Effects of COVID-19 Infection during Pregnancy and Neonatal Prognosis: What Is the Evidence?
Abstract
:1. Introduction
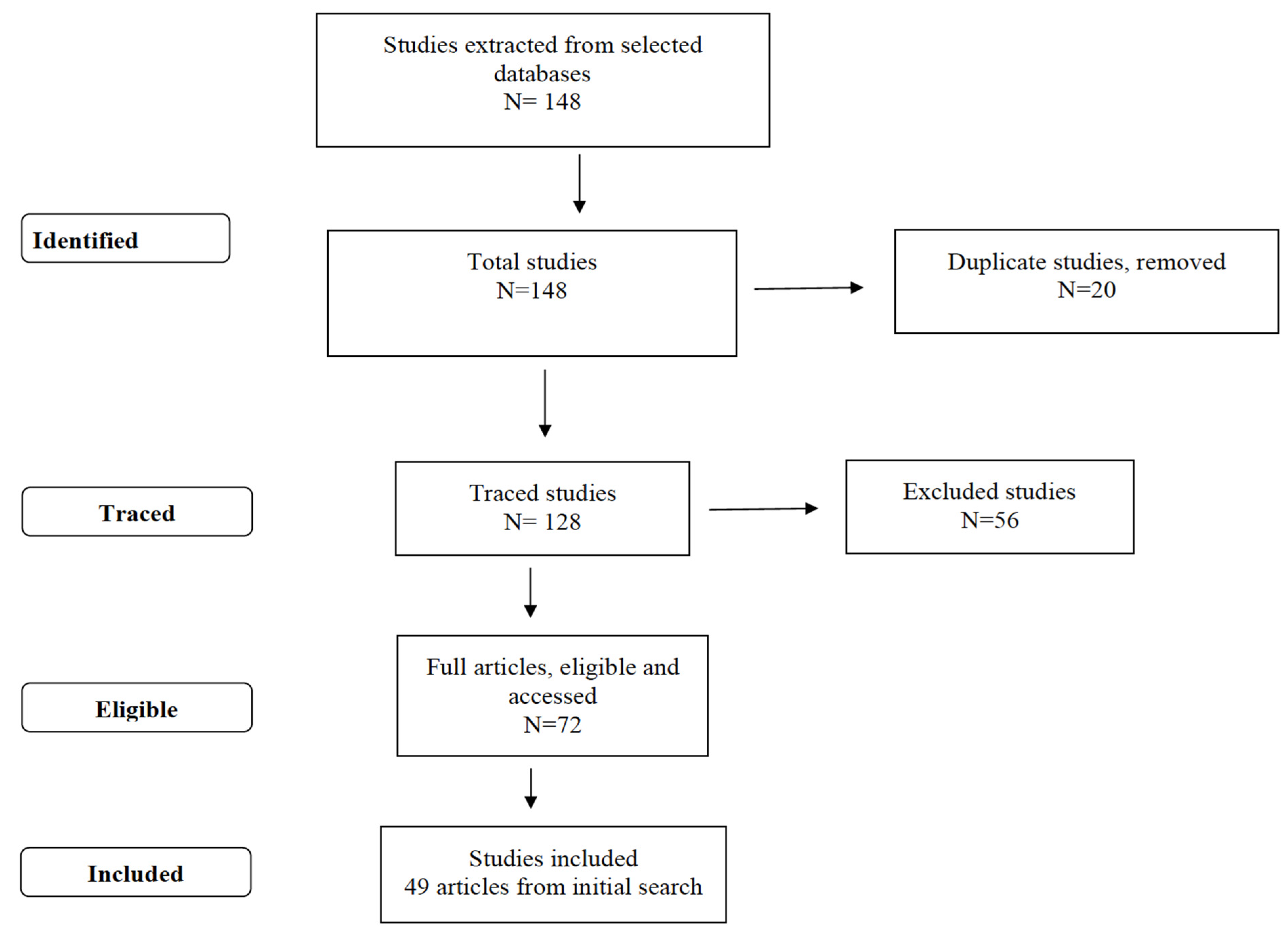
2. Materials and Methods
- Population (P) = pregnant women;
- Exposure (E) = COVID-19 infection;
- Comparison (C) = has not been an object of study;
- Outcome (O) = maternal and/or fetal infection by SARS-CoV-2.
3. Results
3.1. Findings from Case Reports
3.2. Findings from Descriptive Studies
3.3. Findings from Cross-Sectional Analytical Studies
3.4. Findings from Longitudinal Studies
4. Discussion
4.1. Characterization of Pregnant Women
4.2. Clinical Findings in Pregnant Women
4.3. Childbirth
4.4. Newborns
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus Disease 2019 (COVID-19). Situation Report—80. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200409-sitrep-80-covid-19.pdf?sfvrsn=1b685d64_2 (accessed on 10 May 2020).
- Centers for Disease Control and Prevention (CDC). Coronavirus. Available online: https://www.cdc.gov/coronavirus/index.html (accessed on 10 May 2020).
- Perlman, S. Another decade, another coronavirus. N. Engl. J. Med. 2020, 882, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical feature of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and infection. N. Engl. J. Med. 2014, 370, 2211–2218. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Qiao, J. What are the risks of COVID-19 infection in pregnant women? Lancet 2020, 395, 760–762. [Google Scholar] [CrossRef] [Green Version]
- Kontou, P.I.; Braliou, G.G.; Dimou, N.L.; Nikolopoulos, G.; Bagos, P.G. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics 2020, 10, 319. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.M.; McInerney, P.; Soares, C.B.; Khalil, H.; Parker, D. Methodology for JBI scoping reviews. In The Joanna Briggs Institute Reviewers’ Manual 2015; The Joanna Briggs Institute: Adelaide, Australia, 2015. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.; Lei, D.; Fang, C.; Li, C.; Wang, M.; Liu, Y.; Bao, Y.; Sun, Y.; Huang, J.; Guo, Y.; et al. Perinatal Transmission of COVID-19 Associated SARS-CoV-2: Should We Worry? Clin. Infect. Dis. 2020, ciaa226. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Huang, B.; Luo, D.J.; Li, X.; Yang, F.; Zhao, Y.; Nie, X.; Huang, B.X. Pregnant women with new coronavirus infection: A clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi 2020, 49, E005. [Google Scholar]
- Li, Y.; Zhao, R.; Zheng, S.; Chen, X.; Wang, J.; Sheng, X.; Zhou, J.; Cai, H.; Fang, Q.; Fu, F.; et al. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg Infect Dis. 2020, 26. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.; Kim, E.; Woo, K.; Park, H.; An, J. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) confirmed patient. Korean J. Anesth. 2020, in press. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Zhang, Z.; Zhu, F.; Tang, Y.; Shen, X. A case of 2019 Novel Coronavirus in a pregnant woman with preterm delivery. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambrano, L.; Fuentes-Barahona, I.C.; Bejarano-Torres, D.A.; Bustillo, C.; Gonzales, G.; Vallecillo-Chinchilla, G.; Sanchez-Martínez, F.E.; Valle-Reconco, J.A.; Sierra, M.; Bonilla-Aldana, D.K. A pregnant woman with COVID-19 in Central America. Travel Med. Infect. Dis. 2020, 101639. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.N.; Overcash, R.; Mokhtari, N.; Saeed, H.; Gold, S.; Auguste, T.; Mirza, M.U.; Ruiz, M.E.; Chahine, J.J.; Waga, M.; et al. An Uncomplicated Delivery in a Patient with Covid-19 in the United States. N. Engl. J. Med. 2020, 382, e34. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, R.; He, H.; Yao, Y.; Zheng, Y.; Wen, X.; Zhu, S. Anesthesia management in cesarean section for a patient with coronavirus disease 2019. J. Zhejiang Univ. 2020, 49, 249–252. [Google Scholar]
- Lu, D.; Sang, L.; Du, S.; Li, T.; Chang, Y.; Yang, X. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J. Med. Virol. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Yang, H.; Kong, J.; Yang, H. Chest CT Findings in a Pregnant Patient with 2019 Novel Coronavirus Disease. Balkan Med. J. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Raffaelli, F.; Tamburrini, E.; Biasucci, D.G.; Salvi, S.; Smargiassi, A.; Inchingolo, R.; Scambia, G.; Lanzone, A.; Testa, A.C.; et al. Clinical role of lung ultrasound for the diagnosis and monitoring of COVID-19 pneumonia in pregnant women. Ultrasound Obstet Gynecol. 2020, in press. [Google Scholar] [CrossRef]
- Lowe, B.; Bopp, B. COVID-19 vaginal delivery—A case report. Aust. N. Z. J. Obstet. Gynaecol. 2020, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Peng, L.; Siddique, R.; Nabi, G.; Nawsherwan; Xue, M.; Liu, J.; Han, G. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect. Control Hosp. Epidemiol. 2020, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalafat, E.; Yaprak, E.; Cinar, G.; Varli, B.; Ozisik, S.; Uzun, C.; Azap, A.; Koc, A. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obs. Gynecol. 2020, in press. [Google Scholar] [CrossRef] [Green Version]
- Karami, P.; Naghavi, M.; Feyzi, A.; Aghamohammadi, M.; Novin, M.S.; Mobaien, A.; Qorbanisani, M.; Karami, A.; Norooznezhad, A.H. Mortality of a pregnant patient diagnosed with COVID-19: A case report with clinical, radiological, and histopathological findings. Travel Med. Infect. Dis. 2020, in press. [Google Scholar] [CrossRef]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, R.; Ocampo, P.J.; González, B.L.; Santana-Cabrera, L. Pregnancy and perinatal outcome of a woman with COVID-19 infection. Rev. Clin. Esp. 2020, in press. [Google Scholar]
- Alzamora, M.C.; Paredes, T.; Caceres, D.; Webb, C.M.; Valdez, L.M.; Rosa, M.L. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am. J. Perinatol. 2020, in press. [Google Scholar]
- Xiong, X.; Wei, H.; Zhang, Z.; Chang, J.; Ma, X.; Gao, X.; Chen, Q.; Pang, Q. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19. J. Med. Virol. 2020, in press. [Google Scholar]
- Baud, D.; Greub, G.; Favre, G.; Gengler, C.; Jaton, K.; Dubruc, E.; Pomar, L. Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. JAMA 2020, e207233, in press. [Google Scholar] [CrossRef] [PubMed]
- Kirtsman, M.; Diambomba, Y.; Poutanen, S.M.; Malinowski, A.K.; Vlachodimitropoulou, E.; Parks, W.T.; Erdman, L.; Morris, S.K.; Shah, P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020, in press. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Tang, K.; Guo, Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020, piiS0163-4453, 30109–2, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Yu, N.; Li, W.; Kang, Q.; Xiong, Z.; Wang, S.; Lin, X.; Liu, Y.; Xiao, J.; Liu, H.; Deng, D.; et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020, 20, 559–564, in press. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. JAMA 2020, in press. [Google Scholar] [CrossRef]
- Chen, S.; Liao, E.; Shao, Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J. Med. Virol. 2020, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Li, L.; Wu, X.; Zheng, D.; Wang, J.; Yang, L.; Zheng, C. Pregnancy and Perinatal Outcomes of Women With Coronavirus Disease (COVID-19) Pneumonia: A Preliminary Analysis. Am. J. Roentgenol. 2020, 18, 1–6, in press. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef]
- Chen, L.; Qin, L.; Zheng, D.; Jiang, H.; Wei, Y.; Zou, L.; Feng, L.; Xiong, G.; Sun, G.; Wang, H.; et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. N. Engl. Med. 2020, 1–3. [Google Scholar] [CrossRef]
- Yan, J.; Guo, J.; Fan, C.; Juan, J.; Yu, X.; Li, J.; Feng, L.; Li, C.; Chen, H.; Qiao, Y.; et al. Coronavirus disease 2019 (COVID-19) in pregnant women: A report based on 116 cases. Am. J. Obs. Gynecol. 2020, in press. [Google Scholar] [CrossRef]
- Breslin, N.; Baptiste, C.; Gyamfi-Bannerman, C.; Miller, R.; Martinez, R.; Bernstein, K.; Ring, L.; Landau, R.; Purisch, S.; Friedman, A.M.; et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obs. Gynecol. MFM 2020, in press. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Huang, L.; Cheng, B.; Xia, Z.; Meng, Q. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: A case series of 17 patients. Can. J. Anaesth. 2020, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrazi, E.; Frigerio, L.; Savasi, V.; Vergani, P.; Prefumo, F.; Barresi, S.; Bianchi, S.; Ciriello, E.; Facchinetti, F.; Gervasi, M.T.; et al. Vaginal delivery in SARS-CoV-2 infected pregnant women in Northern Italy: A retrospective analysis. BJOG 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Wang, S.; Yang, Q.; Fan, C.; Liu, Y.; He, W.; Jiang, M.; Liu, C.; Zeng, W.; Wu, J.; et al. Clinical features and the maternal and neonatal outcomes of pregnant women with coronavirus disease 2019. medRxiv 2020. [Google Scholar] [CrossRef]
- Khan, S.; Jun, L.; Nawsherwan; Siddique, R.; Li, Y.; Han, G.; Xue, M.; Nabi, G.; Liu, J. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin. Microbiol. Infect. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Hantoushzadeh, S.; Shamshirsaz, A.A.; Aleyasin, A.; Seferovic, M.D.; Aski, S.K.; Arian, S.E.; Pooransari, P.; Ghotbizadeh, F.; Aalipour, S.; Soleimani, Z.; et al. Maternal Death Due to COVID-19 Disease. Am. J. Obstet. Gynecol. 2020, in press. [Google Scholar] [CrossRef]
- Xu, Q.; Shen, J.; Pan, L.; Lei, H.; Jiang, X.; Lu, W.; Yang, G.; Li, S.; Wang, Z.; Xiong, G.; et al. Coronavirus disease 2019 in pregnancy. Int. J. Infect. Dis. 2020, 95, 376–383. [Google Scholar]
- Wu, Y.; Liu, C.; Dong, L.; Zhang, C.; Chen, Y.; Liu, J.; Zhang, C.; Duan, C.; Zhang, H.; Mol, B.W.; et al. Coronavirus disease 2019 among pregnant Chinese women: Case series data on the safety of vaginal birth and breastfeeding. BJOG 2020, in press. [Google Scholar] [CrossRef]
- Yang, H.; Hu, B.; Zhan, S.; Yang, L.; Xiong, G. Effects of SARS-CoV-2 infection on pregnant women and their infants: A retrospective study in Wuhan, China. Arch. Pathol. Lab. Med. 2020, in press. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Li, W.; Zhou, Z.; Liu, S.; Rong, Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front. Med. 2020, 14, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiang, Y.; Wai, M.; Cheng, B.; Zhou, X.; Li, J.; Tian, J.; Dong, L.; Hu, R. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi 2020, 55, E009. [Google Scholar]
- Liu, H.; Liu, F.; Li, J.; Zhang, T.; Wang, D.; Lan, W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J. Infect. 2020, 80, E7–E13, in press. [Google Scholar] [CrossRef]
- Liao, J.; He, X.; Gong, Q.; Yang, L.; Zhou, C.; Li, J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID-19 pandemic. Int. J. Gynaecol. Obs. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, R.; Chen, J.; Xie, Y.; Zhang, S.; Wang, X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int. J. Gynaecol. Obs. 2020, in press. [Google Scholar] [CrossRef]
- Yue, L.; Han, L.; Li, Q.; Zhong, M.; Wang, J.; Wan, Z.; Chu, C.; Zeng, Y.; Peng, M.; Yang, L.; et al. Anaesthesia and infection control in cesarean section of pregnant women with coronavirus disease 2019 (COVID-19). medRxiv 2020. [Google Scholar] [CrossRef]
- Yang, H.; Sun, G.; Tang, F.; Peng, M.; Gao, Y.; Peng, J.; Xie, H.; Zhao, Y.; Jin, Z. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J. Infect. 2020, in press. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, in press. [Google Scholar] [CrossRef]
- Li, N.; Han, L.; Peng, M.; Lv, Y.; Ouyang, Y.; Liu, K.; Yue, L.; Li, Q.; Sun, G.; Chen, L.; et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: A case-control study. Clin. Infect. Dis. 2020, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, M.; Zhang, L.; Deng, G.; Han, C.; Shen, M.; Sun, H.; Zeng, F.; Zhang, W.; Chen, L.; Luo, Q.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection During Pregnancy In China: A Retrospective Cohort Study. medRxiv 2020. [Google Scholar]
- Laake, I.; Tunheim, G.; Robertson, A.H.; Hungnes, O.; Waalen, K.; Håberg, S.E.; Mjaaland, S.; Trogstad, L. Risk of pregnancy complications and adverse birth outcomes after maternal A(H1N1) pdm 09 influenza: A Norwegian population-based cohort study. BMC Infect. Dis. 2018, 18, 525. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Graham, A.L. Potential Maternal and Infant Outcomes from (Wuhan) Coronavirus 2019-nCoV Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 2020, 12, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidlöf, S.; Savchenko, J.; Brune, T.; Josefsson, H. COVID-19 in pregnancy with comorbidities: More liberal testing strategy is needed. Acta Obstet. Gynecol. Scand. 2020, in press. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Interim Considerations for Infection Prevention and Control of Coronavirus Disease 2019 (COVID-19) in Inpatient Obstetric Healthcare Settings. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html (accessed on 10 June 2020).
- WHO. Coronavirus Disease 2019 (COVID-19). Situation Report—72. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2 (accessed on 10 June 2020).
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, in press. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health. Special Considerations in Pregnancy and Post-Delivery. 12 May 2020. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/pregnancy-and-post-delivery/ (accessed on 10 June 2020).
- Ashokka, B. Care of the Pregnant Woman with COVID-19 in Labor and Delivery: Anesthesia, Emergency cesarean delivery, Differential diagnosis in the acutely ill parturient, Care of the newborn, and Protection of the healthcare personnel. Am. J. Obst. Gynec. 2020, in press. [Google Scholar]
- Groß, R.; Conzelmann, C.; Müller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Münch, J. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020. [Google Scholar] [CrossRef]
| Code | Reference | Study Design | Location | Level of Evidence | Limitations |
|---|---|---|---|---|---|
| 1 | Fan et al. (2020) [13] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 2 | Chen et al. (2020) [14] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 3 | Li et al. (2020) [15] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 4 | Lee et al. (2020) [16] | Case report | Asia | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 5 | Xiaotong et al. (2020) [17] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 6 | Zambrano et al. (2020) [18] | Case report | Honduras | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 7 | Iqbal et al. (2020) [19] | Case report | United States of America | Very Low | Small sample size; single setting; without long-term follow-up; without additional assessments of the virus in amniotic fluid, umbilical cord blood or placenta tissue |
| 8 | Kang et al. (2020) [20] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 9 | Lu et al. (2020) [21] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 10 | Liao et al. (2020) [22] | Case report | China | Very Low | Small sample size; only third-trimester pregnant women; without information on the delivery or the isolation conditions of newborns after delivery |
| 11 | Buonsenso et al. (2020) [23] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 12 | Lowe e Bopp (2020) [24] | Case report | Australia | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 13 | Khan et al. (2020) [25] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 14 | Kalafat et al. (2020) [26] | Case report | Turkey | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 15 | Karami et al. (2020) [27] | Case report | Iran | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 16 | Dong et al. (2020) [28] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 17 | González et al. (2020) [29] | Case report | Spain | Very Low | Small sample size; only third-trimester pregnant women; without information on the delivery or the isolation conditions of the newborn after delivery |
| 18 | Alzamora et al. (2020) [30] | Case report | Peru | Very Low | Small sample size; single setting; without long-term follow-up; without additional assessments of the virus in amniotic fluid, umbilical cord blood or placenta tissue |
| 19 | Xiong et al. (2020) [31] | Case report | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 20 | Baud et al. (2020) [32] | Case report | Switzerland | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 21 | Kirtsman et al. (2020) [33] | Case report | Canada | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 22 | Liu et al. (2020) [34] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 23 | Chen et al. (2020) [35] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 24 | Yu et al. (2020) [36] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 25 | Zeng et al. (2020) [37] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 26 | Chen, Liao and Shao (2020) [38] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 27 | Liu et al. (2020) [39] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up |
| 28 | Zhu et al. (2020) [40] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; with little information on the exams performed |
| 29 | Chen et al. (2020) [41] | Cross-sectional descriptive | China | Very Low | Small sample size; without long-term follow-up |
| 30 | Yan et al. (2020) [42] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; without long-term follow-up |
| 31 | Breslin et al. (2020) [43] | Cross-sectional descriptive | United States of America | Very Low | Small sample size; without long-term follow-up |
| 32 | Chen et al. (2020) [44] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; without long-term follow-up |
| 33 | Ferrazzi et al. (2020) [45] | Cross-sectional descriptive | Italia | Low | Without long-term follow-up; not all newborns were tested immediately after birth; without additional assessments of the virus in amniotic fluid, umbilical cord blood or placenta tissue |
| 34 | Nie et al. (2020) [46] | Cross-sectional descriptive | China | Very Low | Small sample size; not all newborns were tested for SARS-CoV-2; without long-term follow-up. |
| 35 | Khan et al. (2020) [47] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up. |
| 36 | Hantoushzadeh et al. (2020) [48] | Cross-sectional descriptive | Iran | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up. |
| 37 | Qianchenga et al. (2020) [49] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up; absence of criteria for allocation to groups |
| 38 | Wu et al. (2020) [50] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; without long-term follow-up |
| 39 | Yang et al. (2020) [51] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; without additional assessments of the virus in amniotic fluid, umbilical cord blood or placenta tissue |
| 40 | Liu et al. (2020) [52] | Cross-sectional descriptive | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; without long-term follow-up; absence of criteria for allocation to groups |
| 41 | Zhang et al. (2020) [53] | Cross-sectional analytical | China | Low | Small sample size; single setting; lack of clear criteria to include control groups; little information on postpartum care provided to newborns |
| 42 | Liu et al. (2020) [54] | Cross-sectional analytical | China | Low | Small sample size; no clear criteria for inclusion in the control group; absence of tomography images for monitoring therapeutic effects in ambulatory pregnant women |
| 43 | Liao et al. (2020) [55] | Cross-sectional analytical | China | Low | Small sample size; single setting; without long-term follow-up; only third-trimester pregnant women |
| 44 | Wu et al. (2020) [56] | Cross-sectional analytical | China | Very Low | Small sample size; single setting; without long-term follow-up |
| 45 | Yue et al. (2020) [57] | Cross-sectional analytical | China | Low | Small sample size; single setting; only third-trimester pregnant women |
| 46 | Yang et al. (2020) [58] | Cross-sectional analytical | China | Low | Small sample size; single setting; only third-trimester pregnant women; not all newborns were tested for SARS-CoV-2 |
| 47 | Shanes et al. (2020) [59] | Cross-sectional analytical | United States of America | Low | Small sample size; single setting; not all newborns were tested for SARS-CoV-2 |
| 48 | Lin et al. (2020) [60] | Case-control | China | Very Low | Small sample size; single setting; only third-trimester pregnant women; retrospective; without clear inclusion criteria for cases and controls |
| 49 | Yin et al. (2020) [61] | Cohort | China | Low | Small sample size; single setting; without long-term follow-up; incomplete information on pregnant women; controls are 35 non-pregnant women of fertile age |
| Code | Study Design | Setting and Participants | Clinical, Laboratory and Imaging Findings of Women and Type of Delivery | Newborns’ Clinical and Laboratory Findings |
|---|---|---|---|---|
| [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] | Case report | Setting: China (11/50%), Asia (1/5%), The Netherlands (1/5%), United States of America (1/5%), Turkey (1/5%), Iran (1/5%), Peru (1/5%), Italy (1/5%), Australia (1/5%), Switzerland (1/5%), and Canada (1/5%); - Pregnant women: 29 COVID-19 infected pregnant women with laboratory confirmation (29/100%), in the second (5/17%) or third (24/83%) trimester of pregnancy, mild or moderate (22/75%) and severe (5/17%) pneumonia; - 27 newborns: tested for SARS-CoV-2 (26/96%). | - Pregnancy associated comorbidity (27/93% assessed): no comorbidity (17/63%), fetal distress (5/16%), placenta previa (2/7%), gestational diabetes (1/4%), gestational hypertension (1/4%), prelabor rupture of membranes (1/4%), and thalassemia (1/4%); - Pre-existing diseases not related to pregnancy: Obesity (1/4%) and familial neutropenia (1/4%); - Signs and symptoms (29/100% assessed women): asymptomatic (1/3%); fever at admission (21/72%), cough (18/62%), post-partum fever (6/21%), myalgia (6/21%), dyspnea (5/17%), shivers (4/14%), sore throat (3/10%), chest pain (3/10%), fatigue (2/7%), malaise (1/3%), loss of taste and/or smell (1/3%); - Imaging (25/86% assessed women): suggestive chest CAT scan (24/96%); - Laboratory exams (26/90% assessed women): increased reactive C protein (18/69%), lymphocytopenia (15/58%), leukocytosis (6/23%), neutrophilia (5/19%), interleukin 6 (2/8%), elevated alanine transaminase (1/4%), immunoglobulin G (1/4%) and immunoglobulin M (1/4%); - Delivery (27/100 deliveries): C-section (21/78%)—due to comorbidities associated with pregnancy (10/50%) and (10/50%) due to the infection; vaginal (6/22%). - Maternal death (1/4%). | - 27 newborns - 24 assessed: normal APGAR index (22/92%), normal weight (18/75%), premature (10/41%); - 26 newborns tested: negative for SARS-CoV-2 (24/92%); positive (2/8%); - Neonatal death (2/7%); - Isolation of mother and newborn (13/48%); - Analysis of the placenta of 6 newborns: no alterations infection-related (6/100%). |
| [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] | Cross-sectional Descriptive | - Settings: China (16/85%), United States of America (1/5%), Italy (1/5%), and Iran (1/5%); - Pregnant women: 546 COVID-19 infected pregnant women, laboratorial diagnosis (409/75%), clinical diagnosis (109/20%), first trimester of pregnancy (39/7%), second trimester of pregnancy (44/8%), third trimester of pregnancy (463/85%), mild or moderate (482/88%) and severe (41/7%) pneumonia; - 429 newborns tested for SARS-CoV-2 (345/81%). | - Pregnancy related comorbidities (398/73% assessed): no comorbidity (139/35%), fetal distress (30/8%), gestational diabetes (30/7%), prelabor rupture of membranes (16/4%), gestational hypertension (12/3%), preeclampsia (7/2%), anemia (5/1%), uterine scar (4/1%), umbilical cord prolapse (4/1%), complete placenta previa (1/0,3%), thalassemia (1/0.3%), and multiple organ dysfunction syndrome/stillbirth (1/0.3%); - Pre-existing diseases not related to pregnancy: hepatitis B infection (4/1%), blood coagulation disorder (2/0.5%), influenza (2/0.5%), hypothyroidism (2/0.5%), schistosomiasis infection (1/0.3%), and hypoproteinemia (1/0.3%); - Signs and symptoms (512/94% assessed women): asymptomatic (55/11%), fever at admission (290/57%), cough (230/45%), dyspnea (65/13%), fatigue (55/11%), myalgia (45/9%), chest pain (38/7%), post-partum fever (23/5%), diarrhea (27/5%), sore throat (19/4%), malaise (5/1%), coryza (2/0.3%), and expectoration (2/0.3%); - Imaging (404/74% assessed women): suggestive chest CAT scan (377/93%); - Laboratory exams (402/74% assessed women): increased Reactive C protein (226/56%), lymphocytopenia (160/40%), leukocytosis (107/27%), elevated alanine transaminase (38/9%), elevated aspartate transaminase (38/9%), neutrophilia (9/2%), immunoglobulin G (5/1%), immunoglobulin M (4/1%), and interleukin 6 (4/1%); - Deliveries (421 deliveries): C-section (273/64%)—due to comorbidities associated with pregnancy (128/46%), due to the infection (74/27%), no information on motive (71/25%); vaginal (148/35%). - Maternal death (7/1%). | - Spontaneous abortion on the 5th week of pregnancy (1) - 429 newborns: normal APGAR index (417/97%), premature (74/17%); - Weight (307/72%): normal birth weight (292/95%), low birth weight (16/5%); - Newborns tested (345/80%): SARS-CoV-2 negative (338/98%), positive (7/2%); - Neonatal death: (8/2%); - 107 pregnant women assessed concerning isolation of which 97 were isolated from newborns; - Analysis of the placenta of 32 newborns: no alterations (32/100%). |
| [53,54,55,56,57,58,59] | Cross-sectional analytical | - Setting: China (6/86%) and United States of America (1/14%); - Pregnant women: 133 COVID-19 infected pregnant women, laboratorial diagnosis (104/78%), clinical diagnosis (29/22%), first trimester of pregnancy (3/2%), second trimester of pregnancy (23/17%), third trimester of pregnancy (107/80%), mild or moderate pneumonia (132/99%), or severe pneumonia (1/1%); - 108 newborns tested for SARS-CoV-2 (102/94%). | - Pregnancy related comorbidities (116/87% assessed women): no comorbidity (75/65%), gestational diabetes (8/7%), prelabor rupture of membranes (6/5%), gestational hypertension (4/3%), threat of abortion (3/3%), fetal distress (3/3%), uterine scar (2/2%), B-Lynch suture or other compression suture (2/2%), preeclampsia (1/1%), asphyxia (1/1%), and gestational cholestasis (1/1%); - Pre-existing diseases not related to pregnancy: asthma (2/2%) and hepatitis B infection (1/1%). - Signs and symptoms (101/75% assessed women): asymptomatic (18/19%), fever at admission (31/31%), post-partum fever (29/29%), cough (27/27%), dyspnea (5/5%), fatigue (5/5%), chest pain (1/1%); - Imaging (101/75% assessed women): suggestive chest CAT scan (96/95%); - Laboratory exams (74/63% assessed women): increased Reactive C protein (58/78%), lymphocytopenia (57/77%), neutrophilia (57/77%), and leukocytosis (17/23%); - Deliveries (106 deliveries): C-section (59/65%)—due to comorbidities associated with pregnancy (10/17%), due to infection (19/32%), no information on motive (30/51%); vaginal (32/35%). | - 108 newborns: normal APGAR index (107/100%); - Weight (71/76%): normal birth weight (65/92%) and low birth weight (6/8%); - Prematurity: premature (8/7%); - Newborns tested (102/94%): SARS-CoV-2 negative test (102/100%); - Neonatal death (0); - 23 pregnant women were assessed concerning isolation and 20 were isolated from newborns; - Analysis of the placenta of 16 newborns: no alterations (16/100%). |
| [60] | Case-control | - Setting: China; - Pregnant women: 16 pregnant women infected by COVID-19, laboratory diagnosis (16/100%), in the third trimester of pregnancy (16/100%), mild or moderate pneumonia (16/100%); - 17 newborns: tested for SARS-CoV-2 (3/18%). | - Pregnancy related comorbidities (16/100% pregnant women assessed): no comorbidities (5/31%), gestational diabetes (3/19%), gestational hypertension (2/13%), preeclampsia (1/6%), prelabor rupture of membranes (1/6%), Hepatitis B (1/6%); - Medical background: hypothyroidism (2/13%) and sinus tachycardia (1/6%); - Signs and symptoms (16/100% pregnant women studied): asymptomatic (4/25%), fever at admission (4/25%), post-partum fever (8/50%); - Imaging (16/100% pregnant women studied): suggestive chest CAT scan (10/63%); - Laboratory exams (16/100% pregnant women studied): increased reactive C protein (16/100%), neutrophilia (16/100%), lymphocytopenia (2/13%); - Deliveries (16 deliveries): C-section (14/87%)—does not mention the indication; vaginal (3/13%). | - 17 newborns: normal APGAR index (17/100%), normal weight (14/82%), premature (3/18%); - Newborns tested (3/18%): SARS-CoV-2 negative (3/100%); - Neonatal deaths (0); - pregnant women assessed for isolation (0); - Analysis of the placenta (0). |
| [61] | Cohort | - Setting: China - Pregnant women: 31 pregnant women infected by COVID-19, laboratory diagnosis (31/100%), in the first trimester of pregnancy (4/13%), in the second trimester (5/16%), in the third trimester (22/71%), mild or moderate pneumonia (21/68%), severe pneumonia (10/32%); - 17 newborns tested for SARS-CoV-2 (17/100%). | - Pregnancy related comorbidities (31/100% pregnant women assessed): no comorbidities (28/90%) and gestational hypertension (1/3%). - Medical background: cardiovascular disease (1/3%) and diabetes (1/3%); - Signs and symptoms (31/100% pregnant women studied): asymptomatic (5/16%), fever at admission (17/55%), cough (15/48%), dyspnea (8/26%), fatigue (6/19%), expectoration (5/16%), myalgia (3/10), diarrhea (2/6%); - Imaging (31/100% pregnant women studied): Suggestive CT scan (31/100%); - Laboratory exams (31/100% pregnant women studied): neutrophilia (10/32%), increased aspartate transaminase (7/23%), - Deliveries (17): C-section (13/76%)—no description on the indication; vaginal (4/24%). | - 17 newborns: normal APGAR index (16/94%), normal weight (16/94%), premature (5/29%); - Newborns tested (17/100%): SARS-CoV-2 negative (17/100%); - neonatal death (0); - Pregnant women analyzed for isolation (0); - Analysis of the placenta (0). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes de Sousa, Á.F.; Carvalho, H.E.F.d.; Oliveira, L.B.d.; Schneider, G.; Camargo, E.L.S.; Watanabe, E.; de Andrade, D.; Fernandes, A.F.C.; Mendes, I.A.C.; Fronteira, I. Effects of COVID-19 Infection during Pregnancy and Neonatal Prognosis: What Is the Evidence? Int. J. Environ. Res. Public Health 2020, 17, 4176. https://doi.org/10.3390/ijerph17114176
Lopes de Sousa ÁF, Carvalho HEFd, Oliveira LBd, Schneider G, Camargo ELS, Watanabe E, de Andrade D, Fernandes AFC, Mendes IAC, Fronteira I. Effects of COVID-19 Infection during Pregnancy and Neonatal Prognosis: What Is the Evidence? International Journal of Environmental Research and Public Health. 2020; 17(11):4176. https://doi.org/10.3390/ijerph17114176
Chicago/Turabian StyleLopes de Sousa, Álvaro Francisco, Herica Emilia Félix de Carvalho, Layze Braz de Oliveira, Guilherme Schneider, Emerson Lucas Silva Camargo, Evandro Watanabe, Denise de Andrade, Ana Fátima Carvalho Fernandes, Isabel Amélia Costa Mendes, and Inês Fronteira. 2020. "Effects of COVID-19 Infection during Pregnancy and Neonatal Prognosis: What Is the Evidence?" International Journal of Environmental Research and Public Health 17, no. 11: 4176. https://doi.org/10.3390/ijerph17114176
APA StyleLopes de Sousa, Á. F., Carvalho, H. E. F. d., Oliveira, L. B. d., Schneider, G., Camargo, E. L. S., Watanabe, E., de Andrade, D., Fernandes, A. F. C., Mendes, I. A. C., & Fronteira, I. (2020). Effects of COVID-19 Infection during Pregnancy and Neonatal Prognosis: What Is the Evidence? International Journal of Environmental Research and Public Health, 17(11), 4176. https://doi.org/10.3390/ijerph17114176











