Assessment of Ketamine and its Enantiomers in an Organophosphate-Based Rat Model for Features of Gulf War Illness
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. DFP Exposure
2.4. Experimental Design
2.5. Open Field Test (OFT)
2.6. Forced Swim Test (FST)
2.7. Sucrose Preference Test (SPT)
2.8. Elevated Plus Maze (EPM)
2.9. Data Analysis
3. Results
3.1. Chronic Effects of Repeated, Low-Dose DFP Exposure on Rat Behavior
3.2. Effects of KET on Locomotor Behavior in GWI Rats
3.3. Acute Effect of KET on FST Behavior in GWI Rats
3.4. Sustained Effect of KET on FST Behavior in GWI Rats
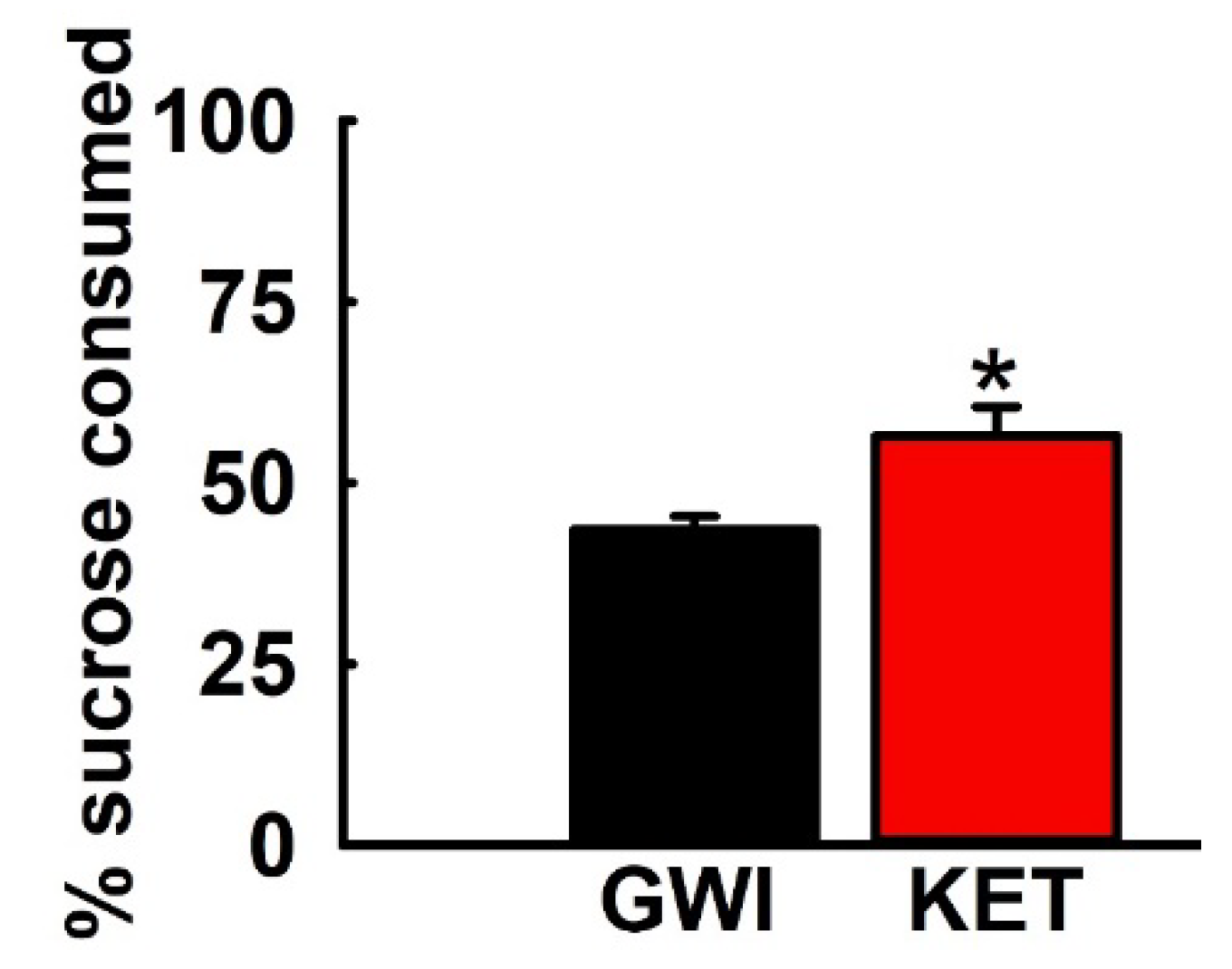
3.5. Effect of KET on Sucrose Comsumption in GWI Rats
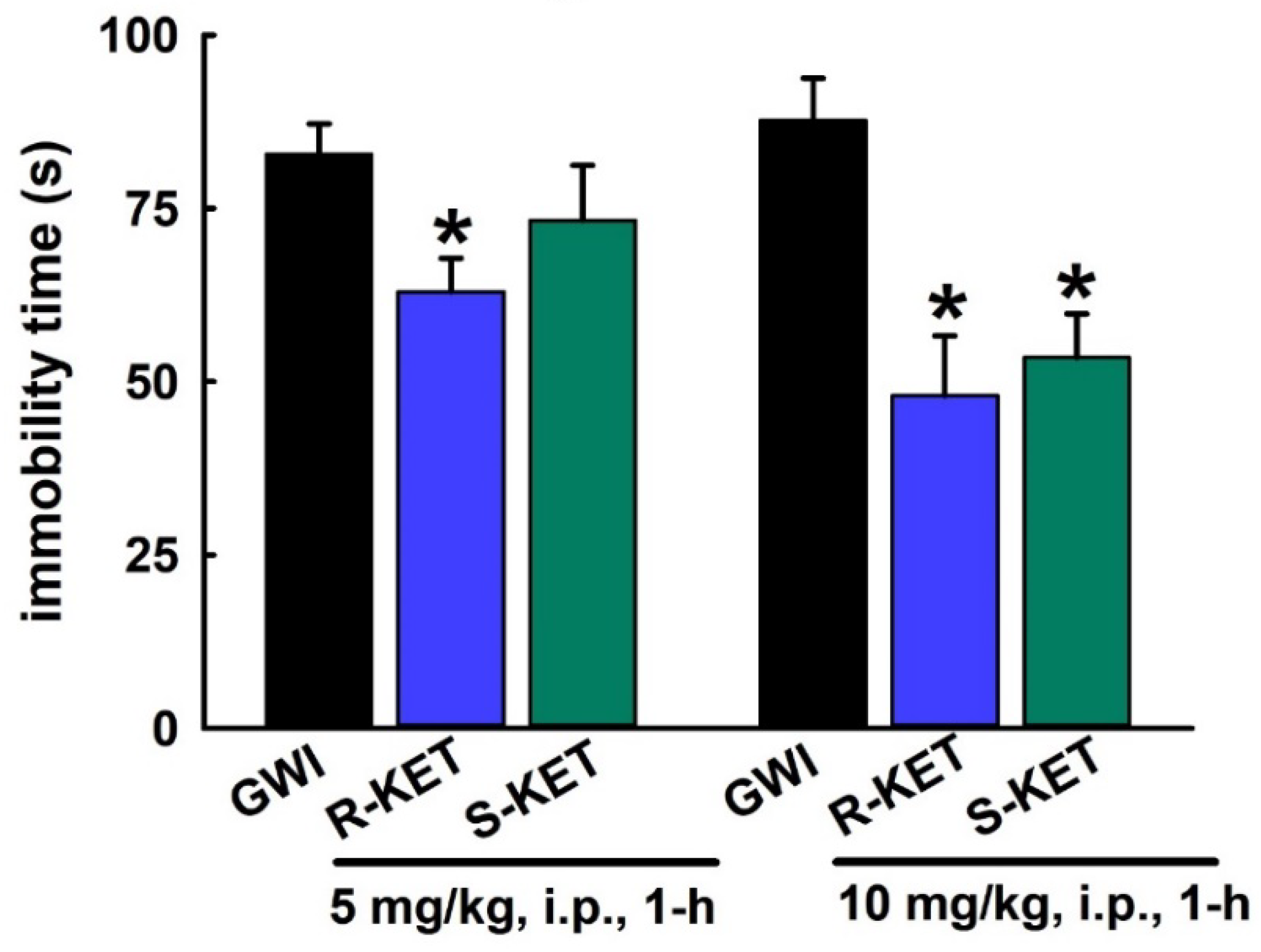
3.6. Acute Effect of R-KET and S-KET on FST Behavior in GWI Rats
3.7. Sustained Effect of R-KET and S-KET on FST Behavior in GWI Rats
3.8. Effects of KET, R-KET, S-KET on EPM Performance in GWI Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steele, L. Prevalence and patterns of Gulf War illness in Kansas veterans: Association of symptoms with characteristics of person, place, and time of military service. Am. J. Epidemiol. 2000, 152, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Update of Health Effects of Serving in the Gulf War. In Gulf War and Health; Cory-Slechta, D., Wedge, R., Eds.; The National Academies Press: Washington, DC, USA, 2016; Volume 10. Available online: https://www.ncbi.nlm.nih.gov/books/NBK355346/ (accessed on 5 May 2020).
- Blanchard, M.S.; Eisen, S.A.; Alpern, R.; Karlinsky, J.; Toomey, R.; Reda, D.J.; Murphy, F.M.; Jackson, L.W.; Kang, H.K. Chronic multisymptom illness complex in Gulf War I veterans 10 years later. Am. J. Epidemiol. 2006, 163, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Depleted Uranium, Sarin, Pyridostigmine Bromide, and Vaccines. In Gulf War and Health; The National Academies Press: Washington, DC, USA, 2000; Volume 1. [Google Scholar]
- Institute of Medicine. Insecticides and Solvents. In Gulf War and Health; The National Academies Press: Washington, DC, USA, 2003; Volume 2. [Google Scholar]
- White, R.F.; Steele, L.; O’Callaghan, J.P.; Sullivan, K.; Binns, J.H.; Golomb, B.A.; Bloom, F.E.; Bunker, J.A.; Crawford, F.; Graves, J.C.; et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 2016, 74, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Sastre, A.; Gerkovich, M.M.; Cook, M.R. Complex factors in the etiology of Gulf War illness: Wartime exposures and risk factors in veteran subgroups. Environ. Health Perspect. 2012, 120, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Brimfield, A.A. Chemicals of military deployments: Revisiting Gulf War Syndrome in light of new information. Prog. Mol. Biol. Transl. Sci. 2012, 112, 209–230. [Google Scholar]
- Golomb, B.A. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc. Natl. Acad. Sci. USA 2008, 105, 4295–4300. [Google Scholar] [CrossRef]
- Fricker, R.D.; Reardon, E.; Spektor, D.M.; Cotton, S.K.; Hawes-Dawson, J.; Pace, J.E.; Hosek, S.D. Pesticide Use During the Gulf War: A Survey of Gulf War Veterans; MR-1018/12-OSD; RAND Corporation: Santa Monica, CA, USA, 2000; Available online: https://www.rand.org/pubs/monograph_reports/MR1018z12.html (accessed on 17 June 2020).
- Cecchine, G.; Golomb, B.A.; Hilborne, L.H.; Spektor, D.M.; Anthony, C.R. A Review of the Scientific Literature as it Pertains to Gulf War Illnesses, Volume 8: Pesticides; MR-1018/8-OSD; RAND Corporation: Santa Monica, CA, USA, 2020; Available online: https://www.rand.org/pubs/monograph_reports/MR1018z8.html (accessed on 17 June 2020).
- Haley, R.W.; Tuite, J.J. Epidemiologic evidence of health effects from long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemiology 2013, 40, 178–189. [Google Scholar] [CrossRef]
- Chao, L.L.; Zhang, Y.; Buckley, S. Effects of low-level sarin and cyclosarin exposure on white matter integrity in Gulf War Veterans. Neurotoxicology 2015, 48, 239–248. [Google Scholar] [CrossRef]
- Wesseling, C.; Keifer, M.; Ahlbom, A.; McConnell, R.; Moon, J.D.; Rosenstock, L.; Hogstedt, C. Long-term neurobehavioral effects of mild poisonings with organophosphate and n-methyl carbamate pesticides among banana workers. Int. J. Occup. Environ. Health 2002, 8, 27–34. [Google Scholar] [CrossRef]
- Nishiwaki, Y.; Maekawa, K.; Ogawa, Y.; Asukai, N.; Minami, M.; Omae, K.; Sarin Health Effects Study Group. Effects of sarin on the nervous system in rescue team staff members and police officers 3 years after the Tokyo subway sarin attack. Environ. Health Perspect. 2001, 109, 1169–1173. [Google Scholar] [CrossRef]
- Cherry, N.; Mackness, M.; Durrington, P.; Povey, A.; Dippnall, M.; Smith, T.; Mackness, B. Paraoxonase (PON1) polymorphisms in farmers attributing ill health to sheep dip. Lancet 2002, 359, 763–764. [Google Scholar] [CrossRef]
- Davies, D.R.; Mrcpsych, M.; Ahmed, G.M.; Freer, T. Chronic Organophosphate Induced Neuropsychiatric Disorder (COPIND): Results of Two Postal Questionnaire Surveys. J. Nutr. Environ. Med. 1999, 9, 123–134. [Google Scholar] [CrossRef]
- Bajgar, J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar] [PubMed]
- Broomfield, C.A.; Kirby, S.D. Progress on the road to new nerve agent treatments. J. Appl. Toxicol. 2001, 21 (Suppl. 1), S43–S46. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef]
- Ikin, J.F.; McKenzie, D.P.; Gwini, S.M.; Kelsall, H.L.; Creamer, M.; McFarlane, A.C.; Clarke, D.M.; Wright, B.; Sim, M. Major depression and depressive symptoms in Australian Gulf War veterans 20 years after the Gulf War. J. Affect. Dis. 2016, 189, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Blore, J.D.; Sim, M.R.; Forbes, A.B.; Creamer, M.C.; Kelsall, H.L. Depression in Gulf War veterans: A systematic review and meta-analysis. Psychol. Med. 2015, 45, 1565–1580. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Board on the Health of Select Populations. In Gulf War and Health: Treatment of Chronic Multisymptom Illness; The National Academies Press: Washington, DC, USA, 2013; Available online: http://www.iom.edu/Reports/2013/Gulf-War-and-HealthTreatment-for-Chronic-Multisymptom-Illness.aspx (accessed on 17 June 2020).
- John, R.L., Jr.; Antai-Otong, D. Contemporary Treatment Approaches to Major Depression and Bipolar Disorders. Nurs. Clin. N. Am. 2016, 51, 335–351. [Google Scholar] [CrossRef]
- Ferrari, F.; Villa, R.F. The Neurobiology of Depression: An Integrated Overview from Biological Theories to Clinical Evidence. Mol. Neurobiol. 2016, 54, 4847–4865. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Warden, D.; Trivedi, M.H.; Wisniewski, S.R.; Fava, M.; Rush, A.J. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr. Serv. 2009, 60, 1439–1445. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A., Jr.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chang, L.; Hashimoto, K. A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol. Biochem. Behav. 2020, 190, 172870. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psych. 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Kaufman, M.B. Pharmaceutical Approval Update. Pharm. Ther. 2019, 44, 251–254. [Google Scholar]
- US FDA News Release. FDA Approves New Nasal Spray Medication for Treatment-Resistant Depression; Available Only at a Certified Doctor’s Office or Clinic, 5 March 2019. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified (accessed on 5 May 2020).
- Phillips, K.F.; Deshpande, L.S. Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology 2016, 52, 127–133. [Google Scholar] [CrossRef]
- Phillips, K.F.; Deshpande, L.S. Chronic Neurological Morbidities and Elevated Hippocampal Calcium Levels in a DFP-Based Rat Model of Gulf War Illness. Mil. Med. 2018, 183, 552–555. [Google Scholar] [CrossRef]
- Phillips, K.F.; Santos, E.; Blair, R.E.; Deshpande, L.S. Targeting Intracellular Calcium Stores Alleviates Neurological Morbidities in a DFP-Based Rat Model of Gulf War Illness. Toxicol. Sci. 2019, 169, 567–578. [Google Scholar] [CrossRef]
- Overstreet, D.H. Modeling depression in animal models. Methods Mol. Biol. 2012, 829, 125–144. [Google Scholar]
- Behan, P.O. Chronic Fatigue Syndrome as a Delayed Reaction to Chronic Low-dose Organophosphate Exposure. J. Nutr. Environ. Med. 1996, 6, 341–350. [Google Scholar] [CrossRef]
- Chan, J.Y.; Chan, S.H.; Dai, K.Y.; Cheng, H.L.; Chou, J.L.; Chang, A.Y. Cholinergic-receptor-independent dysfunction of mitochondrial respiratory chain enzymes, reduced mitochondrial transmembrane potential and ATP depletion underlie necrotic cell death induced by the organophosphate poison mevinphos. Neuropharmacology 2006, 51, 1109–1119. [Google Scholar] [CrossRef]
- Koslik, H.J.; Hamilton, G.; Golomb, B.A. Mitochondrial dysfunction in Gulf War illness revealed by 31Phosphorus Magnetic Resonance Spectroscopy: A case-control study. PLoS ONE 2014, 9, e92887. [Google Scholar] [CrossRef]
- Castagne, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011, 8, 8. [Google Scholar] [CrossRef]
- Bogdanova, O.V.; Kanekar, S.; D’Anci, K.E.; Renshaw, P.F. Factors influencing behavior in the forced swim test. Physiol. Behav. 2013, 118, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Connor, T.J.; Kelly, J.P.; Leonard, B.E. Forced swim test-induced neurochemical endocrine, and immune changes in the rat. Pharmacol. Biochem. Behav. 1997, 58, 961–967. [Google Scholar] [CrossRef]
- Scheggi, S.; De Montis, M.G.; Gambarana, C. Making Sense of Rodent Models of Anhedonia. Int. J. Neuropsychopharmacol. 2018, 21, 1049–1065. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Boakye, E.A.; Buchanan, P.; Wang, J.; Stringer, L.; Geneus, C.; Scherrer, J.F. Self-Reported Lifetime Depression and Current Mental Distress Among Veterans Across Service Eras. Mil. Med. 2017, 182, e1691–e1696. [Google Scholar] [CrossRef]
- Stimpson, N.J.; Thomas, H.V.; Weightman, A.L.; Dunstan, F.; Lewis, G. Psychiatric disorder in veterans of the Persian Gulf War of 1991. Systematic review. Br. J. Psychiatry 2003, 182, 391–403. [Google Scholar] [CrossRef]
- Price, R.B.; Nock, M.K.; Charney, D.S.; Mathew, S.J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psych. 2009, 66, 522–526. [Google Scholar] [CrossRef]
- DiazGranados, N.; Ibrahim, L.A.; Brutsche, N.E.; Ameli, R.; Henter, I.D.; Luckenbaugh, D.A.; Machado-Vieira, R.; Zarate, C.A., Jr. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry 2010, 71, 1605–1611. [Google Scholar] [CrossRef]
- Lasram, M.M.; Dhouib, I.B.; Annabi, A.; El Fazaa, S.; Gharbi, N. A review on the molecular mechanisms involved in insulin resistance induced by organophosphorus pesticides. Toxicology 2014, 322, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Czajka, M.; Matysiak-Kucharek, M.; Jodlowska-Jedrych, B.; Sawicki, K.; Fal, B.; Drop, B.; Kruszewski, M.; Kapka-Skrzypczak, L. Organophosphorus pesticides can influence the development of obesity and type 2 diabetes with concomitant metabolic changes. Environ. Res. 2019, 178, 108685. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Wang, L.; Monk, J.M.; Bright, A.T.; Koslik, H.J.; Ritchie, J.B.; Golomb, B.A. Metabolic features of Gulf War illness. PLoS ONE 2019, 14, e0219531. [Google Scholar] [CrossRef] [PubMed]
- Herzog, D.P.; Beckmann, H.; Lieb, K.; Ryu, S.; Müller, M.B. Understanding and Predicting Antidepressant Response: Using Animal Models to Move Toward Precision Psychiatry. Front. Psychiatry 2018, 9, 512. [Google Scholar] [CrossRef]
- Gambarana, C.; Scheggi, S.; Tagliamonte, A.; Tolu, P.; De Montis, M.G. Animal models for the study of antidepressant activity. Brain Res. Brain Res. Protoc. 2001, 7, 11–20. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Gideons, E.S.; Kavalali, E.T.; Monteggia, L.M. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc. Natl. Acad. Sci. USA 2014, 111, 8649–8654. [Google Scholar] [CrossRef] [PubMed]
- Monteggia, L.M.; Gideons, E.; Kavalali, E.T. The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol. Psych. 2013, 73, 1199–1203. [Google Scholar] [CrossRef]
- Veilleux-Lemieux, D.; Castel, A.; Carrier, D.; Beaudry, F.; Vachon, P. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 567–570. [Google Scholar]
- Deyama, S.; Duman, R.S. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol. Biochem. Behav. 2020, 188, 172837. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Luo, A.; Hashimoto, K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl. Psych. 2019, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.R.; Schatzberg, A.F. NMDA antagonist treatment of depression. Curr. Opin. Neurobiol. 2016, 36, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Miller, O.H.; Yang, L.; Wang, C.C.; Hargroder, E.A.; Zhang, Y.; Delpire, E.; Hall, B.J. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife 2014, 3, e03581. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef]
- Zhang, J.C.; Li, S.X.; Hashimoto, K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol. Biochem. Behav. 2014, 116, 137–141. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef]
- Muller, J.; Pentyala, S.; Dilger, J.; Pentyala, S. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther. Adv. Psychopharmacol. 2016, 6, 185–192. [Google Scholar] [CrossRef]
- Yang, C.; Shirayama, Y.; Zhang, J.C.; Ren, Q.; Yao, W.; Ma, M.; Dong, C.; Hashimoto, K. R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psych. 2015, 5, e632. [Google Scholar] [CrossRef]
- Pesic, V.; Petrovic, J.; Jukic, M.M. Molecular Mechanism and Clinical Relevance of Ketamine as Rapid-Acting Antidepressant. Drug. Dev. Res. 2016, 77, 414–422. [Google Scholar] [CrossRef]
- Du Jardin, K.G.; Muller, H.K.; Elfving, B.; Dale, E.; Wegener, G.; Sanchez, C. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog. Neuro-Psychopharmacol. Biol. Psych. 2016, 71, 27–38. [Google Scholar] [CrossRef]
- Duman, R.S.; Li, N.; Liu, R.J.; Duric, V.; Aghajanian, G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012, 62, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A. A Review of the Scientific Literature As It Pertains to Gulf War Illnesses: Volume 2: Pyridostigmine Bromide; MR-1018/2-OSD; RAND Corporation: Santa Monica, CA, USA, 1999; Available online: https://www.rand.org/pubs/monograph_reports/MR1018z2.html (accessed on 17 June 2020).
- Khan, W.A.; Dechkovskaia, A.M.; Herrick, E.A.; Jones, K.H.; Abou-Donia, M.B. Acute Sarin Exposure Causes Differential Regulation of Choline Acetyltransferase, Acetylcholinesterase, and Acetylcholine Receptors in the Central Nervous System of the Rat. Toxicol. Sci. 2000, 57, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Abou-Donia, M.B.; Dechkovskaia, A.M.; Goldstein, L.B.; Bullman, S.L.; Khan, W.A. Sensorimotor deficit and cholinergic changes following coexposure with pyridostigmine bromide and sarin in rats. Toxicol. Sci. 2002, 66, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Torres-Altoro, M.I.; Mathur, B.N.; Drerup, J.M.; Thomas, R.; Lovinger, D.M.; O’Callaghan, J.P.; Bibb, J.A. Organophosphates dysregulate dopamine signaling, glutamatergic neurotransmission, and induce neuronal injury markers in striatum. J. Neurochem. 2011, 119, 303–313. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.; Abou-Donia, S.; El-Masry, E.; Shetty, A.; Abou-Donia, M. Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. J. Toxicol. Environ. Health A 2004, 67, 163–192. [Google Scholar] [CrossRef]
- Abdullah, L.; Evans, J.E.; Bishop, A.; Reed, J.M.; Crynen, G.; Phillips, J.; Pelot, R.; Mullan, M.A.; Ferro, A.; Mullan, C.M.; et al. Lipidomic profiling of phosphocholine-containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to Gulf War agents. Neuromol. Med. 2012, 14, 349–361. [Google Scholar] [CrossRef]
- Parihar, V.K.; Hattiangady, B.; Shuai, B.; Shetty, A.K. Mood and Memory Deficits in a Model of Gulf War Illness Are Linked with Reduced Neurogenesis, Partial Neuron Loss, and Mild Inflammation in the Hippocampus. Neuropsychopharmacology 2013, 38, 2348–2362. [Google Scholar] [CrossRef]
- Speed, H.E.; Blaiss, C.A.; Kim, A.; Haws, M.E.; Melvin, N.R.; Jennings, M.; Eisch, A.J.; Powell, C.M. Delayed reduction of hippocampal synaptic transmission and spines following exposure to repeated subclinical doses of organophosphorus pesticide in adult mice. Toxicol. Sci. 2012, 125, 196–208. [Google Scholar] [CrossRef]
- Megahed, T.; Hattiangady, B.; Shuai, B.; Shetty, A.K. Parvalbumin and neuropeptide Y expressing hippocampal GABA-ergic inhibitory interneuron numbers decline in a model of Gulf War illness. Front. Cell. Neurosci. 2014, 8, 447. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Hawkins, E.; Phillips, K.; Deshpande, L.S. Assessment of Ketamine and its Enantiomers in an Organophosphate-Based Rat Model for Features of Gulf War Illness. Int. J. Environ. Res. Public Health 2020, 17, 4710. https://doi.org/10.3390/ijerph17134710
Zhu J, Hawkins E, Phillips K, Deshpande LS. Assessment of Ketamine and its Enantiomers in an Organophosphate-Based Rat Model for Features of Gulf War Illness. International Journal of Environmental Research and Public Health. 2020; 17(13):4710. https://doi.org/10.3390/ijerph17134710
Chicago/Turabian StyleZhu, Jackie, Elisa Hawkins, Kristin Phillips, and Laxmikant S. Deshpande. 2020. "Assessment of Ketamine and its Enantiomers in an Organophosphate-Based Rat Model for Features of Gulf War Illness" International Journal of Environmental Research and Public Health 17, no. 13: 4710. https://doi.org/10.3390/ijerph17134710
APA StyleZhu, J., Hawkins, E., Phillips, K., & Deshpande, L. S. (2020). Assessment of Ketamine and its Enantiomers in an Organophosphate-Based Rat Model for Features of Gulf War Illness. International Journal of Environmental Research and Public Health, 17(13), 4710. https://doi.org/10.3390/ijerph17134710




