Contamination Level, Distribution Characteristics, and Ecotoxicity of Tetrabromobisphenol A in Water and Sediment from Weihe River Basin, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection
2.3. Sample Extraction and Cleanup
2.4. Instrumental Analysis
2.5. Quality Assurance and Quality Control
2.6. Ecological Risk Assessment
3. Results and Discussion
3.1. Pollution Characteristics of TBBPA in the Weihe River Basin
3.1.1. Pollution Characteristics of TBBPA in Sediment
3.1.2. Pollution Characteristics of TBBPA in Water Sample
3.2. Spatial Distribution Characteristics and Source Analysis of TBBPA in the Weihe River Basin
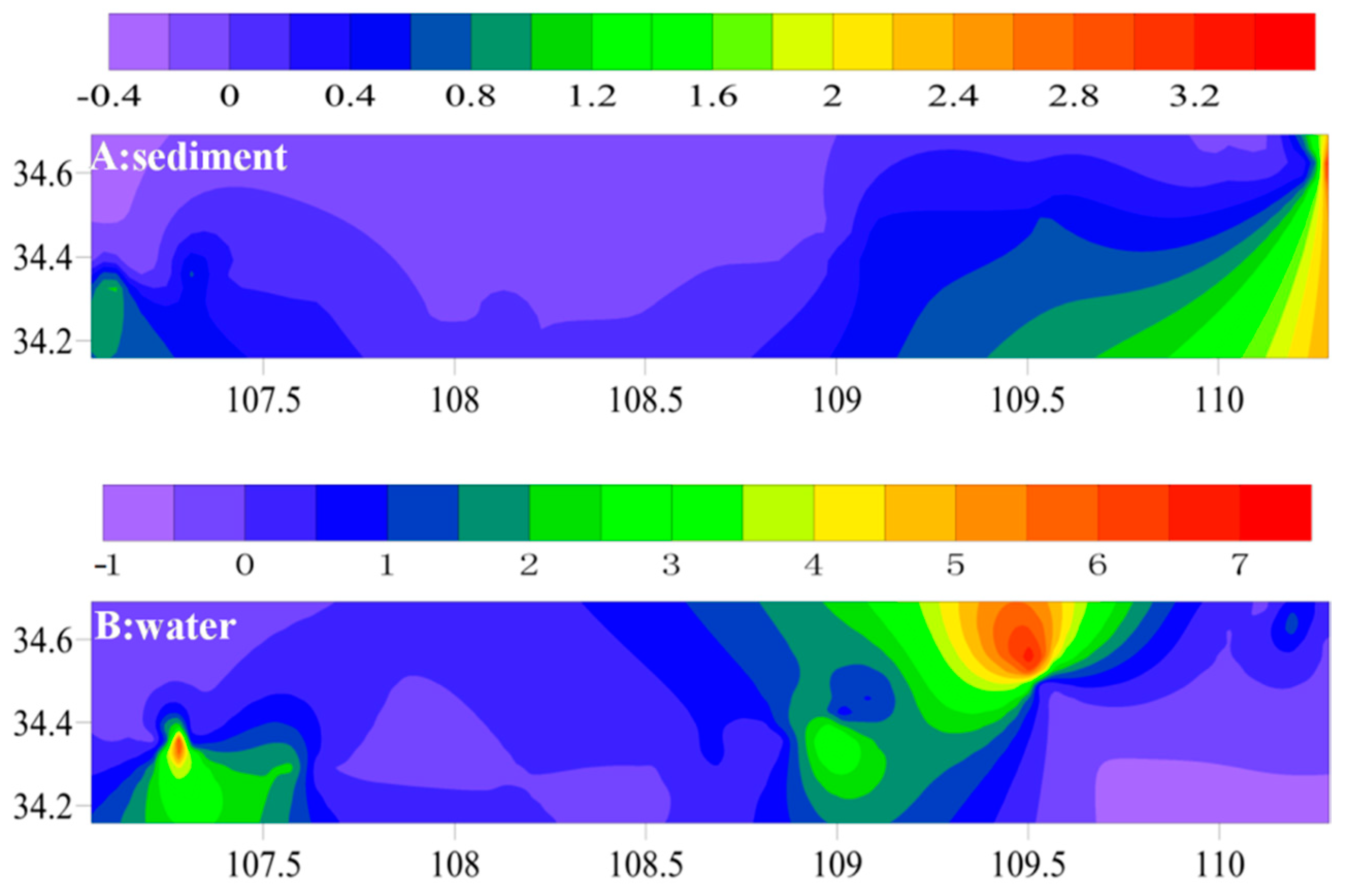
3.2.1. Spatial Distribution Characteristics of TBBPA in Sediments
3.2.2. Spatial Distribution Characteristics of TBBPA in Water Sample
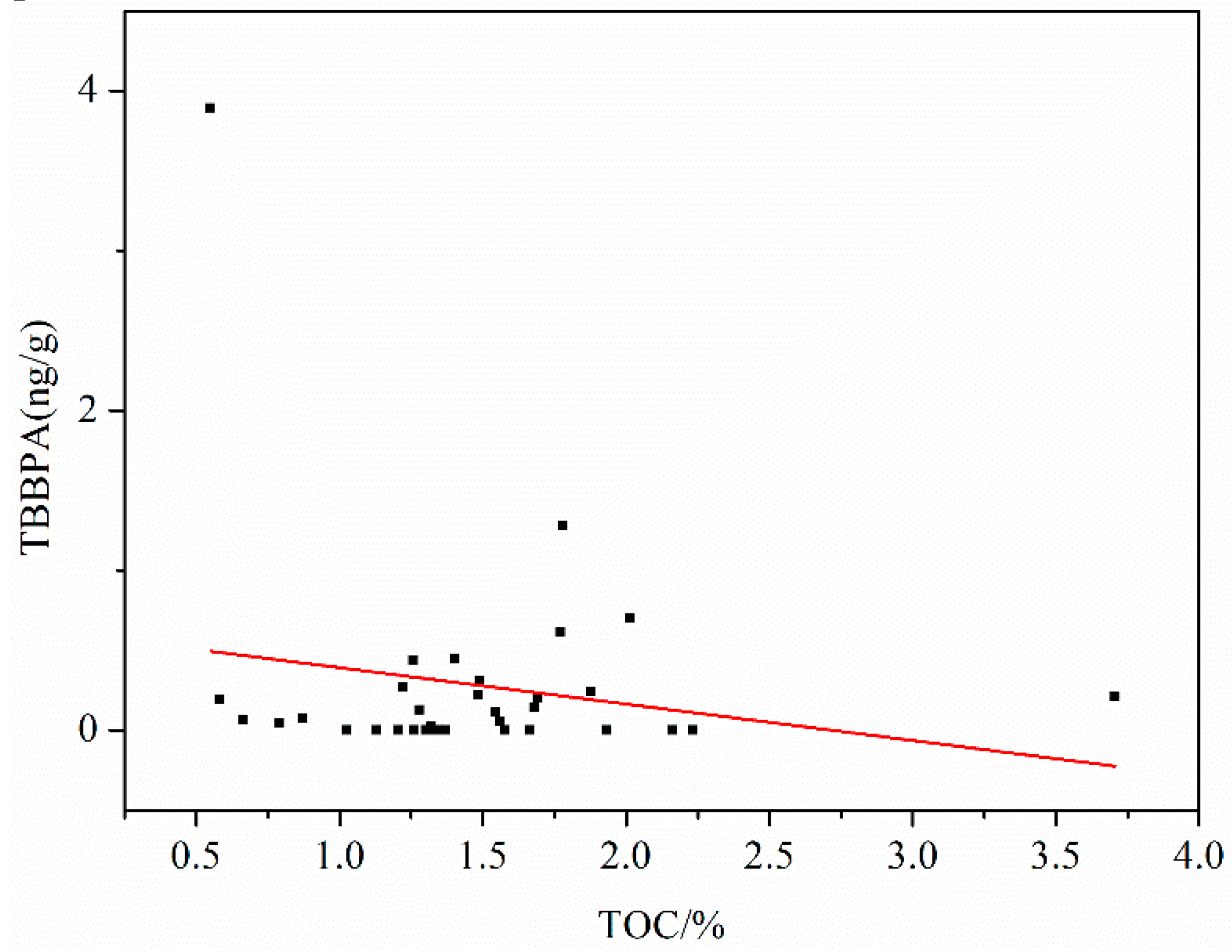
3.2.3. Correlation between TBBPA and TOC in Sediments
3.2.4. Source Analysis
3.3. Ecological Risk Assessment
3.3.1. Screening, Acquisition, and Collation of Toxicological Data
3.3.2. The Calculation of PNECwat in Water Body
3.3.3. The Calculation of PNECsed in Sediments
3.3.4. Risk Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, M.D.; Yang, S.W.; Li, H.P.; Xu, F.F.; Sun, F.C.; Zhang, J.J. Environmental exposure level of tetrabromobisphenol A in surface sediments and fish in the Bohai Sea. Environ. Res. 2013, 26, 160–165. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA J. 2011, 9, 2477. [Google Scholar] [CrossRef]
- Crawford, E.S.; Guarasci, D.T.; Larson, S.A. A Survey of Thyroid Gland Scintigraphy. J. Nucl. Med. Technol. 2009, 37, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Liu, L.L.; Guo, J.; Zhou, X.Y.; Deng, J.J.; Liu, Y.C.; Fu, X.X.; Zhang, W.; Lin, Y.F. Pollution and distribution characteristics of brominated flame retardants in Sichuan and Tibet. J. Environ. Sci. 2014, 34, 2823–2831. [Google Scholar]
- Yang, S.W.; Wang, S.R.; Yan, Z.G.; Zhang, P.Q. Tissue distribution and bioconcentration factors of tetrabromobisphenol A in five species of Chaohu fish. J. Environ. Sci. 2012, 33, 1852–1857. [Google Scholar]
- Zhang, X.-L.; Luo, X.; Chen, S.-J.; Wu, J.-P.; Mai, B.-X. Spatial distribution and vertical profile of polybrominated diphenyl ethers, tetrabromobisphenol A, and decabromodiphenylethane in river sediment from an industrialized region of South China. Environ. Pollut. 2009, 157, 1917–1923. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, D.Y.; Mei, J.; Hu, J.F.; Peng, P.G. Research on pollution level of chlorinated/brominated dioxins and tetrabromobisphenol A in atmospheric particulates near an electronic waste dismantling point in Guiyu. J. Environ. Sci. 2012, 32, 1142–1148. [Google Scholar]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Heal. Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef]
- Hidetaka, T.; Go, S.; Yasuhiro, H.; Shin-Ichi, S. Transfer of brominated flame retardants from components into dust inside television cabinets. Chemosphere 2008, 73, 161–169. [Google Scholar]
- Wang, D.; Yang, S.; Wang, G.; Gao, L.; Wang, Y.; Jiang, Q.; Chen, Y. Residues and Distributions of Organochlorine Pesticides in China’s Weihe River. Pol. J. Environ. Stud. 2016, 25, 1285–1292. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, R.; Yang, S. Distribution and Source of Polycyclic Aromatic Hydrocarbons (PAHs) in Water Dissolved Phase, Suspended Particulate Matter and Sediment from Weihe River in Northwest China. Int. J. Environ. Res. Public Heal. 2015, 12, 14148–14163. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yuan, X.Y.; Yang, S.K.; Zhao, Y.Q. Concentrations, Distributions, and Risk Assessment of HBCD in Sediment in the Weihe River Basin in Northwest China. IJERPH 2018, 15, 2340. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.L.; Emilien, P. LC-ESI-MS-MS method for the analysis of tetrabromobisphenol A in sediment and sewage sludge. Analyst 2004, 129, 724–730. [Google Scholar]
- Paula, G.; Ethel, E.; Damià, B. Simultaneous determination of hexabromocyclododecane, tetrabromobisphenol A, and related compounds in sewage sludge and sediment samples from Ebro River basin (Spain). Anal. Bioanal. Chem. 2010, 397, 2817–2824. [Google Scholar]
- Harrad, S.; Abdallah, M.A.-E.; Rose, N.L.; Turner, S.; Davidson, T.A. Current-Use Brominated Flame Retardants in Water, Sediment, and Fish from English Lakes. Environ. Sci. Technol. 2009, 43, 9077–9083. [Google Scholar] [CrossRef]
- Verslycke, T.; Vethaak, A.D.; Arijs, K.; Janssen, C.R. Flame retardants, surfactants and organotins in sediment and mysid shrimp of the Scheldt estuary (The Netherlands). Environ. Pollut. 2005, 136, 19–31. [Google Scholar] [CrossRef]
- Pierre, L.; Khawla, T.; Fabrice, A.; Catherine, B.; Annie, D.; Marc, C. Development of analytical procedures for trace-level determination of polybrominated diphenyl ethers and tetrabromobisphenol A in river water and sediment. Anal. Bioanal. Chem. 2010, 396, 865–875. [Google Scholar]
- Yang, S.; Wang, S.; Liu, H.; Yan, Z. Tetrabromobisphenol A: Tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ. Sci. Pollut. Res. 2012, 19, 4090–4096. [Google Scholar] [CrossRef]
- Xu, T.; Wang, J.; Liu, S.-Z.; Lu, C.; Shelver, W.L.; Li, Q.X.; Li, J. A highly sensitive and selective immunoassay for the detection of tetrabromobisphenol A in soil and sediment. Anal. Chim. Acta 2012, 751, 119–127. [Google Scholar] [CrossRef]
- Zhao, M.D. Study on exposure of tetrabromobisphenol A in environmental media in Erhai Lake. Ph.D. Thesis, Chang Sha University of Science and Technology, Changsha, China, 2013. [Google Scholar]
- Xu, J.; Zhang, Y.; Guo, C.; He, Y.; Li, L.; Meng, W. Levels and distribution of tetrabromobisphenol A and hexabromocyclododecane in Taihu Lake, China. Environ. Toxicol. Chem. 2013, 32, 2249–2255. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Guo, M.; Xu, H.; Zhang, S.; Shi, L.; Yao, C. Occurrence, distribution, and risk assessment of alkylphenols, bisphenol A, and tetrabromobisphenol A in surface water, suspended particulate matter, and sediment in Taihu Lake and its tributaries. Mar. Pollut. Bull. 2016, 112, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.-H.; Chen, S.-J.; Chen, M.-Y.; He, M.-J.; Luo, X.; Mai, B.-X. Hexabromocyclododecane (HBCD) and tetrabromobisphenol A (TBBPA) in riverine and estuarine sediments of the Pearl River Delta in southern China, with emphasis on spatial variability in diastereoisomer- and enantiomer-specific distribution of HBCD. Mar. Pollut. Bull. 2012, 64, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, L.; Shi, M.; Liu, G. Persistent organic pollutants in typical lake ecosystems. Ecotoxicol. Environ. Saf. 2019, 180, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Meng, Z.; Zhu, Y.; Song, M.; Wang, H. Dummy molecularly imprinted polymer for selective screening of trace bisphenols in river water. Anal. Methods 2011, 3, 173–180. [Google Scholar] [CrossRef]
- He, M.-J.; Luo, X.-J.; Yu, L.-H.; Wu, J.-P.; Chen, S.-J.; Mai, B.-X. Diasteroisomer and enantiomer-specific profiles of hexabromocyclododecane and tetrabromobisphenol A in an aquatic environment in a highly industrialized area, South China: Vertical profile, phase partition, and bioaccumulation. Environ. Pollut. 2013, 179, 105–110. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Yan, S.; Zhang, W.; Li, Y.; Han, D. A review of status of tetrabromobisphenol A (TBBPA) in China. Chemosphere 2016, 148, 8–20. [Google Scholar] [CrossRef]
- Gouin, T. Modelling the environmental fate of the polybrominated diphenyl ethers. Environ. Int. 2003, 29, 717–724. [Google Scholar] [CrossRef]
- Nam, J.J.; Gustafsson, Ö.; Kurt-Karakus, P.B.; Breivik, K.; Steinnes, E.; Jones, K.C. Relationships between organic matter, black carbon and persistent organic pollutants in European background soils: Implications for sources and environmental fate. Environ. Pollut. 2008, 156, 809–817. [Google Scholar] [CrossRef]
- Zou, M.Y.; Gong, J.; Yan, Y. Distribution and environmental behavior of soil polybrominated diphenyl ethers (PBDEs) in the Pearl River Delta. J. Eco-Environ. 2009, 18, 122–127. [Google Scholar]
- Meng, X.-Z.; Duan, Y.-P.; Yang, C.; Pan, Z.-Y.; Wen, Z.-H.; Chen, L. Occurrence, sources, and inventory of hexabromocyclododecanes (HBCDs) in soils from Chongming Island, the Yangtze River Delta (YRD). Chemosphere 2011, 82, 725–731. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Liu, L.-Y.; Zhang, K.; Liang, B.; Li, G.-L.; Chen, T.-H. Halogenated organic contaminants (HOCs) in sediment from a highly eutrophicated lake, China: Occurrence, distribution and mass inventories. Chemosphere 2012, 89, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hua, Z.; Wang, L.; Wang, Y.; Xie, Z.; Zhu, T. Relative effects of wind-induced disturbances and vegetation on tetrabromobisphenol A cycling in shallow lakes: Direct and indirect effects. Environ. Pollut. 2019, 252, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.B.; Wang, J.N.; Xu, Z.C.; Zhang, X.Y. Evaluation of Regional Aquatic Ecological Risk by DEHP Using Species Sensitivity Distribution. J. Eco-Environ. 2012, 21, 1082–1087. [Google Scholar]
- Li, Y.; Liu, Z.T.; Liu, X.H.; Zhang, Y.H.; Li, Y.D.; Gao, C. Derivation of environmental effect prediction for freshwater sediments in the Philippines. Chin. J. Environ. Res. 2014, 27, 790–796. [Google Scholar]
- Hu, W.X.; Liu, X.Y.; Chen, A.X.; Li, M.; Wang, X.P. Pollution characteristics and ecological risk assessment of DBP in Xi ’an section of Weihe River basin. Chin. J. Environ. Pollut. 2020, 46, 84–90. [Google Scholar]
- Zhang, H.W. Multi-Species Water Ecological Hazard Assessment of Tetrabromobisphenol A and 4-Tert-Butylphenol. Ph.D. Thesis, Northeast Normal University, Xi’an, China, 2014. [Google Scholar]
- Zhou, S.Y.; Zhang, Y.; Wei, C.; Ni, F.; Quan, X. Study on the toxic effects of tetrabromobisphenol A on swimming behavior of Brachydanio rerio var. Chin. J. Saf. Environ. 2016, 16, 387–391. [Google Scholar]
- Vighi, M.; Finizio, A.; Villa, S. The evolution of the Environmental Quality concept: From the US EPA Red Book to the European Water Framework Directive. Environ. Sci. Pollut. Res. 2006, 13, 9–14. [Google Scholar] [CrossRef]
- European Commission. Technical Guidance Document on Risk Assessment. Available online: https://echa.europa.eu/documents/10162/16960216/tgdpart2_2ed_en.pdf (accessed on 15 March 2020).
- US Environmental Protection Agency (EPA). Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses; US Environmental Protection Agency: Washington, DC, USA, 1985. [Google Scholar]
- The Organisation for Economic Co-operation and Development. Guidance Document for Aquatic Effects Assessment; Technical Report; Organization for Economic Co-operation and Development: Paris, France, 1995. [Google Scholar]
- Zhao, W.; Guo, Y.; Lu, S.; Yan, P.; Sui, Q. Recent advances in pharmaceuticals and personal care products in the surface water and sediments in China. Front. Environ. Sci. Eng. 2016, 10, 2. [Google Scholar] [CrossRef]
- Li, B.; Liu, R.; Gao, H.; Tan, R.; Zeng, P.; Song, Y. Spatial distribution and ecological risk assessment of phthalic acid esters and phenols in surface sediment from urban rivers in Northeast China. Environ. Pollut. 2016, 219, 409–415. [Google Scholar] [CrossRef]
- Davis, J.W.; Gonsior, S.; Marty, G.; Ariano, J. The transformation of hexabromocyclododecane in aerobic and anaerobic soils and aquatic sediments. Water Res. 2005, 39, 1075–1084. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, X.; Shen, Y.; Di, M.; Wang, J. Antibiotics in surface water and sediments from Hanjiang River, Central China: Occurrence, behavior and risk assessment. Ecotoxicol. Environ. Saf. 2018, 157, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, L.-H.; Liu, G.-Q.; Shi, L.; Guo, Y. Occurrence and Ecological Risk Assessment of Eight Endocrine-Disrupting Chemicals in Urban River Water and Sediments of South China. Arch. Environ. Contam. Toxicol. 2018, 75, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, N.; Guo, R.; Xu, H.; Zhang, Q.; Han, Z.; Feng, M.; Li, D.; Zhang, S.; Chen, J. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): Implication for eco-toxicity risk. Chemosphere 2018, 202, 255–263. [Google Scholar] [CrossRef] [PubMed]

| Zones | Number | Types | Sediment | Water Sample | |
|---|---|---|---|---|---|
| TOC/% | TBBPA (ng/g, dw) | TBBPA (ng/L) | |||
| Upper stream | 1 | Main stream | 1.328 | N.D. | N.D. |
| 2 | Tributary stream | 1.779 | 1.281 | N.D. | |
| 3 | Main stream | 1.544 | 0.111 | N.D. | |
| 4 | Tributary stream | 2.163 | N.D. | N.D. | |
| 5 | Main stream | 1.667 | N.D. | N.D. | |
| 6 | Tributary stream | 2.016 | 0.700 | N.D. | |
| 7 | Main stream | 0.584 | 0.190 | 1.295 | |
| 8 | Tributary stream | 1.279 | 0.127 | N.D. | |
| 9 | Main stream | 1.32 | 0.024 | 12.279 | |
| 10 | Main stream | 1.691 | 0.203 | 2.137 | |
| 11 | Tributary stream | 3.707 | 0.213 | N.D. | |
| 12 | Main stream | 1.931 | N.D. | N.D. | |
| Middle stream | 13 | Main stream | 1.34 | N.D. | N.D. |
| 14 | Tributary stream | 0.87 | 0.069 | N.D. | |
| 15 | Main stream | 1.576 | N.D. | N.D. | |
| 16 | Tributary stream | 0.665 | 0.062 | N.D. | |
| 17 | Main stream | 0.792 | 0.043 | N.D. | |
| 18 | Tributary stream | 2.234 | N.D. | N.D. | |
| 19 | Tributary stream | 1.301 | N.D. | N.D. | |
| 20 | Main stream | 1.562 | 0.052 | 0.716 | |
| 21 | Tributary stream | - | - | N.D. | |
| 22 | Main stream | 1.025 | N.D. | 1.377 | |
| 23 | Tributary stream | - | - | N.D. | |
| 24 | Main stream | 1.207 | N.D. | 1.493 | |
| 25 | Tributary stream | 1.257 | 0.432 | N.D. | |
| 26 | Tributary stream | 1.483 | 0.221 | 4.086 | |
| 27 | Main stream | 1.402 | 0.450 | 0.890 | |
| Lower stream | 28 | Main stream | 1.491 | 0.309 | 7.203 |
| 29 | Tributary stream | 1.772 | 0.614 | N.D. | |
| 30 | Main stream | 1.369 | N.D. | N.D. | |
| 31 | Tributary stream | 1.678 | 0.140 | N.D. | |
| 32 | Main stream | 1.261 | N.D. | N.D. | |
| 33 | Tributary stream | 1.128 | N.D. | N.D. | |
| 34 | Main stream | 1.219 | 0.265 | 1.523 | |
| 35 | Main stream | 1.878 | 0.241 | N.D. | |
| 36 | Main stream | 0.55 | 3.889 | N.D. | |
| Sample | Sample Collection Area | Sample Type | TBBPA/(ng/g) | References |
|---|---|---|---|---|
| China | ||||
| Chaohu Lake | Lake sediments | Max. 518 | [5] | |
| Taihu Lake | Lake sediments | 0.056–2.15 | [21] | |
| Xijiang River | River bottom sediments | N.D. –1.33 | [23] | |
| Beijiang River | River bottom sediments | 0.537–6.2 | [23] | |
| Beijing Qinghe | River bottom sediments | 0.2–22 | [19] | |
| Sediments | Erhai Lake | Lake sediments | 21–53 | [20] |
| Other countries | ||||
| Netherlands Scholt River | River bottom sediments | <0.1 | [16] | |
| Canada St. Lawrence River | River bottom sediments | 300 | [13] | |
| A River in Paris, France | Lake sediments | 0.04–0.13 | [17] | |
| Britain Six major Lakes | Lake sediments | 0.33–3.8 | [15] | |
| Spain Ebro River | River bottom sediments | N.D. –15 | [14] | |
| China | ||||
| Water | Taihu Lake | Lake | N.D. –1.12 | [21] |
| Beijing Qinghe | River | 23.9–224 | [25] | |
| Chaohu Lake | Lake | 850–4870 | [18] | |
| Dongjiang River | River | 1.11–2.83 | [26] | |
| Other countries | ||||
| England | Lake | 0.14–3.2 | [15] | |
| France | River | <0.035–0.068 | [17] |
| Species | Observation Endpoint | Exposure Time/d | Water Type | Experimental Site |
|---|---|---|---|---|
| Algae | NOEC/LOEC | ≥3 | Fresh water | Laboratory test |
| Invertebrate | NOEC/LOEC | ≥7 | Fresh water | Laboratory test |
| Vertebrate | NOEC/LOEC | ≥14 | Fresh water | Laboratory test |
| The Most Sensitive Species | Biological Classification | Endpoint | Standard Concentration mg/L | Data Source |
|---|---|---|---|---|
| Chlorella pyrenoidosa | Chlorophyta Chlorellaceae Chlorella | LOEC | 2.67 | ECOTOX |
| Scenedesmus acutus var. acutus | Chlorophyta Scenedesmaceae Scenedesmus | NOEC | 0.50 | ECOTOX |
| Daphnia magna | Arthropoda Daphniidae Daphnia | NOEC | 1.80 | ECOTOX |
| Carassius auratus | Chordata Cyprinidae Carassius | NOEC | 0.28 | ECOTOX |
| Gobiocypris rarus | Chordata Cyprinidae Gobiocypris | NOEC | 0.05 | [37] |
| Brachionus calyciflorus Pallas | Rotifera Brachionidae Brachionus | NOEC | 1.00 | [37] |
| Brachydanio rerio | Chordata Cyprinidae Danio | EC50 | 22.00 | [38] |
| Gammarus pulex | Arthropoda Gammaridae Gammarus | LC50 | 1.17 | ECOTOX |
| Pseudorasbora parva | Chordata Cyprinidae Pseudorasbora | LC50 | 0.86 | ECOTOX |
| Limnodrilus hoffmeisteri | Annelida Tubificidae Limnodrilus | EC50 | 2.92 | ECOTOX |
| Chironomus plumosus | Arthropoda Chironomidae Chironomus | LC50 | 0.13 | ECOTOX |
| Model | Equation | R2 | Standard Error of Estimate |
|---|---|---|---|
| Exponential Growth | Y = 0.70 + 315.57 × exp (0.0005 × x) | 0.9674 | 0.056 |
| Gaussian | Y = 0.88 × exp (−0.5 × ((x − 1.73/2.812) | 0.9752 | 0.049 |
| Weibull | Y= 1.00 × (1 − exp (−(abs(x + 1.24 + 6.80 × ln(2)^(1 / 3.17)) / 6.80)^3.17))) | 0.9765 | 0.052 |
| Sigmoid | Y = 0.98 / (1 + exp (−(x + 1.33) / 1.26)) | 0.9768 | 0.048 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, C.; Yuan, X.; Yang, S. Contamination Level, Distribution Characteristics, and Ecotoxicity of Tetrabromobisphenol A in Water and Sediment from Weihe River Basin, China. Int. J. Environ. Res. Public Health 2020, 17, 3750. https://doi.org/10.3390/ijerph17113750
Wang X, Li C, Yuan X, Yang S. Contamination Level, Distribution Characteristics, and Ecotoxicity of Tetrabromobisphenol A in Water and Sediment from Weihe River Basin, China. International Journal of Environmental Research and Public Health. 2020; 17(11):3750. https://doi.org/10.3390/ijerph17113750
Chicago/Turabian StyleWang, Xueli, Chenyang Li, Xiaoyu Yuan, and Shengke Yang. 2020. "Contamination Level, Distribution Characteristics, and Ecotoxicity of Tetrabromobisphenol A in Water and Sediment from Weihe River Basin, China" International Journal of Environmental Research and Public Health 17, no. 11: 3750. https://doi.org/10.3390/ijerph17113750
APA StyleWang, X., Li, C., Yuan, X., & Yang, S. (2020). Contamination Level, Distribution Characteristics, and Ecotoxicity of Tetrabromobisphenol A in Water and Sediment from Weihe River Basin, China. International Journal of Environmental Research and Public Health, 17(11), 3750. https://doi.org/10.3390/ijerph17113750




