Comparisons of Muscle Quality and Muscle Growth Factor Between Sarcopenic and Non-Sarcopenic Older Women
Abstract
1. Introduction
2. Materials and Methods
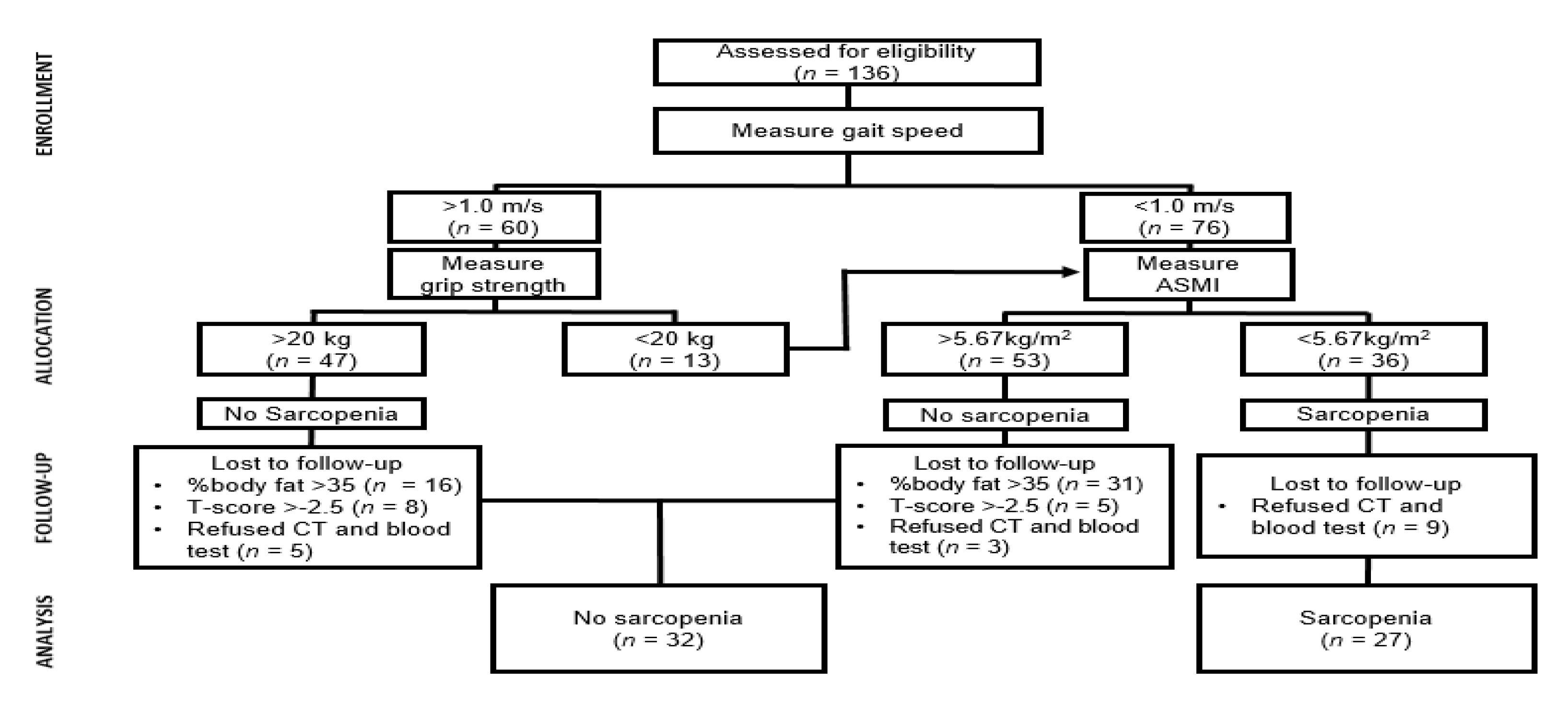
2.1. Participants
2.2. Anthropometric Measurements
2.3. Body Composition and Bone Mineral Density
2.4. Functional Fitness Test
2.5. Muscle Quality Assessment
2.6. Biochemical Markers
2.7. Questionnaire
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., III; Khosla, S.; Crowson, C.S.; O’Connor, M.K.; O’Fallon, W.M.; Riggs, B.L. Epidemiology of sarcopenia. J. Am. Geriatr. Soc. 2000, 48, 625–630. [Google Scholar] [CrossRef]
- Dalal, M.; Ferrucci, L.; Sun, K.; Beck, J.; Fried, L.P.; Semba, R.D. Elevated Serum Advanced Glycation End Products and Poor Grip Strength in Older Community-Dwelling Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Ferrucci, L.; Sun, K.; Fried, L.P.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Oxidative protein damage is associated with poor grip strength among older women living in the community. J. Appl. Physiol. 2007, 103, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.; Pluijm, S.M.F.; Deeg, D.J.H.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Colbert, L.H.; Pahor, M.; Rubin, S.M.; Tylavsky, F.A.; et al. Higher Inflammatory Marker Levels in Older Persons: Associations With 5-Year Change in Muscle Mass and Muscle Strength. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 1183–1189. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Baumgartner, R.N.; Atkinson, H.H.; Penninx, B.W.H.J.; Lenchik, L.; Palla, S.L.; Ambrosius, W.T.; Tracy, R.P.; Pahor, M. Sarcopenia, obesity, and inflammation—Results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am. J. Clin. Nutr. 2005, 82, 428–434. [Google Scholar] [CrossRef]
- Ferrucci, L.; Penninx, B.W.J.H.; Volpato, S.; Harris, T.B.; Bandeen-Roche, K.; Balfour, J.; Leveille, S.G.; Fried, L.P.; Guralnik, J.M. Change in Muscle Strength Explains Accelerated Decline of Physical Function in Older Women With High Interleukin-6 Serum Levels. J. Am. Geriatr. Soc. 2002, 50, 1947–1954. [Google Scholar] [CrossRef]
- Szulc, P.; Duboeuf, F.; Marchand, F.; Delmas, P.D. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS study. Am. J. Clin. Nutr. 2004, 80, 496–503. [Google Scholar] [CrossRef]
- Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Newman, A.B.; Nevitt, M.; Rubin, S.M.; Simonsick, E.M.; Harris, T.B. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 324–333. [Google Scholar] [CrossRef]
- Park, S.; Ham, J.-O.; Lee, B.-K. A positive association of vitamin D deficiency and sarcopenia in 50 year old women, but not men. Clin. Nutr. 2014, 33, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Van Atteveld, V.A.; Van Ancum, J.M.; Reijnierse, E.M.; Trappenburg, M.C.; Meskers, C.G.M.; Maier, A. Erythrocyte sedimentation rate and albumin as markers of inflammation are associated with measures of sarcopenia: A cross-sectional study. BMC Geriatr. 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- Ebner, N.; Steinbeck, L.; Doehner, W.; Anker, S.D.; Von Haehling, S. Highlights from the 7th Cachexia Conference: Muscle wasting pathophysiological detection and novel treatment strategies. J. Cachex Sarcopenia Muscle 2014, 5, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mafi, F.; Biglari, S.; Ghardashi-Afousi, A.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Curcio, F.; Ferro, G.; Basile, C.; Liguori, I.; Parrella, P.; Pirozzi, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Tocchetti, C.G.; et al. Biomarkers in sarcopenia: A multifactorial approach. Exp. Gerontol. 2016, 85, 18. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Abe, T.; Thiebaud, R.S.; Loenneke, J.P.; Loftin, M.; Fukunaga, T. Prevalence of site-specific thigh sarcopenia in Japanese men and women. AGE 2013, 36, 417–426. [Google Scholar] [CrossRef]
- Kara, M.; Ata, A.M.; Çakır, B.; Kaymak, B.; Özçakar, L. The Impact of Cut-Off Values and Adjustments for Muscle Mass and Strength on Diagnosis of Sarcopenia. J. Am. Med. Dir. Assoc. 2019, 20, 1653. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and Validation of Criterion-Referenced Clinically Relevant Fitness Standards for Maintaining Physical Independence in Later Years. Gerontology 2012, 53, 255–267. [Google Scholar] [CrossRef]
- Cohn, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Earlbam Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Kim, M.; Won, C.W. Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: Findings from the Korean Frailty and Aging Cohort Study. Age Ageing 2019, 48, 910–916. [Google Scholar] [CrossRef]
- Nasimi, N.; Dabbaghmanesh, M.H.; Sohrabi, Z. Nutritional status and body fat mass: Determinants of sarcopenia in community-dwelling older adults. Exp. Gerontol. 2019, 122, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Aspray, T.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Cherry, K.E.; Su, L.J.; Kim, S.; Myers, L.; Welsh, D.A.; Jazwinski, S.M.; Ravussin, E. Body Composition, IGF1 Status, and Physical Functionality in Nonagenarians: Implications for Osteosarcopenia. J. Am. Med. Dir. Assoc. 2019, 20, 70–75.e2. [Google Scholar] [CrossRef]
- Rolland, Y.; Gallini, A.; Cristini, C.; Schott, A.M.; Blain, H.; Beauchet, O.; Cesari, M.; Lauwers-Cances, V. Body-composition predictors of mortality in women aged ≥ 75 y: Data from a large population-based cohort study with a 17-y follow-up. Am. J. Clin. Nutr. 2014, 100, 1352–1360. [Google Scholar] [CrossRef]
- Wu, C.-H.; Yang, K.-C.; Chang, H.-H.; Yen, J.-F.; Tsai, K.-S.; Huang, K.-C.; Yang, K.-C. Sarcopenia is Related to Increased Risk for Low Bone Mineral Density. J. Clin. Densitom. 2013, 16, 98–103. [Google Scholar] [CrossRef]
- Lima, R.M.; De Oliveira, R.J.; Raposo, R.; Neri, S.G.R.; Gadelha, A.B. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch. Osteoporos. 2019, 14, 38. [Google Scholar] [CrossRef]
- Tang, T.; Wu, L.; Yang, L.; Jiang, J.; Hao, Q.; Dong, B.; Yang, M. A sarcopenia screening test predicts mortality in hospitalized older adults. Sci. Rep. 2018, 8, 2923. [Google Scholar] [CrossRef]
- Beaudart, C.; Reginster, J.-Y.; Petermans, J.; Gillain, S.; Quabron, A.; Locquet, M.; Slomian, J.; Buckinx, F.; Bruyère, O. Quality of life and physical components linked to sarcopenia: The SarcoPhAge study. Exp. Gerontol. 2015, 69, 103–110. [Google Scholar] [CrossRef]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Heal. 2014, 3, 9. [Google Scholar] [CrossRef]
- Fragala, M.S.; Kenny, A.M.; Kuchel, G.A. Muscle Quality in Aging: A Multi-Dimensional Approach to Muscle Functioning with Applications for Treatment. Sports Med. 2015, 45, 641–658. [Google Scholar] [CrossRef]
- Tsukasaki, K.; Matsui, Y.; Arai, H.; Harada, A.; Tomida, M.; Takemura, M.; Otsuka, R.; Ando, F.; Shimokata, H. Association of Muscle Strength and Gait Speed with Cross-Sectional Muscle Area Determined by Mid-Thigh Computed Tomography—A Comparison with Skeletal Muscle Mass Measured by Dual-Energy X-ray Absorptiometry. J. Frailty Aging 2020, 9, 82–89. [Google Scholar] [PubMed]
- Lang, T.F.; A Cauley, J.; Tylavsky, F.; Bauer, U.; Cummings, S.; Harris, T.B. Computed Tomographic Measurements of Thigh Muscle Cross-Sectional Area and Attenuation Coefficient Predict Hip Fracture: The Health, Aging, and Body Composition Study. J. Bone Miner. Res. 2009, 25, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.W.W.; Husaini, Y.; Manandhar, R.; Lee-Ng, K.K.M.; Zhang, H.P.; Harriott, K.; Jiang, L.; Lin, S.; Sainsbury, A.; Brown, D.A.; et al. Anorexia/cachexia of chronic diseases: A role for the TGF-β family cytokine MIC-1/GDF15. J. Cachex- Sarcopenia Muscle 2012, 3, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Schober-Halper, B.; Oesen, S.; Franzke, B.; Tschan, H.; Bachl, N.; Strasser, E.-M.; Quittan, M.; Wagner, K.-H.; Wessner, B. Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women: The Vienna Active Ageing Study (VAAS). Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 116, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Franz, K.; Ost, M.; Herpich, C.; Otten, L.; Coleman, V.; Klaus, S.; Müller-Werdan, U.; Norman, K. Elevated serum growth differentiation factor 15 levels in geriatric patients–sarcopenia and physical parameters. Innov. Aging 2018, 2, 299. [Google Scholar] [CrossRef]
- Rothenbacher, D.; Dallmeier, D.; Christow, H.; Koenig, W.; Denkinger, M.; Klenk, J.; the ActiFE Study Group; ActiFE Study Group. Association of growth differentiation factor 15 with other key biomarkers, functional parameters and mortality in community-dwelling older adults. Age Ageing 2019, 48, 541–546. [Google Scholar] [CrossRef]
- Ratkevicius, A.; Joyson, A.; Selmer, I.; Dhanani, T.; Grierson, C.; Tommasi, A.M.; Rauchhaus, P.; Crowther, D.; Alesci, S.; Yaworsky, P.; et al. Serum Concentrations of Myostatin and Myostatin-Interacting Proteins Do Not Differ Between Young and Sarcopenic Elderly Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 620–626. [Google Scholar] [CrossRef]
- Tay, L.; Ding, Y.Y.; Leung, B.P.; Ismail, N.H.; Yeo, A.; Yew, S.; Tay, K.S.; Tan, C.H.; Chong, M.S. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. AGE 2015, 37, 1–12. [Google Scholar] [CrossRef]
- Hittel, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.E.; Houmard, J.A. Increased Secretion and Expression of Myostatin in Skeletal Muscle From Extremely Obese Women. Diabetes 2008, 58, 30–38. [Google Scholar] [CrossRef]
- Zhang, C.; Mc Farlane, C.; Lokireddy, S.; Masuda, S.; Ge, X.; Gluckman, P.D.; Sharma, M.; Kambadur, R. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetology 2011, 55, 183–193. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lee, S.J. Suppression of body fat accumulation in myostatin-deficient mice. J. Clin. Investig. 2002, 109, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Loumaye, A.; De Barsy, M.; Nachit, M.; Lause, P.; Frateur, L.; Van Maanen, A.; Tréfois, P.; Gruson, D.; Thissen, J.P. Role of Activin A and Myostatin in Human Cancer Cachexia. J. Clin. Endocrinol. Metab. 2015, 100, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Aukrust, P.; Aakhus, S.; Smith, C.; Endresen, K.; I Birkeland, K.; Gullestad, L.; Johansen, O.E. Activin A and cardiovascular disease in type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2012, 9, 234–237. [Google Scholar] [CrossRef]
- Peng, L.-N.; Lee, W.-J.; Liu, L.-K.; Lin, M.-H.; Chen, L.-K. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J. Cachex- Sarcopenia Muscle 2018, 9, 635–642. [Google Scholar] [CrossRef]
- Miyamoto, T.; Carrero, J.J.; Qureshi, A.R.; Anderstam, B.; Heimbürger, O.; Bárány, P.; Lindholm, B.; Stenvinkel, P. Circulating follistatin in patients with chronic kidney disease: Implications for muscle strength, bone mineral density, inflammation, and survival. Clin. J. Am. Soc. Nephrol. 2011, 6, 1001–1008. [Google Scholar] [CrossRef]
- Liaw, F.; Kao, T.; Fang, W.; Han, D.; Chi, Y.; Yang, W.-S. Increased follistatin associated with decreased gait speed among old adults. Eur. J. Clin. Investig. 2016, 46, 321–327. [Google Scholar] [CrossRef]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef]
| Variable | Sarcopenic (n = 27) | Non-Sarcopenic (n = 32) | t-Value | Cohen’s d | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Anthropo metric | Age (yr) | 71.4 ± 4.88 | 71.6 ± 4.11 | −0.15 | 0.04 | −2.16 | 2.52 |
| Height (cm) | 153.1 ± 5.23 | 153.2 ± 5.89 | −0.08 | 0.02 | −2.82 | 3.04 | |
| Weight (kg) | 52.7 ± 4.88 | 55.5 ± 5.77 | −1.97 | 0.52 | −0.05 | 5.58 | |
| BMI (kg·m−2) | 22.5 ± 1.80 | 23.6 ± 2.28 | −2.14 * | 0.54 | 0.07 | 2.23 | |
| WC (cm) | 76.3 ± 4.82 | 78.8 ± 6.87 | −1.58 | 0.42 | −0.67 | 5.63 | |
| HC (cm) | 90.2 ± 3.92 | 91.6 ± 4.20 | −1.36 | 0.34 | −0.69 | 3.57 | |
| WHR (ratios) | 0.85 ± 0.04 | 0.86 ± 0.06 | 0.98 | 0.20 | −0.01 | 0.04 | |
| Body compositon | Fat mass (kg) | 18.1 ± 3.28 | 16.6 ± 3.01 | 1.91 | 0.48 | −3.21 | 0.07 |
| % Body fat (%) | 34.9 ± 3.92 | 30.3 ± 3.55 | 4.74 *** | 1.23 | −6.56 | −2.67 | |
| LBM (kg) | 32.0 ± 2.43 | 36.1 ± 3.42 | −5.30 *** | 1.38 | 2.60 | 5.75 | |
| ASM (kg) | 12.6 ± 1.02 | 14.6 ± 1.47 | −5.82 *** | 1.58 | 1.27 | 2.62 | |
| Bone mineral density | WBMD(g·cm−2) | 0.93 ± 0.06 | 1.00 ± 0.07 | −3.68 *** | 1.27 | 0.03 | 0.10 |
| WBMD Tscore | −1.92 ± 0.70 | −1.20 ± 0.79 | −3.64 *** | 0.96 | 0.32 | 1.11 | |
| LBMD (g·cm−2) | 0.80 ± 0.10 | 0.88 ± 0.12 | −3.01 *** | 0.79 | 0.03 | 0.15 | |
| LBMD T-score | −1.82 ± 0.87 | −1.05 ± 1.01 | −3.10 *** | 0.82 | 0.27 | 1.27 | |
| FBMD (g·cm−2) | 0.70 ± 0.10 | 0.74 ± 0.08 | −1.69 | 0.44 | −0.01 | 0.87 | |
| FBMD T-score | −1.30 ± 0.88 | −0.96 ± 0.69 | −1.66 | 0.43 | −0.07 | 0.75 | |
| Physical activity | PA level (MET-min·week−1) | 1690.2 ± 1128.69 | 1784.4 ± 1553.49 | −0.262 | 0.07 | −625.86 | 814.30 |
| Nutritional intake | TDI (kcal·day−1) | 1222.9 ± 301.99 | 1412.1 ± 225.94 | −2.750 ** | 0.71 | 51.44 | 327.07 |
| Carbohydrate (g) | 198.0 ± 49.61 | 214.3 ± 38.29 | −1.420 | 0.37 | −6.67 | 39.19 | |
| Lipid (g) | 29.0 ± 11.10 | 38.2 ± 15.04 | −2.633 * | 0.70 | 2.21 | 16.22 | |
| Protein (g) | 47.9 ± 13.46 | 56.4 ± 10.14 | −2.746 ** | 0.71 | 2.29 | 14.60 | |
| Variable | Sarcopenic (n = 27) | Non-sarcopenic (n = 32) | t-Value | Cohen’s d | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| 30-s chair stand (n) | 14.6 ± 4.15 | 14.8 ± 2.79 | −0.21 | 0.06 | −1.70 | 2.09 |
| 30-s arm curl (n) | 15.9 ± 3.66 | 17.6 ± 2.64 | −2.06 * | 0.53 | 0.043 | 3.44 |
| Chair sit-and-reach (cm) | 15.5 ± 11.19 | 15.6 ± 9.55 | −0.05 | 0.00 | −5.28 | 5.53 |
| Back scratch (cm) | −2.4 ± 6.70 | −4.1 ± 7.56 | 0.90 | 0.24 | −5.44 | 2.07 |
| 8-foot up-and-go (s) | 5.8 ± 0.57 | 5.7 ± 0.81 | 0.23 | 0.14 | −0.41 | 0.33 |
| 2-min step test (n) | 89.9 ± 11.50 | 92.3 ± 15.61 | −0.67 | 0.18 | −4.85 | 9.69 |
| Grip strength (kg) | 20.4 ± 3.27 | 24.3 ± 3.47 | −4.39 *** | 1.16 | 2.11 | 5.65 |
| Gait speed (m·s−1) | 0.96 ± 0.12 | 1.07 ± 0.14 | −3.22 ** | 0.84 | 0.04 | 0.18 |
| Variable | Sarcopenic (n = 27) | Non-Sarcopenic (n = 32) | t-Value | Cohen’s d | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Muscle quality | MVIC (N·m) | 111.0 ± 28.59 | 135.6 ± 27.37 | 3.374 ** | 0.88 | 10.01 | 39.25 |
| RMVIC(N·m·kg−1) | 212.5 ± 57.56 | 246.9 ± 57.07 | 2.301 * | 0.60 | 4.48 | 64.44 | |
| TTV (cm2) | 143.7 ± 18.31 | 153.1 ± 20.40 | 1.844 | 0.48 | −0.8 | 19.58 | |
| TFV (cm2) | 63.2 ± 13.67 | 56.3 ± 13.51 | −1.932 | 0.51 | −13.96 | 0.25 | |
| TMV (cm2) | 75.5 ± 8.35 | 90.7 ± 12.42 | 5.614 *** | 1.44 | 9.65 | 20.90 | |
| TSFV (cm2) | 46.2 ± 13.29 | 41.9 ± 12.35 | −1.262 | 0.34 | −10.91 | 2.47 | |
| IMAT (cm2) | 17.1 ± 5.37 | 14.4 ± 4.02 | −2.240 * | 0.57 | −5.19 | −0.29 | |
| Muscle growth factor | GDF-15 (pg·mL−1) | 835.67 ± 335.03 | 741.54 ± 349.00 | 1.051 | 0.28 | −273.45 | 85.20 |
| GDF-8 (pg·mL−1) | 2006.99 ± 729.75 | 2064.24 ± 869.18 | −0.271 | 0.07 | −365.85 | 480.36 | |
| Activin A (pg·mL−1) | 380.17 ± 90.23 | 351.26 ± 97.45 | 1.174 | 0.31 | −78.21 | 20.40 | |
| Follistatin (pg·mL−1) | 2092.43 ± 562.30 | 2203.62 ± 588.78 | −0.738 | 0.19 | −190.67 | 413.04 | |
| Variable | Odds Ratio | p-Value | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Muscle quality | MVIC (N·m) | 0.968 | 0.004 | 0.947 | 0.990 |
| RMVIC (N·m·kg−1) | 0.989 | 0.032 | 0.979 | 0.999 | |
| TTV (cm2) | 0.974 | 0.077 | 0.947 | 1.003 | |
| TFV (cm2) | 1.039 | 0.063 | 0.998 | 1.081 | |
| TMV (cm2) | 0.836 | 0.000 | 0.756 | 0.923 | |
| TSFV (cm2) | 1.027 | 0.212 | 0.985 | 1.072 | |
| IMAT (cm2) | 1.138 | 0.037 | 1.008 | 1.284 | |
| Muscle growth factor | GDF-15 (pg·mL−1) | 1.001 | 0.303 | 0.999 | 1.002 |
| GDF-8 (pg·mL−1) | 1.000 | 0.783 | 0.999 | 1.001 | |
| Activin A (pg·mL−1) | 1.003 | 0.245 | 0.998 | 1.009 | |
| Follistatin (pg·mL−1) | 1.000 | 0.458 | 0.999 | 1.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.-W.; Jung, S.-W.; Kim, S.-W.; Jung, H.C.; Kim, D.-Y.; Song, J.K. Comparisons of Muscle Quality and Muscle Growth Factor Between Sarcopenic and Non-Sarcopenic Older Women. Int. J. Environ. Res. Public Health 2020, 17, 6581. https://doi.org/10.3390/ijerph17186581
Seo M-W, Jung S-W, Kim S-W, Jung HC, Kim D-Y, Song JK. Comparisons of Muscle Quality and Muscle Growth Factor Between Sarcopenic and Non-Sarcopenic Older Women. International Journal of Environmental Research and Public Health. 2020; 17(18):6581. https://doi.org/10.3390/ijerph17186581
Chicago/Turabian StyleSeo, Myong-Won, Sung-Woo Jung, Sung-Woo Kim, Hyun Chul Jung, Deog-Yoon Kim, and Jong Kook Song. 2020. "Comparisons of Muscle Quality and Muscle Growth Factor Between Sarcopenic and Non-Sarcopenic Older Women" International Journal of Environmental Research and Public Health 17, no. 18: 6581. https://doi.org/10.3390/ijerph17186581
APA StyleSeo, M.-W., Jung, S.-W., Kim, S.-W., Jung, H. C., Kim, D.-Y., & Song, J. K. (2020). Comparisons of Muscle Quality and Muscle Growth Factor Between Sarcopenic and Non-Sarcopenic Older Women. International Journal of Environmental Research and Public Health, 17(18), 6581. https://doi.org/10.3390/ijerph17186581






