Lung Function Testing and Prediction Equations in Adult Population from Maputo, Mozambique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Data Analysis
2.4. Ethics Approval and Consent to Participate
3. Results
3.1. Characteristics of the Study Participants
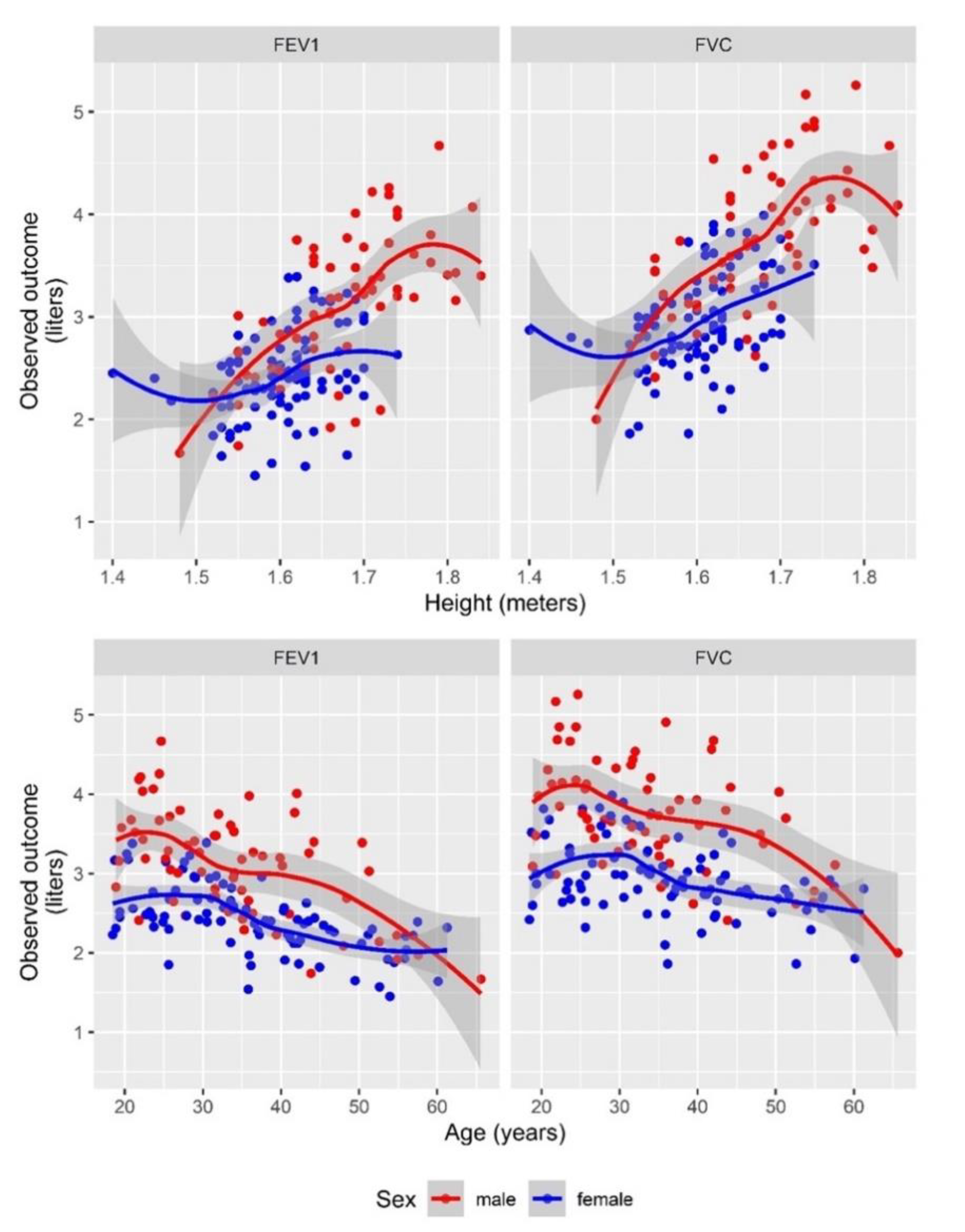
3.2. Mozambican Spirometric Reference Equations
3.3. Lung Impairment in Mozambican Sample: Type and Severity Grading
3.4. Comparison of Spirometry Results Based on Different Reference Standards
3.5. Validation of Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brusasco, V.; Crapo, R.; Viegi, G.; American Thoracic Society; European Respiratory Society. Coming together: The ATS/ERS consensus on clinical pulmonary function testing. Eur. Respir. J. 2005, 26, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.G.; Stocks, J.; Hall, G.L.; Culver, B.; Steenbruggen, I.; Carter, K.W.; Thompson, B.R.; Graham, B.L.; Miller, M.R.; Ruppel, G.; et al. The Global Lung Function Initiative (GLI) Network: Bringing the world’s respiratory reference values together. Breathe 2017, 13, e56–e64. [Google Scholar] [CrossRef]
- Barr, R.G.; Stemple, K.J.; Mesia-Vela, S.; Basner, R.C.; Derk, S.J.; Henneberger, P.K.; Milton, D.K.; Taveras, B. Reproducibility and validity of a handheld spirometer. Respir. Care 2008, 53, 433–441. [Google Scholar] [PubMed]
- Myers, J.E. Differential ethnic standards for lung functions, or one standard for all? S. Afr. Med. J. 1984, 65, 768–772. [Google Scholar] [PubMed]
- Khosa, C.; Bhatt, N.; Massango, I.; Azam, K.; Saathoff, E.; Bakuli, A.; Riess, F.; Ivanova, O.; Hoelscher, M.; Rachow, A. Development of chronic lung impairment in Mozambican TB patients and associated risks. BMC Pulm. Med. 2020, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Rachow, A.; Ivanova, O.; Wallis, R.; Charalambous, S.; Jani, I.; Bhatt, N.; Kampmann, B.; Sutherland, J.; Ntinginya, N.E.; Evans, D.; et al. TB sequel: Incidence, pathogenesis and risk factors of long-term medical and social sequelae of pulmonary TB—A study protocol. BMC Pulm. Med. 2019, 19, 4. [Google Scholar] [CrossRef]
- Cooper, B.G. An update on contraindications for lung function testing. Thorax 2011, 66, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Leuppi, J.D.; Miedinger, D.; Chhajed, P.N.; Buess, C.; Schafroth, S.; Bucher, H.C.; Tamm, M. Quality of spirometry in primary care for case finding of airway obstruction in smokers. Respiration 2010, 79, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Musafiri, S.; Van Meerbeeck, J.P.; Musango, L.; Derom, E.; Brusselle, G.; Joos, G.; Rutayisire, C. Spirometric reference values for an east-African population. Respiration 2013, 85, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Fawibe, A.E.; Odeigah, L.O.; Saka, M.J. Reference equations for spirometric indices from a sample of the general adult population in Nigeria. BMC Pulm. Med. 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigby, R.A.; Stasinopoulos, D.M. Generalized additive models for location, scale and shape. J. R. Stat. Soc. Ser. C (Appl. Stat.) 2005, 54, 507–554. [Google Scholar] [CrossRef] [Green Version]
- Quanjer, P.H.; Stocks, J.; Cole, T.J.; Hall, G.L.; Stanojevic, S. Global Lungs Initiative Influence of secular trends and sample size on reference equations for lung function tests. Eur. Respir. J. 2011, 37, 658–664. [Google Scholar] [CrossRef] [Green Version]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An. Introduction to Statistical Learning: With Applications in R.; Springer Texts in Statistics; Springer: New York, NY, USA, 2013; Volume 103, ISBN 978-1-4614-7137-0. [Google Scholar]
- Fox, J. Applied Regression Analysis and Generalized Linear Models; SAGE: Los Angeles, CA, USA, 2016; ISBN 1452205663. [Google Scholar]
- Fox, J.; Weisberg, S.; Fox, J. An. R Companion to Applied Regression; SAGE: Thousand Oaks, CA, USA, 2019; ISBN 141297514X. [Google Scholar]
- Efron, B.; Tibshirani, R. An. Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1994; ISBN 0412042312. [Google Scholar]
- Stanojevic, S.; Quanjer, P.; Miller, M.R.; Stocks, J. The Global Lung Function Initiative: Dispelling some myths of lung function test interpretation. Breathe 2013, 9, 462–474. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; Van Der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- C-P Criée, A.; Baur, X.; Berdel, D.; Bösch, D.; Gappa, M.; Haidl, P.; Husemann, K.; Jörres, R.A.; Kabitz, H.; Kardos, P.; et al. Leitlinie zur Spirometrie Leitlinie der Deutschen Atemwegsliga, der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin und der Deutschen Gesellschaft für Arbeitsmedizin und Umweltmedizin zur Spirometrie of Occupational and Environmental Medicine. Pneumologie 2015, 69, 147–164. [Google Scholar]
- Van Schalkwyk, E.M.; Schultz, C.; Joubert, J.R.; White, N.W. Guideline for Office Spirometry in Adults, 2004. S. Afr. Med. J. 2004, 94, 576–587. [Google Scholar]
- Schwartz, J.; Katz, S.A.; Fegley, R.W.; Tockman, M.S. Sex and race differences in the development of lung function. Am. Rev. Respir. Dis. 1988, 138, 1415–1421. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S. Do the Global Lung Function Initiative 2012 equations fit my population? Eur. Respir. J. 2016, 48, 1782–1785. [Google Scholar] [CrossRef]
- Miller, M.R. Choosing and using lung function prediction equations. Eur. Respir. J. 2016, 48, 1535–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C.; Steyerberg, E.W. The number of subjects per variable required in linear regression analyses. J. Clin. Epidemiol. 2015, 68, 627–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Saad, H.; Tfifha, M.; HarrabI, I.; Tabka, Z.; Guenard, H.; Hayot, M.; Zbidi, A. Facteurs influençant les variables ventilatoires des Tunisiennes âgées de 45 ans et plus. Rev. Mal. Respir. 2006, 23, 324–338. [Google Scholar] [CrossRef]
- Pérez-Padilla, R.; Valdivia, G.; Muiño, A.; López, M.V.; Márquez, M.N.; Montes de Oca, M.; Tálamo, C.; Lisboa, C.; Pertuzé, J.; Jardim, J.R.B.; et al. Spirometric Reference Values in 5 Large Latin American Cities for Subjects Aged 40 Years or Over. Arch. Bronconeumol. (Engl. Ed.) 2006, 42, 317–325. [Google Scholar] [CrossRef]
- Swanney, M.P.; Miller, M.R. Adopting universal lung function reference equations. Eur. Respir. J. 2013, 901–903. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Male (n = 63) | Female (n = 92) | Total (n = 155) |
|---|---|---|---|
| Age (years) | 33.83 (SD 10.74) | 36.13 (SD 11.12) | 35.20 (SD 10.99) |
| Height (meters) | 1.67 (SD 0.08) | 1.60 (SD 0.06) | 1.63 (SD 0.08) |
| Weight (kg s) | 65.52 (SD 9.80) | 69.24 (SD 15.68) | 67.73 (SD 13.69) |
| BMI (kg/sq. meters) | 23.43 (SD 3.70) | 26.95 (SD 5.60) | 25.52 (SD 5.20) |
| Age group | |||
| <30 years | 27 (42.86%) | 30 (32.61%) | 57 (36.77%) |
| 30–40 years | 20 (31.75%) | 28 (30.43%) | 48 (30.97%) |
| ≥40 years | 16 (25.39%) | 34 (39.96%) | 50 (32.26%) |
| BMI Class * | |||
| Underweight | 2 (3.17%) | 2 (2.17%) | 4 (2.58%) |
| Normal | 45 (71.43%) | 38 (41.30%) | 83 (53.55%) |
| Overweight | 11 (17.46%) | 28 (30.43%) | 39 (25.16%) |
| Obese | 5 (7.94%) | 24 (26.09%) | 29 (18.71%) |
| Smoking | |||
| Never Smoked | 49 (77.78%) | 87 (94.57%) | 136 (87.74%) |
| Past Smoker | 8 (12.70%) | 5 (5.43%) | 13 (8.39%) |
| Current Smoker | 6 (9.52%) | 0 (0%) | 6 (3.87%) |
| Marital Status | |||
| Single | 25 (39.68%) | 39 (42.39%) | 64 (41.29%) |
| Married | 11 (17.46%) | 11 (11.96%) | 22 (14.19%) |
| Living with spouse/partner | 25 (39.68%) | 37 (40.22%) | 62 (40.00%) |
| Widowed | 2 (3.17%) | 5 (5.43%) | 7 (4.52%) |
| Education | |||
| No formal education | 0 (0%) | 2 (2.17%) | 2 (1.29%) |
| Grades 1–5 | 5 (7.94%) | 24 (26.09%) | 29 (18.71%) |
| Grades 6–10 | 27 (42.86%) | 43 (46.74%) | 70 (45.16%) |
| Grades 11–12 | 17 (26.98%) | 20 (21.74%) | 37 (23.87%) |
| Vocational | 8 (12.70%) | 2 (2.17%) | 10 (6.45%) |
| University | 6 (9.52%) | 1 (1.09%) | 7 (4.52%) |
| HIV status (self-reported) (n = 108, 47 missing observations) | |||
| Negative | 27 (72.97%) | 40 (56.34%) | 67 (62.04%) |
| Positive | 10 (27.03%) | 31 (43.66%) | 41 (37.96%) |
| Worked in Mines | |||
| No | 61 (96.83%) | 92 (100%) | 153 (98.71%) |
| Yes | 2 (3.17%) | 0 (0%) | 2 (1.29%) |
| Spirometric Parameters ** | |||
| FVC (L) | 3.77 (SD 0.69) | 2.94 (SD 0.46) | 3.28 (SD 0.70) |
| FVC (% of predicted) | 90.82 (SD 11.43) | 88.62 (SD 11.76) | 89.51 (SD 11.65) |
| FEV1 (L) | 3.12 (SD 0.67) | 2.43 (SD 0.42) | 2.71 (SD 0.63) |
| FEV1 (% of predicted) | 91.28 (SD 13.69) | 95.53 (SD 13.02) | 93.80 (SD 13.41) |
| FEV1/FVC ratio | 0.83 (SD 0.06) | 0.83 (SD 0.06) | 0.83 (SD 0.06) |
| Outcome (Sex Specific) | South African (Black) Population | Mozambique (Local) Population |
|---|---|---|
| FVC (Males) | −3.08 − 0.024 × Age + 4.8 × Height; RSS = 0.54 | −2.271 − 0.019 × Age + 3.989 × Height; RSS = 0.43; adj Rsquare = 0.61 |
| FVC (Females) | −3.04 − 0.023 × Age + 4.5 × Height; RSS = 0.41 | −2.761 − 0.019 × Age + 3.989 × Height; RSS = 0.43; adj Rsquare = 0.61 |
| FEV1 (Males) | −0.54 − 0.027 × Age + 2.9 × Height; RSS = 0.46 | −3.504 − 0.023 × Age + 4.426 × Height; RSS = 0.37; adj Rsquare = 0.65 |
| FEV1 (Females) | −1.87 − 0.028 × Age + 3.4 × Height; RSS = 0.39 | −0.170 − 0.023 × Age + 2.150 × Height; RSS = 0.37; adj Rsquare = 0.65 |
| Ratio FEV1/FVC (Not sex specific) | - | 0.921 − 0.0027 × Age; RSS = 0.06; adj Rsquare = 0.22 |
| Impairment Type and Severity Grading, N = 155 | Mozambique—Local % (n/N) | GLI—Others % (n/N) | South Africa—Black % (n/N) |
|---|---|---|---|
| Normal | 89.7 (139/155) | 72.9 (113/155) | 74.8 (116/155) |
| Obstruction—Mild | 5.2 (8/155) | 2.6 (4/155) | 5.8 (9/155) |
| Obstruction—Moderate | 0.6 (1/155) | 1.9 (3/155) | 0.6 (1/155) |
| Obstruction—Severe | 0.0 (0/155) | 1.3 (2/155) | 0.0 (0/155) |
| Restriction—Mild | 4.5 (7/155) | 15.5 (24/155) | 12.9 (20/155) |
| Restriction—Moderate | 0.0 (0/155) | 3.2 (5/155) | 3.9 (6/155) |
| Restriction—Severe | 0.0 (0/155) | 0.0 (0/155) | 0.0 (0/155) |
| Mixed—Mild | 0.0 (0/155) | 0.0 (0/155) | 0.0 (0/155) |
| Mixed—Moderate | 0.0 (0/155) | 0.6 (1/155) | 1.3 (2/155) |
| Mixed—Severe | 0.0 (0/155) | 1.9 (3/155) | 0.6 (1/155) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, O.; Khosa, C.; Bakuli, A.; Bhatt, N.; Massango, I.; Jani, I.; Saathoff, E.; Hoelscher, M.; Rachow, A. Lung Function Testing and Prediction Equations in Adult Population from Maputo, Mozambique. Int. J. Environ. Res. Public Health 2020, 17, 4535. https://doi.org/10.3390/ijerph17124535
Ivanova O, Khosa C, Bakuli A, Bhatt N, Massango I, Jani I, Saathoff E, Hoelscher M, Rachow A. Lung Function Testing and Prediction Equations in Adult Population from Maputo, Mozambique. International Journal of Environmental Research and Public Health. 2020; 17(12):4535. https://doi.org/10.3390/ijerph17124535
Chicago/Turabian StyleIvanova, Olena, Celso Khosa, Abhishek Bakuli, Nilesh Bhatt, Isabel Massango, Ilesh Jani, Elmar Saathoff, Michael Hoelscher, and Andrea Rachow. 2020. "Lung Function Testing and Prediction Equations in Adult Population from Maputo, Mozambique" International Journal of Environmental Research and Public Health 17, no. 12: 4535. https://doi.org/10.3390/ijerph17124535






