Value | p-Value | r-Value | p-Value | r-Value | p-Value | |
| pH | −0.0915 | 0.222 | 0.1434 | 0.055 | 0.0875 | 0.243 |
| EC | −0.1886 | 0.011 | −0.1367 | 0.067 | 0.3134 | 0.00 |
| TDS | −0.1901 | 0.011 | −0.1772 | 0.017 | 0.3133 | 0.000 |
| SAL | −0.1790 | 0.016 | −0.1430 | 0.055 | 0.2709 | 0.000 |
| TEMP | 0.2416 | 0.001 | 0.3816 | 0.000 | 0.4228 | 0.000 |
| TSS | 0.4639 | 0.000 | 0.3572 | 0.000 | 0.3633 | 0.000 |
| TBS | 0.4483 | 0.000 | 0.3512 | 0.000 | 0.3581 | 0.000 |
| DO | −0.2644 | 0.000 | −0.4084 | 0.000 | −0.4975 | 0.000 |
| BOD | 0.3079 | 0.000 | 0.0234 | 0.755 | 0.4202 | 0.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adewoyin, M.A.; Okoh, A.I. Seasonal Shift in Physicochemical Factors Revealed the Ecological Variables that Modulate the Density of Acinetobacter Species in Freshwater Resources. Int. J. Environ. Res. Public Health 2020, 17, 3606. https://doi.org/10.3390/ijerph17103606
Adewoyin MA, Okoh AI. Seasonal Shift in Physicochemical Factors Revealed the Ecological Variables that Modulate the Density of Acinetobacter Species in Freshwater Resources. International Journal of Environmental Research and Public Health. 2020; 17(10):3606. https://doi.org/10.3390/ijerph17103606
Chicago/Turabian StyleAdewoyin, M. A., and A. I. Okoh. 2020. "Seasonal Shift in Physicochemical Factors Revealed the Ecological Variables that Modulate the Density of Acinetobacter Species in Freshwater Resources" International Journal of Environmental Research and Public Health 17, no. 10: 3606. https://doi.org/10.3390/ijerph17103606
APA StyleAdewoyin, M. A., & Okoh, A. I. (2020). Seasonal Shift in Physicochemical Factors Revealed the Ecological Variables that Modulate the Density of Acinetobacter Species in Freshwater Resources. International Journal of Environmental Research and Public Health, 17(10), 3606. https://doi.org/10.3390/ijerph17103606





