Abstract
The experience of craving via exposure to drug-related cues often leads to relapse in drug users. This study consolidated existing empirical evidences of cue reactivity to methamphetamine to provide an overview of current literature and to inform the directions for future research. The best practice methodological framework for conducting scoping review by Arkey and O’Malley was adopted. Studies that have used a cue paradigm or reported on cue reactivity in persons with a history of methamphetamine use were included. Databases such as Medline, EMBASE, PsycINFO and CINAHL were searched using key terms, in addition to citation check and hand search. The search resulted in a total of 32 original research articles published between 2006 to 2020. Three main themes with regard to cue reactivity were identified and synthesized: (1) effects of cue exposure, (2) individual factors associated with cue reactivity, and (3) strategies that modulate craving or reactivity to cues. Exposure to methamphetamine-associated cues elicits significant craving and other autonomic reactivity. Evidence suggests that drug cue reactivity is strongly associated with indices of drug use and other individual-specific factors. Future studies should focus on high quality studies to support evidence-based interventions for reducing cue reactivity and to examine cue reactivity as an outcome measure.
1. Introduction
Several theoretical models of drug-use behaviors, such as the expectancy model, the dual-affect model, and the cognitive processing models have proposed that external environmental cues can serve as triggers for drug use [1,2]. Cues can produce symptoms of withdrawal in drug users, even after abstinence or detoxification [3,4]. A vast amount of empirical research has demonstrated that stimuli associated with the drug or its administration (e.g., bottle of preferred alcohol, syringe, lighter) can elicit subjective reports of craving and patterns of physiological responding in persons who have a history of drug use [5]. This phenomenon is often referred to as cue reactivity [5].
Drug cue reactivity is one of the hallmark characteristics in addiction research and numerous attempts have been made to elucidate the underlying learning mechanisms [5]. Drug cue reactivity was earlier proposed to be attributed to the formation of a direct association between the stimulus (i.e., the cue) and the response [6] but later theories began to support the view that drug cues elicit expectations of the drug, which drive drug-seeking behaviors [7]. With repeated drug experience, the drug user associates the rewarding effects of a drug with cues present at the time of consumption and this is known as classical conditioning, or Pavlovian conditioning [8]. In other words, formation of associations between cues and drugs is largely based on the premise of classical conditioning, during which initially neutral cues that are repeatedly paired with drugs (the unconditioned stimulus) acquire conditioned incentive properties and become the conditioned stimuli [7,9]. It is therefore through these Pavlovian associations that innocuous environmental stimuli become salient mediators of drug-seeking behaviors.
Cue reactivity paradigm is a valuable and versatile tool that aids the studying of cue-elicited drug craving. It typically involves the exposure of current or abstinent drug users to visual and/or auditory drug-related cues in order to monitor their reactions to these cues [10]. Exposure to drug-associated cues in laboratory settings has been shown to reliably induce drug craving and physiological reactions amongst drug users [5]. Such cue paradigms have been widely employed in addiction research across individuals who are addicted to various drugs including methamphetamine, heroin, cocaine and alcohol [5,10]. Furthermore, the application of cue reactivity paradigm has been examined in addiction research and cue reactivity studies have been proposed to offer insights into understanding the nature of drug dependence; predicting relapse and as a method of evaluating treatment efficacy [10,11,12].
Methamphetamine, a highly addictive illicit drug that is also otherwise known as ice, speed, meth and crystal, is an amphetamine-type stimulant that heightens stimulation in the central nervous system [13]. While the short-term rewarding effects include euphoria, elevated mood, reduced fatigue, increased alertness and increased libido, long-term methamphetamine use is accompanied by devastating health consequences such as having neurological abnormalities and damage, cognitive deficits, drug-induced psychosis, increased depressive and anxiety symptoms, and elevated risk of drug overdose and transmission of blood-borne diseases through sharing of needles [14,15]. Furthermore, the effects of methamphetamine were found to last longer as compared to other drugs and the drug’s high lipid solubility allows it to be transferred to the brain more readily [13]. Moreover, methamphetamine is also popularly consumed before or during sex, especially by homosexual men, to improve sexual experiences, prolong sexual performance and reduce sexual inhibition [16]. This further promotes risky sexual behaviors and the proliferation of sexually transmitted diseases amongst the drug users. More worryingly, methamphetamine has increasingly become a global public health concern It was estimated in 2017 that there were approximately 28.9 million amphetamine-type stimulant (including methamphetamine) users over the last two decades [16]. Beyond the constant evolving and expansive trends of methamphetamine manufacturing and trafficking activities across different regions of the world, methamphetamine use continues to increase in North America and Asia [16,17].
Despite amphetamine-type stimulants (mainly methamphetamine) being the second most widely used class of illicit drugs worldwide [18], no effective pharmacotherapeutic agents are available for the treatment of methamphetamine dependence, nor is there any medication approved by the regulatory authorities for such treatment till date [19]. Cue reactivity studies in methamphetamine may in turn represent a good tool of considerable utility for investigating addictive phenomena. Much research efforts in the area of cue reactivity have been made with other substances such as opiates, cocaine and alcohol, but those relating to methamphetamine appear to remain in infancy [5,10,12,20]. The current study therefore intends to consolidate and review existing empirical evidences of cue reactivity to methamphetamine so as to provide an overview on current literature and inform the directions for future research.
2. Materials and Methods
The current review was conducted in line with Arksey and O’Malley’s framework for scoping methodology [21] (Step 1: identify the research questions; Step 2: identify relevant studies; Step 3: study selection; Step 4: chart the data; and Step 5: collate, summarize and report the results) and the PRISMA-ScR (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews) [22] checklist was used for reporting findings.
Scoping reviews tend to have a very broadly defined research question and we therefore formulated the following: “What does cue reactivity have to offer to methamphetamine research?” to guide our review. Based on knowledge from existing literature, specific areas of applications of cue reactivity paradigms were pre-defined, but not limited to:
- Method of understanding the nature of methamphetamine dependence
- Predictor of relapse
- Method of studying treatment effects
2.1. Search Strategy and Databases
Cue reactivity has been a frequently used method in addiction research since the 1990s with the theory and practice of cue exposure being put into perspective by Drummond (1995) [23]. An electronic search of articles published between January 1995 and November 2019 was therefore performed on EMBASE, MEDLINE (PubMed), PsycINFO and CINAHL (with full text). The main search of the databases was conducted on 15 November 2019. A prior search limit was set to include English publications and studies involving humans only. Hand searching of references from key papers and citations from the web (e.g., Cochrane Central Register of Controlled Trials, Google Scholar; last updated on 9 March 2020 to screen for new, potential studies) were also performed. Authors were also contacted for full-text articles if they were not publicly available (e.g., accepted paper but yet published) and clarification of information. Search terms (“cue” AND “methamphetamine” only) were selected to provide extensive coverage. All references were stored and managed in EndNote X7 (bibliographic software).
A two-stage review strategy—first at the title/abstract level followed by full-text level—was adopted and each potentially relevant result was examined by two authors (ES and WJ) who worked independently at each stage. Disagreements between the two authors were discussed or consulted with the third author (MS) until a consensus was reached.
2.2. Inclusion and Exclusion Criteria
Studies were screened and included if they had used a cue paradigm or reported on cue reactivity in persons with a history of methamphetamine use (i.e., current or previous users). Studies that were conducted in animals or among healthy participants only were excluded. In addition, studies that recruited participants with polysubstance (cocaine, cannabis, heroin etc.) use and had not reported findings specific to exposure of methamphetamine cues or the use of methamphetamine separately, were also excluded. Upon full-text screening, we decided to further exclude reviews, study protocols, extracts from books or other non-scientific publications, case reports and those that were only available as abstracts (e.g., conference or dissertation abstracts), and to include only primary studies.
2.3. Data Charting, Collating, Summarizing and Reporting
Data was extracted and tabulated to include the Population, Intervention, Comparison and Outcome (PICO) elements by one author (ES) and verified for completeness and accuracy by a second reviewer (WJ). Details of study population and context, cue reactivity paradigm, type of intervention and comparator (if applicable), outcome measures, and findings of interest were extracted and recorded. In order to chart the data, the studies were classified according to the broad areas of application of the cue paradigms and sorted by date of publication. Quality of evidence or formal risk-of-bias assessment for each individual study was not evaluated, as consistent with the scoping review methodology. To summarize the findings, data was synthesized and reported according to key themes. Within each broader theme, data was further sub-categorized to group common effects, factors and intervention types so as to present a more meaningful narrative account of the existing literature. All team members reviewed the themes and consensus was reached for the label of each theme.
3. Results
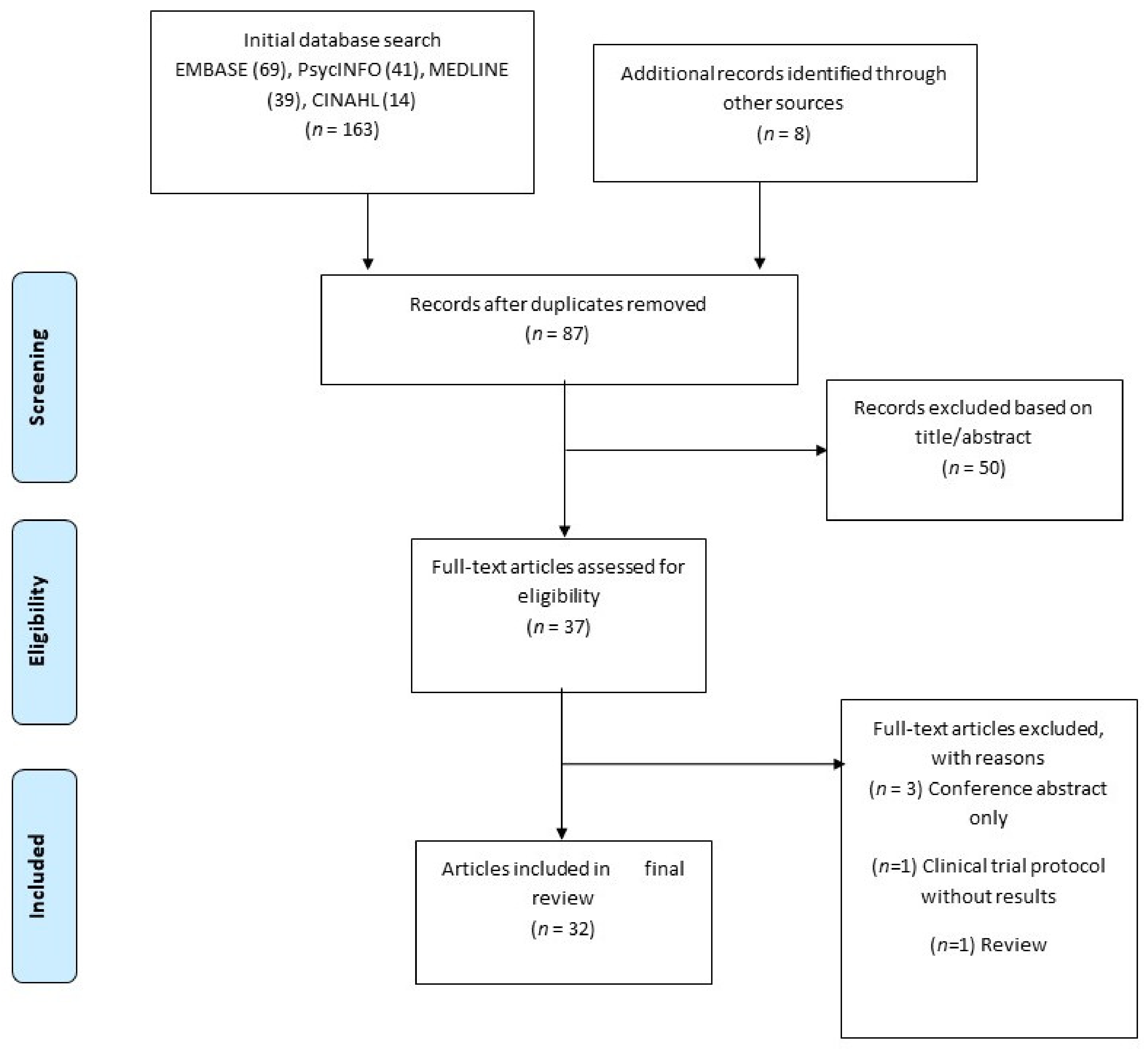
The combined search identified a total of 163 references, of which 84 were duplicates and were removed. A total of 87 citations (including an additional 8 articles found through hand search) were therefore screened on the basis of title and abstract (see Figure 1) to ensure that they addressed the appropriate population and had a focus on drug cue reactivity. 50 references did not meet our criteria at title/abstract screening and a further 5 were eventually excluded at full text-screening. Of the final 32 relevant articles accepted, 16 were from the USA, 11 were from China, 4 from Iran and 1 from Georgia. Two of these articles reported on two separate studies each, leading to a total of 34 studies being reviewed.
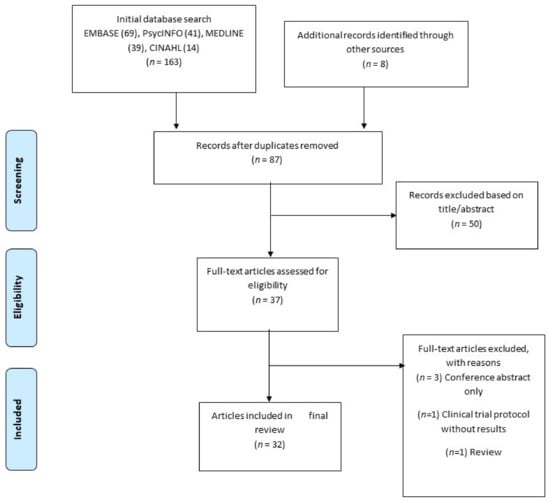
Figure 1.
Summary of search strategy.
3.1. Summary of Included Studies
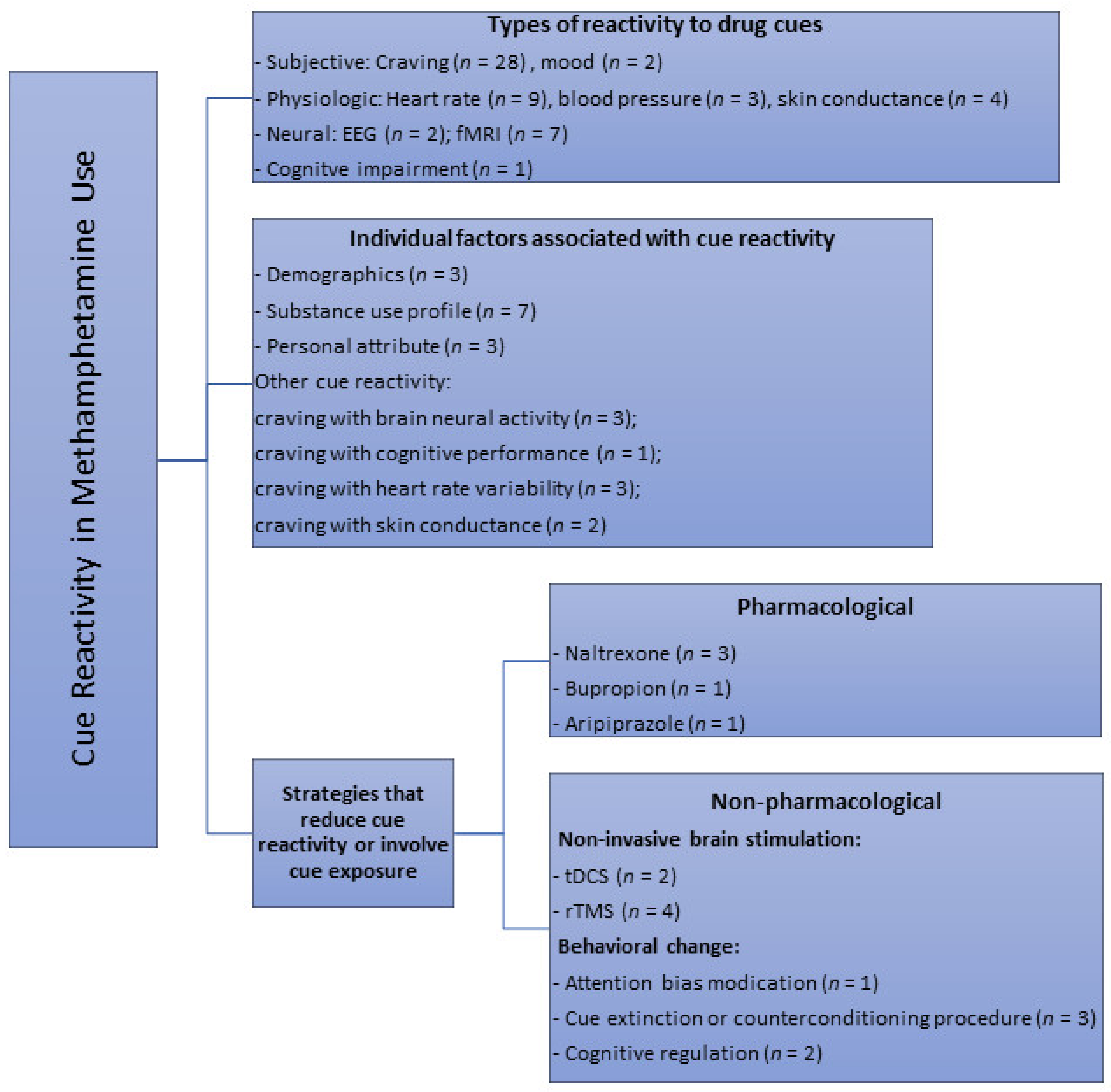
Visual (photographs/images and videos) (n = 28), in vivo paraphernalia and/or simulated drug (n = 10) and imagery cues (e.g., audiotaped scripts, recalling of last drug use) (n = 4) were the different modalities used in all the studies to elicit responses in a cue reactivity paradigm. Several studies also designed and employed the use of a methamphetamine virtual reality (methamphetamine-VR) cue model to assess self-reported cravings and physiological changes [24,25,26,27]. A total of 18 studies compared effects of methamphetamine-related cues with neutral cues; some of which involved the use of cues related to nature scenes (n = 4), beach (n = 1), images of artifacts and normal daily actions (n = 2), footage of tropical fish in tank/aquarium (n = 2) or the handling of a glass of water (n = 2) and pine cones, shells, and rocks (n = 1). Few studies also used control cues such as sexual (n = 2), food (n = 1) visual cues, as well as “happy” and “sad” stimuli based on subjective evaluations of the emotional valence of the images (n = 1). Only 8 studies recruited a control group with healthy participants for comparison purpose. Studies (n = 3) that used both control cue(s) and a control group were those that looked at difference in neural reactivity to methamphetamine vs. control vs. neutral cues using functional magnetic resonance imaging, and compared them between drug users and healthy controls. Most studies employed a single item visual analogue scale to assess cue-induced craving (n = 14). Established scales such as the general craving scale (GCS, n = 1), within session rating scale (WSRS, n = 4), brief substance craving scale (BSCS, n = 1) and methamphetamine urge questionnaire (MAUQ, n = 2) were also utilized to assess cue-induced drug craving. Depending on the aim of the studies, these measures were administered to participants at different time points (e.g., prior, during and after cue exposure depending on the study). Overall, this scoping review identified several themes with regard to the main applications of drug cue paradigms. As shown in Figure 2, the three main themes synthesized were: (1) effects of cue exposure, (2) individual factors associated with cue-induced cravings or other cue reactivity, and (3) strategies and measures that modulate drug craving or reactivity to cues.
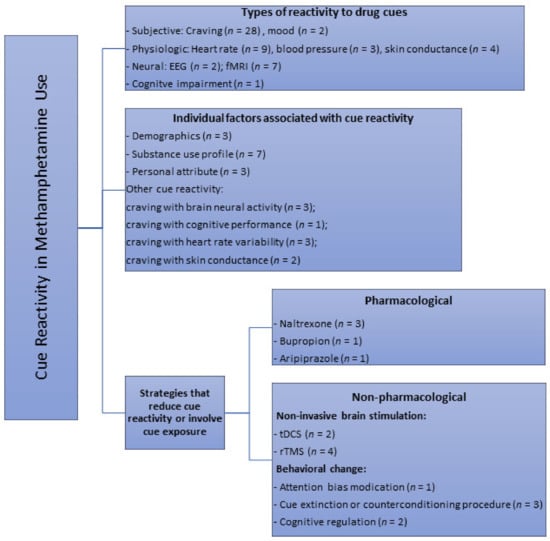
Figure 2.
Key themes of cue reactivity or areas of cue paradigm applications identified from primary studies in methamphetamine research through the scoping review. n = number of available studies.
3.2. Effects of Cue Exposure
The majority of the included studies assessed the impact of cue exposure on cue-elicited craving as the primary study outcome. A handful of studies also included more than one measure of cue reactivity such as self-reported craving with neural activation or autonomic arousal recorded at baseline, during and after cue processing (see Table 1).

Table 1.
Details of included studies that looked at the effects of drug cue exposure and factors associated with change in cravings upon cue exposure.
3.2.1. Subjective and Physiological Responses
Studies looking at cue reactivity found that methamphetamine stimuli are generally reported to increase levels of drug craving, “anxious” mood and other physiological arousal such as heart rate, blood pressure and skin conductance variability among participants who had a history of methamphetamine use. Further, two studies revealed differences in reactivity to different modalities of methamphetamine-related cues. For example, a study by Tolliver and colleagues suggested that relative to baseline, presentation of methamphetamine-associated photo, video, and paraphernalia cues elicited significant increases in subjective craving (from fewer than half of the participants at baseline to approximately 70% of participants after cue exposure) and skin conductance while heart rate was significantly decreased only after viewing methamphetamine-related photos or video, but not after exposure to paraphernalia [28]. Methamphetamine virtual reality condition was also found to induce the greatest change in subjective reports of “crave methamphetamine”, “desire methamphetamine” and “want methamphetamine” at all-time points compared to methamphetamine-video and other neutral test conditions, as well as higher increase in “anxiety” rating compared to neutral virtual reality condition [24].
3.2.2. Neural Reactivity
Besides subjective and physiological responses, the scoping review also found studies examining cue reactivity with functional magnetic resonance imaging (fMRI) and electroencephalogram (EEG). Seven studies that examined fMRI evidence reported functional abnormalities in the brain of those with methamphetamine use disorder, compared to healthy participants. Blood-oxygen-level dependent measures of methamphetamine cue reactivity revealed activation of a broad set of regions, particularly the mesocorticolimbic system which includes the ventral and dorsal striatum, the cingulate cortex, prefrontal cortex (PFC) and insula [29,30,31,32,33,34,35]. Two studies looked at EEG recordings: one study utilized event-related potential (ERP) technique and found higher neural responses to drug-related visual stimuli among users compared to controls in the P300 components [36], while the other study measured gamma current density and found gamma activity in medial prefrontal cortex (mPFC)/orbitofrontal cortex (OFC) and right dorsolateral prefrontal cortex (DLPFC) to decrease after cue exposure [27].
3.2.3. Cognitive Function
The cognitive effects of cue exposure in chronic methamphetamine abusers have been examined in one study. Tolliver and colleagues found the exposure of methamphetamine-related cues to impair participants’ performances (increased rates of both response errors and inhibition errors) on an auditory dual task Go–No Go cognitive test requiring divided attention and inhibition of distracting information [37].
3.3. Factors Associated with Drug Cue Reactivity
The scoping review identified and characterized major factors that were found to modulate craving upon presentation of methamphetamine cues in users. In this section, we have reviewed available evidence and discussed individual-specific factors that were associated with, or in some cases, predictive of cue-elicited craving (see Table 1).
3.3.1. Demographics
Three studies explored the relationship between demographics and cue reactivity and reported that differences in cue-induced cravings (across individuals or cue modalities) were not associated with examined sociodemographic variables such as age, education, employment etc. [28,38,39]. These findings proposed that methamphetamine craving was largely due to cue exposure and not influenced by demographic keys. Only one study, however, found age to positively and education to negatively predict craving changes [40].
3.3.2. Substance Use Profile
Findings from six out of seven studies that examined the relationship between variables relating to drug use and cue reactivity indicated significant effects of substance use-related variables on cue reactivity (except [39]). Ekhtiari et al. (2009) found age of onset of drug abuse to be negatively correlated with level of craving responsiveness [38]. Lopez et al. (2015) also revealed that the strength of cue-induced craving for methamphetamine can be moderated by users’ route of administration, such that individuals who preferred to smoke methamphetamine reported significantly stronger craving for smoking stimuli, whereas those who preferred the intranasal route reported stronger craving for intranasal stimuli [41]. The most robust predictor of cue-induced craving was found to be baseline craving for methamphetamine in the study by Tolliver and colleagues, who noted that the degree of craving at baseline was strongly associated with the frequency and amount of methamphetamine use in the 60 days prior to study entry [28]. Wang et al. (2013) also found the effects of length of methamphetamine abstinence on cue-induced craving, which increased as the length of abstinence increased until 3 months but decreased with 6 months and 1 year of abstinence. This effect was, however, not observed on cardiovascular measures including heart rate, systolic and diastolic blood pressure [42]. In terms of neural cue reactivity, Malcolm et al. (2016) similarly found the increased brain activation in the ventral striatum in response to methamphetamine cues compared to rest condition, to correlate significantly and negatively with the days since last use of methamphetamine, supporting the notion of reduced cue reactivity with longer period of abstinence [31]. Lastly, Huang et al. (2018) also found activation in the left lateral anterior cingulate cortex (ACC) region of the bilateral medial prefrontal cortex (mPFC) to methamphetamine-related image cues in users to be positively associated with previous drug use frequency [30].
3.3.3. Personality Attributes
Three studies explored the relationship between personality attributes and craving in response to drug cues. Saladin et al. (2012) examined the factor alexithymia—a personality attribute characterized by a difficulty identifying and describing emotions—and predicted that it may contribute to addicted persons’ failure to report cue-elicited cravings. Contrary to their hypothesis, higher scores on the Toronto Alexithymia Scale factor 1 (a measure of difficulty in identifying feelings) were positively associated with cue-elicited craving and authors suggested that their results may need to be replicated [43]. In a study by Chen et al. (2020), both attentional and non-planning impulsivity were found to correlate negatively with methamphetamine cue-related activation among short-term (but not long-term) methamphetamine users, though these findings did not survive Bonferroni correction [35]. However, methamphetamine users who rated zero increase in craving upon cue exposure did not differ from those who rated non-zero increase in terms of impulsiveness and emotional stability [39].
3.3.4. Other Cue Reactivity Responses
Several studies found an association between cue-elicited craving and other measures of cue reactivity. For neural cue reactivity, for example, three studies revealed craving response to be significantly associated with increased activation in different regions of the brain related to the mesolimbic reward pathway during methamphetamine cue processing [29,30,33]. In an attempt to examine impaired performance on a cognitive task due to the exposure of methamphetamine-related cues, Tolliver et al. (2012) found response error rates, but not inhibition error rates or reaction times, to correlate with cue-elicited craving scores in methamphetamine users [37]. For physiological reactivity, two studies found a positive relationship between changes in subjective craving response and heart rate variability measure upon exposure to cue [24,42]. In contrast, Tolliver et al. (2010) reported a lack of correlation between change in heart rate and skin conductance after cue exposure, and between either measure with cue-induced craving [28]. Tan et al. (2012) found cue-induced changes in brain electrophysiological response (gamma activity) to associate with changes in skin conductance level, but not self-reported craving while neither the change in skin conductance nor heart rate variability was correlated with craving increase [27].
3.4. Strategies or Interventions that Modulate Drug Cue Reactivity
We reviewed eighteen studies that assessed efficacy of measures that targeted methamphetamine cue reactivity and organized them into broad categories of “pharmacological” and “non-pharmacological” methods. Studies using “non-pharmacological” methods were further grouped into non-invasive brain stimulation or behavioral techniques (see Table 2).

Table 2.
Details of included studies that looked at methods that modulate cue-elicited cravings and reactivity or involve cue exposure.
3.4.1. Pharmacological Measures
Five studies investigated the role of medications in the treatment of participants with methamphetamine abuse or dependence by targeting cue reactivity. These studies assessed oral naltrexone, aripiprazole and bupropion using slightly different randomized trial designs (crossover or parallel group) in clinical samples. Three studies assessed the potential effects of 50 mg naltrexone (NTX) in attenuating cue reactivity and generally found NTX to be a promising pharmacotherapy through reducing cue-induced craving or other neural and physiological cue reactivity and altering functional connectivity [33,44,45]. Furthermore, NTX was found to moderate the associations between cue-elicited craving with precuneus connectivity to the sensorimotor and frontal regions [33] and post-infusion subjective positive methamphetamine effects (i.e., good effects, feel drug, high) [45], thereby suggesting brain-activity dependent or behavioral mechanisms by which naltrexone may be efficacious in treating methamphetamine use disorder. Only one study examined the effectiveness of oral aripiprazole (15 mg) treatment, which did not reduce cue-elicited craving and cardiovascular response and the authors proposed to conduct further research with lower doses of aripiprazole before ruling it out as a treatment for methamphetamine dependence [46]. The effects of bupropion treatment were also examined in a single study and bupropion was found to block cue-induced craving [47].
3.4.2. Non-Pharmacological Measures
Non-Invasive Brain Stimulation
Six studies assessed either repetitive transcranial magnetic stimulation (rTMS) (n = 4) or transcranial direct current stimulation (tDCS) (n = 2) of the dorsolateral prefrontal cortex (DLPFC) to evaluate changes in cue-induced craving [40,48,49,50,51,52]. Li et al. (2013) found low-frequency rTMS of 1 Hz to transiently increase cue-induced craving [48] while Su et al. (2017) found high frequency rTMS of 10 Hz to reduce cue-induced craving in methamphetamine users [40]. Despite these conflicting results with regard to the effect of rTMS on left DLPFC, it was proposed that low frequency rTMS (<1 Hz) tends to produce inhibitory effect while high frequency rTMS (>5 Hz) tends to increase cortical excitability, and therefore high frequency of 10 Hz rTMS is generally most used in the treatment of substance addiction [40]. This is also in line with the “inter-inhibition between two hemispheres” theory in neuro-rehabilitation field which proposed that excitation of unilateral cortical region leads to suppression of the contralateral side. In another words, “high frequency on the left” equals to “low frequency on the right” and therefore high frequency stimulation would result in opposing effects to low frequency treatment [53]. Yet, a later study by Liu and colleagues demonstrated conflicting results where minimal differences across the different combinations between left/right hemisphere and high/low frequency rTMS were reported and all the four protocols (10 Hz L-DLPFC, 10 Hz R-DLPFC, 1 Hz L-DLPFC, 1 Hz R-DLPFC) were found to be effective in managing cue-induced craving. The authors suggested potential explanations to their results such as the use of different rTMS modes or stimulation targets and individual differences in reaction to plasticity induction protocols [49]. A newer form of rTMS, known as the intermittent theta burst stimulation (iTBS) that can induce long-term potentiation but deliver the same number of pulses in a shorter time with excitatory effects similar to traditional 10 Hz stimulation, was also found to reduce cue-induced craving significantly over four weeks of intervention in a recent study [52]. Two studies looking at tDCS (current intensity = 2 mA) of the DLPFC also showed inconsistent results, with both revealing a state dependent effect on methamphetamine cravings (induced vs non-induced). A single session of anodal tDCS on the right DLPFC decreased immediate subjective craving at rest after 10 min but increased craving rating upon exposure to methamphetamine cues compared to the sham condition, with the more provocative cues inducing significantly more cravings [50]. On the other hand, repeated sessions of bilateral tDCS (anodal stimulation of right hemisphere and cathodal stimulation of left hemisphere) reduced cue-induced craving but did not alter instant craving [51]. It was proposed that the different numbers of tDCS sessions or montages used by the two studies may have contributed to such discrepancy in findings [51].
Behavioral Interventions
The efficacy of an attentional bias modification (ABM) program to reduce attention bias towards drug-related stimuli was tested in one study which indicated that the ABM training did not lead to reductions in craving for methamphetamine or in attentional bias to methamphetamine-related stimuli [34]. The authors, however, highlighted several study limitations including the need to improve measurement of attention bias, that may have led to the discouraging results of the ABM training. Three studies reported on interventions related to cue exposure therapy or counterconditioning procedure, i.e., the use of cue paradigms to extinguish conditioned responses to drug cues. DeSantis et al. (2009) found that participation in a human laboratory cue reactivity paradigm was associated with longitudinal (14 days after study) decreased odds of drug use among methamphetamine-dependent participants [54]. Price et al. (2010) also examined drug cue reactivity and response extinction in a laboratory setting where participants underwent a total of six cue exposure sequences, and data revealed a mean percentage change of −84.4% in craving score from Sequence 1 to end of the last cue sequence, with11 of the 20 participants reported no craving at the end of Sequence 6. Lastly, another study using virtual reality counterconditioning procedure (VRCP) and its computerized version found participants in the intervention groups to show a significantly larger decrease on methamphetamine craving and liking in a group of patients, as well as in heart rate variability on time domain and non-linear domain from baseline to follow-up assessments in a separate sample [26]. These results therefore indicated that extinction of drug-cue conditioned responding can occur in methamphetamine-using individuals, offering promise for the development of extinction- or counterconditioning-based treatment strategies. Findings from two studies suggested that applying behavioral self-regulation can also be helpful in reducing cue-elicited cravings. In a study by Lopez et al. (2015), participants reported significantly lower cue-induced craving when focusing on the negative consequences associated with methamphetamine use (e.g., how tired or sad they might feel the next day, how much money they spend on methamphetamine use, and any damage to their relationships resulting from use), instead of the positive aspects (e.g., how smoking methamphetamine might cause pleasant physical sensations, increase their energy, and make them feel good) [41]. In another qualitative study, respondents reported using different personalized methods in real life for controlling drug or cue-induced urges such as focusing on the importance of maintaining structure in their lives and resetting limits or obstacles that would make future use difficult [55].
4. Discussion
This review seeks to identify and describe the type of available literature related to cue reactivity and the use of cue paradigms in methamphetamine research. A scoping review was chosen as it helps to provide a framework to (1) map the key concepts and insights, (2) summarize and share existing research findings and (3) determine gaps in the current literature. To the authors’ knowledge, only one article published in 2010 had provided a preliminary review on studies that apply the different cues as main methods of craving induction in laboratory settings among human methamphetamine dependents [12]. Six studies [24,28,37,38,47,56] discussed in the article were also included in our scoping review. This brief report was, however, conducted ad-hoc without following a specific methodology, and may thus lack the thoroughness and rigor compared to a review that conducts its search strategy using a systematic approach.
The current scoping review identified 32 relevant articles that either involved the use of a cue reactivity paradigm in their studies or allowed us to understand more about cue reactivity in methamphetamine abuse or dependence. Our results revealed high variability in the terms of reporting and conduct of the included studies and sources of heterogeneity include differences in sample groups, methodologies, type of cue reactivity paradigms, interventions and comparators, outcome measures and even research purposes across the studies. The included articles were predominantly from two countries—USA (n = 16) followed by China (n = 11), with an under-representation of studies from other parts of the world. Importantly, three overarching themes were identified in this review and they provided us with further knowledge on (1) the effect of methamphetamine cue exposure, (2) possible factors that correlated with cue reactivity, and (3) strategies that could modulate cue reactivity.
Firstly, it is evident from our review that methamphetamine-related cues can result in significant impact associated with exposure of these cues among drug users. These include an increase in subjective craving, physiological responses, activity in specific brain regions, as well as cognitive impairment. Notably, some studies did not report any significant change in physiological reactivity upon cue exposure [24,46] while one study found a decreased heart rate in response to methamphetamine cues [28], which clearly contrasts with results from majority of cue reactivity studies in the current review, as well as for other drugs of abuse. Such variation in findings may be due to methodological heterogeneity across the studies. The increased neural reactivity in response to cues was revealed to occur mainly in the mesocorticolimbic system such as ACC, posterior cingulate cortex (PCC), DLPFC, and orbitofrontal cortex (OFC) involved in relapse mechanism, demonstrating the incentive salience of drug cues. This finding is also consistent with other neuroimaging studies conducted among individuals with other drugs such as nicotine, cocaine, heroin, marijuana and alcohol [57]. Of the studies that looked primarily at the impact of cue exposure, only seven of them had recruited healthy controls for comparison and the lack of this control group could be seen as a shortcoming of many studies. Despite so, healthy controls in all these seven studies did not show significant change in response to methamphetamine cues compared to neutral cues, thus providing support to the theory that cue reactivity may indeed be a result of Pavlovian conditioning. In other words, the lack of drug expectancies among non-drug users lead to lack of cue reactivity. Nonetheless, future studies that aimed to explore effects of cue exposure should include a healthy control group to provide further support for the theory.
The current review also identified several individual-specific and strategy-specific factors that have been shown to affect reactivity to methamphetamine-related cues (see Figure 2). The former includes methamphetamine use profile, personality attributes and the association of cue-elicited craving with neural or physiological reactivity, while the latter includes non-invasive brain stimulation (e.g., rTMS and tDCS) and behavioral techniques (e.g., cognitive regulation, cue extinction and counterconditioning procedures). Individual factors such as demographic characteristics were typically not found to associate with cue reactivity, while interventions such as aripiprazole treatment and attentional bias modification did not significantly reduce cue reactivity. Drug cue reactivity is a complex phenomenon and is not surprisingly modulated by a large number of factors (i.e., main effects) as well as their interactions. In terms of its relationship with individual-specific factors, the significance, direction and magnitude of their associations may require more robust research with larger study samples to confirm on the findings. For modulation due to interventions or strategies, the findings from studies (e.g., on aripiprazole, attentional bias modification, and cognitive regulation) with smaller sample sizes (<20 participants) in particular should be treated with caution as the likelihood of a Type II error is increased in these studies, and therefore decreases the power of the study.
Future Directions on Treatment Options
There is currently no medication with well-established efficacy for the treatment of methamphetamine use disorder, nor is there any medication approved by regulatory authorities (e.g., U.S. Food and Drug Administration) for use in methamphetamine dependence [19]. Craving is an important symptom and server that maintains methamphetamine dependence as it is often elicited by drug-related or contextual cues, and eventually leading to relapse of the drug [58]. As a result, cue-induced craving has always been regarded as a primary target for relapse prevention.
Our review suggested that oral naltrexone (NTX), an opioid receptor antagonist, could be a potential pharmacotherapy for preventing relapse to methamphetamine. Three studies examined the efficacy of NTX in modulating or reducing cue-induced craving and all revealed positive results in favor of the drug [33,44,45]. Furthermore, NTX appeared to be well-tolerated and had very minimal side effects [44]. Despite these promising results, the use of NTX in targeting methamphetamine relapse needs to be supported by more clinical trials and evidence. Scientifically-based approaches to evaluate medications that limit brain exposure to methamphetamine, modulate methamphetamine effects at vesicular monoamine transporter-2, or target dopaminergic, serotonergic, gamma-aminobutyric acid (GABA)-ergic, and/or glutamatergic brain pathways have already been underway [19,59] and despite the increasing efforts made to review medications for the treatment of methamphetamine dependence, many of these trials failed to consider their course of action on cue-induced reactivity.
For behavioral intervention, the use of either cue extinction or counterconditioning strategies appeared to be promising in preventing relapse due to cue reactivity based on findings from our review. Studies among healthy humans have consistently shown that conditioning, the process by which contextual cues becomes associated with methamphetamine through repeated pairing, is the key to understanding addiction and problematic drug use [60,61]. Literature, however, suggests that extinction procedure may not be adequate in suppressing drug craving [62] and it was proposed that the affective component inherent to the drug-related cues tends to hinder the efficacy of cue exposure-based therapy in individuals with substance use disorder [63]. In this sense, the counterconditioning approach has been viewed to be a better alternative as it not only decreases the unconditioned expectancy, but also changes the emotional valence of conditioned stimuli through pairing new unconditioned stimuli with an opposite valence [26]. Previous studies have also supported counterconditioning procedures to exert a stronger suppressing effect on the relapse of memories or the cue-drug association than extinction [64,65]. Future methamphetamine studies could focus on these non-pharmacological strategies and compare the difference between extinction and counterconditioning procedures in reducing cue reactivity. As with research on pharmacological therapies, while literature identified other psychosocial interventions such as cognitive behavioral therapy, counselling or motivational interviewing, and contingency management to show effectiveness in the treatment of methamphetamine dependence [66], there was a paucity of research that addressed cue-induced craving and reactivity to cues as study outcomes. Lastly, there also appears to be a lack of studies looking at the combined effect of pharmacotherapy and psychological treatments in reducing reactivity to methamphetamine-related cues.
5. Limitations
Although it is not the main tenet of a scoping review, it must be acknowledged that we did not formally assess the methodological quality of the studies, particularly those that evaluated interventions. Among which, we also included one qualitative study and few of these studies did not include a control group with only a pre-post study design. The majority of studies had small sample sizes except one, which had over 1000 study participants. The current review also did not include grey literature (e.g., conference abstracts), which may have led us to miss out some relevant studies or possible interventions. The restriction of searches to well-established academic databases and exclusion of grey literature may also lead to a potential publication bias as studies with null findings are less likely to be published in peer-reviewed journals.
6. Conclusions
Cue reactivity studies have been shown to be useful for understanding how craving would lead to continued drug-seeking behaviors and relapse among abusers in a real-life environment. Exposure to methamphetamine-associated cues can significantly induce measurable craving or other autonomic reactivity in laboratory settings. Our scoping review provides insights into the type of cue reactivity, as well as in identifying and characterizing specific factors that modulate this reactivity in methamphetamine research. The use of cue reactivity paradigms also has important implications for the development of new pharmacological and psychosocial interventions for methamphetamine relapse prevention. Further studies on cue-induced craving are necessary to explore the effects that this notion could bring to treatment approaches.
Author Contributions
L.S.E.S. and W.J.O. conducted the searches and wrote the first draft together. M.S., A.H. and P.V.A. provided intellectual input. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Home Affairs, Singapore.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Tiffany, S.T. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol. Rev. 1990, 97, 147–168. [Google Scholar] [CrossRef]
- Drummond, D.C. Theories of drug craving, ancient and modern. Addiction 2001, 96, 33–46. [Google Scholar] [CrossRef]
- Childress, A.R.; McLellan, A.T.; O’Brien, C.P. Abstinent Opiate Abusers Exhibit Conditioned Craving, Conditioned Withdrawal and Reductions in both through Extinction. Br. J. Addict. 1986, 81, 655–660. [Google Scholar] [CrossRef]
- O’Brien, C.P.; Childress, A.R.; McLellan, A.T.; Ehrman, R. Classical conditioning in drug-dependent humans. Ann. N. Y. Acad. Sci. 1992, 654, 400–415. [Google Scholar] [CrossRef]
- Carter, B.L.; Tiffany, S.T. Meta-analysis of cue-reactivity in addiction research. Addiction 1999, 94, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Wikler, A. Conditioning factors in opiate addiction and relapse. J. Subst. Abus. Treat. 1984, 1, 279–285. [Google Scholar] [CrossRef]
- Stewart, J.; de Wit, H.; Eikelboom, R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 1984, 91, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, P.I. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Ann. Neurosci. 2010, 17, 136–141. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Drummond, D.C. What does cue-reactivity have to offer clinical research? Addiction 2000, 95, S129–S144. [Google Scholar] [CrossRef]
- Modesto-Lowe, V.; Kranzler, H.R. Using cue reactivity to evaluate medications for treatment of cocaine dependence: A critical review. Addiction 1999, 94, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Mehrjerdi, Z.A.; Tasnim, S.; Ekhtiari, H. Measurement of cue-induced craving in human methamphetamine dependent subjects new methodological hopes for reliable assessment of treatment efficacy. Basic Clin. Neurosci. 2011, 2, 48–53. [Google Scholar]
- Barr, A.M.; Panenka, W.J.; MacEwan, G.W.; Thornton, A.E.; Lang, D.J.; Honer, W.G.; Lecomte, T. The need for speed: An update on methamphetamine addiction. J. Psychiatry Neurosci. 2006, 31, 301–313. [Google Scholar] [PubMed]
- Radfar, S.R.; Rawson, R.A. Current research on methamphetamine: Epidemiology, medical and psychiatric effects, treatment, and harm reduction efforts. Addict. Health 2014, 6, 146. [Google Scholar] [PubMed]
- Petit, A.; Karila, L.; Chalmin, F. Methamphetamine Addiction: A Review of the Literature. J. Addict. Res. Ther. 2012, 1. [Google Scholar] [CrossRef]
- The United Nations Office on Drugs and Crime: World Drug Report. 2019. Available online: https://wdr.unodc.org/wdr2019/ (accessed on 8 March 2020).
- Stoneberg, D.M.; Shukla, R.K.; Magness, M.B. Global methamphetamine trends: An evolving problem. Int. Crim. Justice Rev. 2018, 28, 136–161. [Google Scholar] [CrossRef]
- The United Nations Office on Drugs and Crime: Patterns and Trends of Amphetamine=Type Stimulants and other Drugs. Available online: http://www.unodc.org/documents/data-and-analysis/WDR2012/WDR_2012_web_small.pdf (accessed on 8 March 2020).
- Vocci, F.J.; Appel, N.M. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction 2007, 102 (Suppl. 1), 96–106. [Google Scholar] [CrossRef]
- Rohsenow, D.J.; Niaura, R.S.; Childress, A.R.; Abrams, D.B.; Monti, P.M. Cue reactivity in addictive behaviors: Theoretical and treatment implications. Int. J. Addict. 1990, 25, 957–993. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Drummond, D.C.; Tiffany, S.T.; Glautier, S.; Remington, B. Cue exposure in understanding and treating addictive behaviours. In Addictive Behaviour: Cue Exposure Theory and Practice; John Wiley & Sons: Oxford, UK, 1995; pp. 1–17. [Google Scholar]
- Culbertson, C.; Nicolas, S.; Zaharovits, I.; London, E.D.; La Garza, R.D.; Brody, A.L.; Newton, T.F. Methamphetamine craving induced in an online virtual reality environment. Pharmacol. Biochem. Behav. 2010, 96, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Shen, Z.H.; Wu, X.C. Detection of patients with methamphetamine dependence with cue-elicited heart rate variability in a virtual social environment. Psychiatry Res. 2018, 270, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Liu, M.H.; Shen, Z.H. A virtual reality counterconditioning procedure to reduce methamphetamine cue-induced craving. J. Psychiatr. Res. 2019, 116, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chen, T.; Du, J.; Li, R.; Jiang, H.; Deng, C.-L.; Song, W.; Xu, D.; Zhao, M. Drug-related Virtual Reality Cue Reactivity is Associated with Gamma Activity in Reward and Executive Control Circuit in Methamphetamine Use Disorders. Arch. Med. Res. 2019, 50, 509–517. [Google Scholar] [CrossRef]
- Tolliver, B.K.; McRae-Clark, A.L.; Saladin, M.; Price, K.L.; Simpson, A.N.; DeSantis, S.M.; Baker, N.L.; Brady, K.T. Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am. J. Drug Alcohol Abus. 2010, 36, 106–113. [Google Scholar] [CrossRef]
- Grodin, E.N.; Courtney, K.E.; Ray, L.A. Drug-Induced Craving for Methamphetamine Is Associated with Neural Methamphetamine Cue Reactivity. J. Stud. Alcohol Drugs 2019, 80, 245–251. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Z.; Dai, Y.; Zhang, C.; Yang, C.; Fan, L.; Liu, J.; Hao, W.; Chen, H. Craving responses to methamphetamine and sexual visual cues in individuals with methamphetamine use disorder after long-term drug rehabilitation. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef]
- Malcolm, R.; Myrick, H.; Li, X.; Henderson, S.; Brady, K.T.; George, M.S.; See, R.E. Regional Brain Activity in Abstinent Methamphetamine Dependent Males Following Cue Exposure. J. Drug Abuse 2016, 2, 16. [Google Scholar] [CrossRef]
- Yin, J.J.; Ma, S.H.; Xu, K.; Wang, Z.X.; Le, H.B.; Huang, J.Z.; Fang, K.M.; Liao, L.M.; Cai, Z.L. Functional magnetic resonance imaging of methamphetamine craving. Clin. Imaging 2012, 36, 695–701. [Google Scholar] [CrossRef]
- Courtney, K.E.; Ghahremani, D.G.; Ray, L.A. The Effects of Pharmacological Opioid Blockade on Neural Measures of Drug Cue-Reactivity in Humans. Neuropsychopharmacology 2016, 41, 2872–2881. [Google Scholar] [CrossRef]
- Dean, A.C.; Nurmi, E.L.; Moeller, S.J.; Amir, N.; Rozenman, M.; Ghahremani, D.G.; Johnson, M.; Berberyan, R.; Hellemann, G.; Zhang, Z.; et al. No effect of attentional bias modification training in methamphetamine users receiving residential treatment. Psychopharmacology 2019, 236, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, S.; Yang, C.; Cai, W.; Chen, H.; Hao, W.; Liu, T.; Wang, X.; Worhunsky, P.D.; Potenza, M.N. Neurofunctional Differences Related to Methamphetamine and Sexual Cues in Men with Shorter and Longer Term Abstinence Methamphetamine Dependence. Int. J. Neuropsychopharmacol. 2019. [Google Scholar] [CrossRef]
- Shahmohammadi, F.; Golesorkhi, M.; Riahi Kashani, M.M.; Sangi, M.; Yoonessi, A.; Yoonessi, A. Neural Correlates of Craving in Methamphetamine Abuse. Basic Clin. Neurosci. 2016, 7, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Tolliver, B.K.; Price, K.L.; Baker, N.L.; LaRowe, S.D.; Simpson, A.N.; McRae-Clark, A.L.; Saladin, M.E.; DeSantis, S.M.; Chapman, E.; Garrett, M.; et al. Impaired cognitive performance in subjects with methamphetamine dependence during exposure to neutral versus methamphetamine-related cues. Am. J. Drug Alcohol Abuse 2012, 38, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ekhtiari, H.; Alam-Mehrjerdi, Z.; Nouri, M.; George, S.; Mokri, A. Designing and evaluation of reliability and validity of visual cue-induced craving assessment task for methamphetamine smokers. Basic Clin. Neurosci. 2010, 1, 34–37. [Google Scholar]
- Liang, Q.; Yuan, T.; Cao, X.; He, H.; Yang, J.; Yuan, J. Assessing the severity of methamphetamine use disorder beyond the subjective craving report: The role of an attention bias test. Gen. Psychiatry 2019, 32, e100019. [Google Scholar] [CrossRef]
- Su, H.; Zhong, N.; Gan, H.; Wang, J.; Han, H.; Chen, T.; Li, X.; Ruan, X.; Zhu, Y.; Jiang, H.; et al. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug Alcohol Depend. 2017, 84–91. [Google Scholar] [CrossRef]
- Lopez, R.B.; Onyemekwu, C.; Hart, C.L.; Ochsner, K.N.; Kober, H. Boundary conditions of methamphetamine craving. Exp. Clin. Psychopharmacol. 2015, 23, 436–444. [Google Scholar] [CrossRef]
- Wang, G.; Shi, J.; Chen, N.; Xu, L.; Li, J.; Li, P.; Sun, Y.; Lu, L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS ONE 2013, 8, e68791. [Google Scholar] [CrossRef]
- Saladin, M.; Santa Ana, E.; La Rowe, S.; Simpson, A.; Tolliver, B.; Price, K.; McRae-Clark, A.; Brady, K. Does Alexithymia Explain Variation in Cue-Elicited Craving Reported by Methamphetamine-Dependent Individuals? Am. J. Addict. 2012, 21, 130–135. [Google Scholar] [CrossRef]
- Ray, L.A.; Bujarski, S.; Courtney, K.E.; Moallem, N.R.; Lunny, K.; Roche, D.; Leventhal, A.M.; Shoptaw, S.; Heinzerling, K.; London, E.D.; et al. The Effects of Naltrexone on Subjective Response to Methamphetamine in a Clinical Sample: A Double-Blind, Placebo-Controlled Laboratory Study. Neuropsychopharmacology 2015, 40, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Roche, D.; Worley, M.; Ray, L. Naltrexone moderates the relationship between cue-induced craving and subjective response to methamphetamine in individuals with methamphetamine use disorder. Neuropsychopharmacology 2016, 41, S606–S607. [Google Scholar] [CrossRef] [PubMed]
- Newton, T.F.; Reid, M.S.; De La Garza, I.R.; Mahoney, I.J.J.; Abad, A.; Condos, R.; Palamar, J.; Halkitis, P.N.; Mojisak, J.; Anderson, A.; et al. Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. Int. J. Neuropsychopharmacol. 2008, 11, 1037–1045. [Google Scholar] [CrossRef]
- Newton, T.F.; Roache, J.D.; De La Garza, R., II; Fong, T.; Wallace, C.L.; Li, S.-H.; Elkashef, A.; Chiang, N.; Kahn, R. Bupropion Reduces Methamphetamine-Induced Subjective Effects and Cue-Induced Craving. Neuropsychopharmacology 2006, 31, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Malcolm, R.J.; Huebner, K.; Hanlon, C.A.; Taylor, J.J.; Brady, K.T.; George, M.S.; See, R.E. Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: A preliminary study. Drug Alcohol Depend. 2013, 133, 641–646. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, Y.; Cao, X.; Li, Y.; Chen, Y.; Yang, W.; Yuan, T.-F. Either at left or right, both high and low frequency rTMS of dorsolateral prefrontal cortex decreases cue induced craving for methamphetamine. Am. J. Addict. 2017, 26, 776–779. [Google Scholar] [CrossRef]
- Shahbabaie, A.; Golesorkhi, M.; Zamanian, B.; Ebrahimpoor, M.; Keshvari, F.; Nejati, V.; Fregni, F.; Ekhtiari, H. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int. J. Neuropsychopharmacol. 2014, 17, 1591–1598. [Google Scholar] [CrossRef]
- Rohani Anaraki, M.; Dolatshahi, B.; Nosratabadi, M.; Nouri Yalghouzaghaji, M.; Rezaei Mashhadi, S. Repeated Transcranial Direct Current Stimulation (tDCS) on Methamphetamine Craving: A Randomized, Sham-controlled Study. Iran. Rehabil. J. 2019, 17, 385–394. [Google Scholar] [CrossRef]
- Su, H.; Chen, T.; Jiang, H.; Zhong, N.; Du, J.; Xiao, K.; Xu, D.; Song, W.; Zhao, M. Intermittent theta burst transcranial magnetic stimulation for methamphetamine addiction: A randomized clinical trial. Eur. Neuropsychopharmacol. 2020, 31, 158–161. [Google Scholar] [CrossRef]
- Kirton, A.; Chen, R.; Friefeld, S.; Gunraj, C.; Pontigon, A.M.; Deveber, G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: A randomised trial. Lancet Neurol. 2008, 7, 507–513. [Google Scholar] [CrossRef]
- Desantis, S.M.; Bandyopadhyay, D.; Back, S.E.; Brady, K.T.; DeSantis, S.M.; Bandyopadhyay, D.; Back, S.E.; Brady, K.T. Non-treatment laboratory stress- and cue-reactivity studies are associated with decreased substance use among drug-dependent individuals. Drug Alcohol Depend. 2009, 105, 227–233. [Google Scholar] [CrossRef][Green Version]
- Bruehl, A.M.; Lende, D.H.; Schwartz, M.; Sterk, C.E.; Elifson, K. Craving and control: Methamphetamine users’ narratives. J. Psychoact. Drugs 2006, 38 (Suppl. 3), 385–392. [Google Scholar] [CrossRef]
- Price, K.L.; Saladin, M.E.; Baker, N.L.; Tolliver, B.K.; DeSantis, S.M.; McRae-Clark, A.L.; Brady, K.T. Extinction of drug cue reactivity in methamphetamine-dependent individuals. Behav. Res. Ther. 2010, 48, 860–865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jasinska, A.J.; Stein, E.A.; Kaiser, J.; Naumer, M.J.; Yalachkov, Y. Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci. Biobehav. Rev. 2014, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hormes, J.M. The clinical significance of craving across the addictive behaviors: A review. Curr. Addict. Rep. 2017, 4, 132–141. [Google Scholar] [CrossRef]
- Karila, L.; Weinstein, A.; Aubin, H.J.; Benyamina, A.; Reynaud, M.; Batki, S.L. Pharmacological approaches to methamphetamine dependence: A focused review. Br. J. Clin. Pharmacol. 2010, 69, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.; De Wit, H. A multidimensional approach to studying responses to a methamphetamine-associated contextual cue in healthy, non-dependent humans. Neuropsychopharmacology 2014, 39, S516. [Google Scholar] [CrossRef][Green Version]
- Cavallo, J.S.; Mayo, L.M.; de Wit, H. Acquisition of Conditioning between Methamphetamine and Cues in Healthy Humans. PLoS ONE 2016, 11, e0161541. [Google Scholar] [CrossRef]
- Conklin, C.A.; Tiffany, S.T. Applying extinction research and theory to cue-exposure addiction treatments. Addiction 2002, 97, 155–167. [Google Scholar] [CrossRef]
- Hone-Blanchet, A.; Wensing, T.; Fecteau, S. The use of virtual reality in craving assessment and cue-exposure therapy in substance use disorders. Front. Hum. Neurosci. 2014, 8, 844. [Google Scholar] [CrossRef]
- Tunstall, B.J.; Verendeev, A.; Kearns, D.N. A comparison of therapies for the treatment of drug cues: Counterconditioning vs. extinction in male rats. Exp. Clin. Psychopharmacol. 2012, 20, 447. [Google Scholar] [CrossRef] [PubMed]
- Newall, C.; Watson, T.; Grant, K.-A.; Richardson, R. The relative effectiveness of extinction and counter-conditioning in diminishing children’s fear. Behav. Res. Ther. 2017, 95, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ciketic, S.; Hayatbakhsh, M.R.; Doran, C.M.; Najman, J.M.; McKetin, R. A review of psychological and pharmacological treatment options for methamphetamine dependence. J. Subst. Use 2012, 17, 363–383. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).