Effects of Inhaled Corticosteroids and Particle Size on Risk of Obstructive Sleep Apnea: A Large Retrospective Cohort Study
Abstract
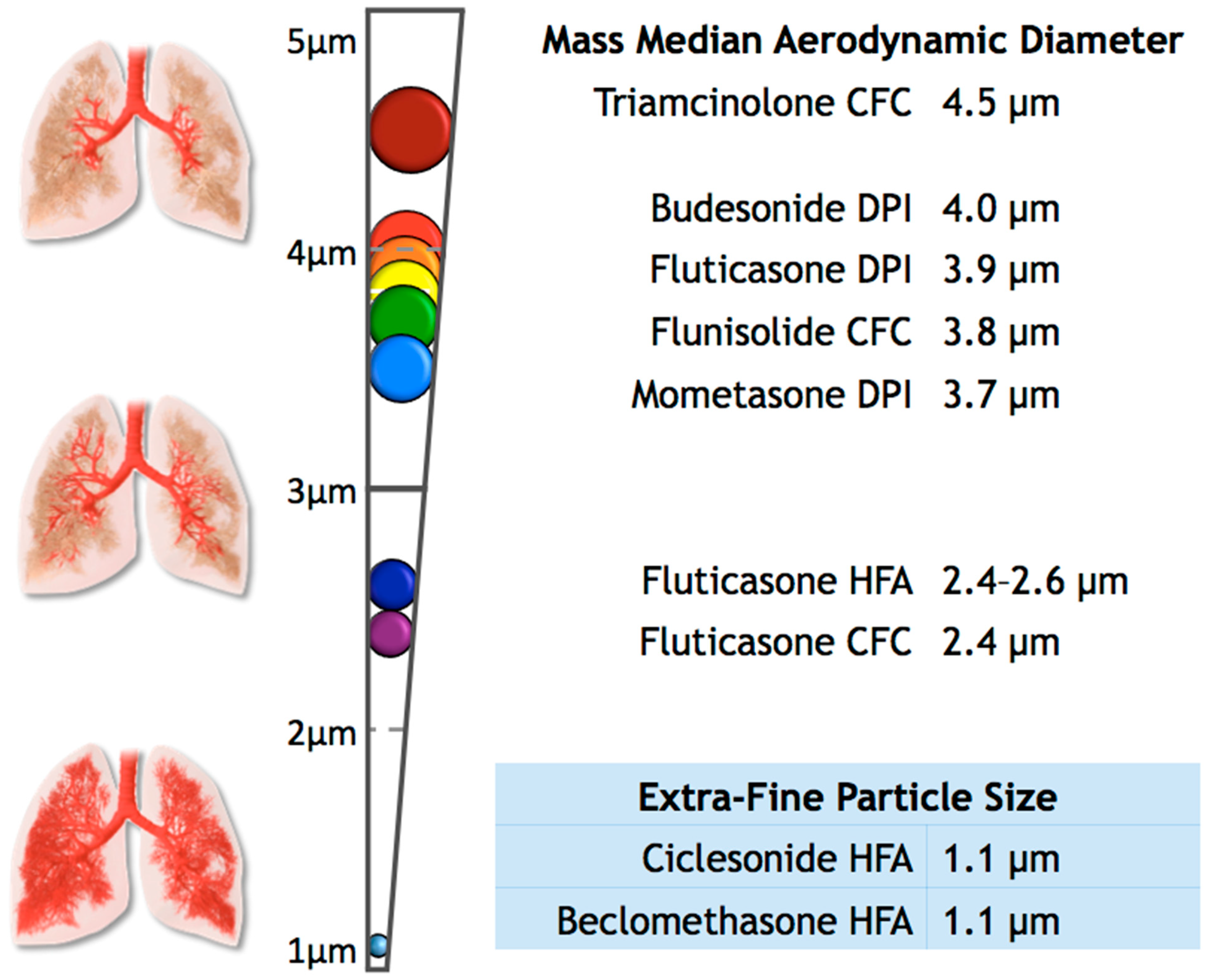
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hudgel, D.W.; Shucard, D.W. COexistence of sleep apnea and asthma resulting in severe sleep hypoxemia. JAMA 1979, 242, 2789–2790. [Google Scholar] [CrossRef]
- Catterall, J.R.; Calverley, P.M.A.; Brezinova, V.; Douglas, N.J.; Brash, H.M.; Shapiro, C.M.; Flenley, D.C. Irregular breathing and hypoxaemia during chronic stable asthma. Lancet 1982, 319, 301–304. [Google Scholar] [CrossRef]
- Fitzpatrick, M.F.; Martin, K.; Fossey, E.; Shapiro, C.M.; Elton, R.A.; Douglas, N.J. Snoring, asthma and sleep disturbance in Britain: A community-based survey. Eur. Respir. J. 1993, 6, 531–535. [Google Scholar] [PubMed]
- Janson, C.; De Backer, W.; Gislason, T.; Plaschke, P.; Björnsson, E.; Hetta, J.; Kristbjarnarson, H.; Vermeire, P.; Boman, G. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: A population study of young adults in three European countries. Eur. Respir. J. 1996, 9, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.K.; Berry, R.B. Asthma and Obstructive Sleep Apnea: At Different Ends of the Same Airway? Chest 2009, 135, 1115–1116. [Google Scholar] [CrossRef]
- Teodorescu, M.; Consens, F.B.; Bria, W.F.; Coffey, M.J.; McMorris, M.S.; Weatherwax, K.J.; Palmisano, J.; Senger, C.M.; Ye, Y.; Kalbfleisch, J.D.; et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest 2009, 135, 1125–1132. [Google Scholar] [CrossRef]
- Teodorescu, M.; Consens, F.B.; Bria, W.F.; Coffey, M.J.; McMorris, M.S.; Weatherwax, K.J.; Durance, A.; Palmisano, J.; Senger, C.M.; Chervin, R.D. Correlates of daytime sleepiness in patients with asthma. Sleep Med. 2006, 7, 607–613. [Google Scholar] [CrossRef]
- Teodorescu, M.; Barnet, J.H.; Hagen, E.W.; Palta, M.; Young, T.B.; Peppard, P.E. Association between asthma and risk of developing obstructive sleep apnea. JAMA 2015, 313, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Asthma Facts: CDC’s National Asthma Control Program Grantees; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- Chmielewska, M.; Akst, L.M. Dysphonia associated with the use of inhaled corticosteroids. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 255–259. [Google Scholar] [CrossRef]
- Teodorescu, M.; Broytman, O.; Curran-Everett, D.; Sorkness, R.L.; Crisafi, G.; Bleecker, E.R.; Erzurum, S.; Gaston, B.M.; Wenzel, S.E.; Jarjour, N.N.; et al. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J. Allergy Clin. Immunol. Pr. 2015, 3, 566–575.e1. [Google Scholar] [CrossRef] [Green Version]
- Newman, S.P.; Chan, H.-K. In vitro/in vivo comparisons in pulmonary drug delivery. J. Aerosol. Med. Pulm. Drug Deliv. 2008, 21, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Leach, C.L.; Kuehl, P.J.; Chand, R.; McDonald, J.D. Respiratory Tract Deposition of HFA-Beclomethasone and HFA-Fluticasone in Asthmatic Patients. J. Aerosol. Med. Pulm. Drug Deliv. 2016, 29, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wolthers, O.D. Extra-fine particle inhaled corticosteroids, pharma-cokinetics and systemic activity in children with asthma. Pediatr. Allergy Immunol. 2016, 27, 13–21. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines and Healthcare (EDQM). Section 2.9.18—Preparations for inhalation: Aerodynamic assessment of fine particles. In European Pharmacopeia; Council of Europe: Strasbourg, France, 2009; pp. 287–300. [Google Scholar]
- Devadason, S.G.; Huang, T.; Walker, S.; Troedson, R.; Le Souëf, P.N. Distribution of technetium-99m-labelled QVAR delivered using an Autohaler device in children. Eur. Respir. J. 2003, 21, 1007–1011. [Google Scholar] [CrossRef]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef]
- Schatz, M.; Sorkness, C.A.; Li, J.T.; Marcus, P.; Murray, J.J.; Nathan, R.A.; Kosinski, M.; Pendergraft, T.B.; Jhingran, P. Asthma Control Test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J. Allergy Clin. Immunol. 2006, 117, 549–556. [Google Scholar] [CrossRef]
- National Asthma Education and Prevention Program. National Heart, Lung, and Blood Institute Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allergy Clin. Immunol. 2007, 120, S94–S138. [Google Scholar] [CrossRef]
- Fuhlbrigge, A.L.; Kitch, B.T.; Paltiel, A.D.; Kuntz, K.M.; Neumann, P.J.; Dockery, D.W.; Weiss, S.T. FEV(1) is associated with risk of asthma attacks in a pediatric population. J. Allergy Clin. Immunol. 2001, 107, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Fuhlbrigge, A.L.; Weiss, S.T.; Kuntz, K.M.; Paltiel, A.D. CAMP Research Group Forced expiratory volume in 1 second percentage improves the classification of severity among children with asthma. Pediatrics 2006, 118, e347–e355. [Google Scholar] [CrossRef]
- Kitch, B.T.; Paltiel, A.D.; Kuntz, K.M.; Dockery, D.W.; Schouten, J.P.; Weiss, S.T.; Fuhlbrigge, A.L. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest 2004, 126, 1875–1882. [Google Scholar] [CrossRef]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [PubMed]
- van Aalderen, W.M.C.; Grigg, J.; Guilbert, T.W.; Roche, N.; Israel, E.; Martin, R.J.; Colice, G.; Postma, D.S.; Hillyer, E.V.; Burden, A.; et al. Small-particle Inhaled Corticosteroid as First-line or Step-up Controller Therapy in Childhood Asthma. J. Allergy Clin. Immunol. Pract. 2015, 3, 721–731.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scichilone, N.; Spatafora, M.; Battaglia, S.; Arrigo, R.; Benfante, A.; Bellia, V. Lung penetration and patient adherence considerations in the management of asthma: Role of extra-fine formulations. J. Asthma Allergy 2013, 6, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, C.; Colice, G.L.; Luskin, A. Particle size of inhaled corticosteroids: Does it matter? J. Allergy Clin. Immunol. 2009, 124, S88–S93. [Google Scholar] [CrossRef]
- Schofield, M.L. Asthma Pharmacotherpay. In Asthma: Screening, Diagnosis, Management, An Issue of Otolaryngologic Clinics of North America; Calhoun, K., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; pp. 55–64. ISBN 9780323266758. [Google Scholar]
- Hoppentocht, M.; Hagedoorn, P.; Frijlink, H.W.; de Boer, A.H. Technological and practical challenges of dry powder inhalers and formulations. Adv. Drug Deliv. Rev. 2014, 75, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Kamin, W.E.S.; Genz, T.; Roeder, S.; Scheuch, G.; Trammer, T.; Juenemann, R.; Cloes, R.M. Mass output and particle size distribution of glucocorticosteroids emitted from different inhalation devices depending on various inspiratory parameters. J. Aerosol Med. 2002, 15, 65–73. [Google Scholar] [CrossRef]
- Yang, T.T.; Li, S.; Wyka, B.; Kenyon, D. Drug delivery performance of the mometasone furoate dry powder inhaler. J. Aerosol Med. 2001, 14, 487–494. [Google Scholar] [CrossRef]
- Cripps, A.; Riebe, M.; Schulze, M.; Woodhouse, R. Pharmaceutical transition to non-CFC pressurized metered dose inhalers. Respir. Med. 2000, 94 (Suppl. B), S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Leach, C.L.; Davidson, P.J.; Boudreau, R.J. Improved airway targeting with the CFC-free HFA-beclomethasone metered-dose inhaler compared with CFC-beclomethasone. Eur. Respir. J. 1998, 12, 1346–1353. [Google Scholar] [CrossRef]
- Newman, S.; Salmon, A.; Nave, R.; Drollmann, A. High lung deposition of 99mTc-labeled ciclesonide administered via HFA-MDI to patients with asthma. Respir. Med. 2006, 100, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Leach, C.L.; Bethke, T.D.; Boudreau, R.J.; Hasselquist, B.E.; Drollmann, A.; Davidson, P.; Wurst, W. Two-dimensional and three-dimensional imaging show ciclesonide has high lung deposition and peripheral distribution: A nonrandomized study in healthy volunteers. J. Aerosol Med. 2006, 19, 117–126. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.; Vos, W.; Vinchurkar, S.; Van Holsbeke, C.; Poli, G.; Claes, R.; Salgado, R.; De Backer, W. The effects of extrafine beclometasone/formoterol (BDP/F) on lung function, dyspnea, hyperinflation, and airway geometry in COPD patients: Novel insight using functional respiratory imaging. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur. J. Pharm. Biopharm. 2015, 96, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total | With Asthma Control Test Scores | With Pulmonary Function Test Values | ||||
|---|---|---|---|---|---|---|
| (n = 29,816) | (n = 4668) | (n = 2428) | ||||
| GENDER | ||||||
| Male | 10,803 | (36.2%) | 1938 | (41.5%) | 824 | (33.9%) |
| Female | 19,013 | (63.8%) | 2730 | (58.5%) | 1604 | (66.1%) |
| AGE † | ||||||
| Mean Age (S.D.) | 42.8 | (±21.1) | 27.9 | (±15.5) | 55.5 | (±18.0) |
| RACE | ||||||
| Black or African America | 2531 | (8.5%) | 384 | (8.2%) | 189 | (7.8%) |
| White or Caucasian | 24,026 | (80.6%) | 3661 | (78.4%) | 2073 | (85.3%) |
| Other | 3260 | (10.9%) | 623 | (13.4%) | 166 | (6.8%) |
| Mean BMI (S.D.) | 29.8 | (±9.0) | 27.1 | (±8.6) | 32.4 | (±9.5) |
| Underweight (BMI < 18) | 1470 | (5.3%) | 512 | (11.0%) | 44 | (1.9%) |
| Normal (BMI 18–25) | 7770 | (28.0%) | 1726 | (37.2%) | 479 | (20.7%) |
| Overweight (BMI 25–30) | 6593 | (30.3%) | 1018 | (21.9%) | 537 | (23.2%) |
| Obese (BMI 30–40) | 8393 | (30.3%) | 1000 | (21.5%) | 827 | (35.8%) |
| Morbidly Obese (BMI ≥ 40) | 3498 | (12.6%) | 387 | (8.3%) | 424 | (18.4%) |
| OTHER | ||||||
| Current Tobacco Users | 6250 | (21.0%) | 694 | (14.9%) | 598 | (24.6%) |
| Inhaled Corticosteroid Users | 14,428 | (48.4%) | 3355 | (71.9%) | 1814 | (74.7%) |
| ICS Non-Users | ICS Users | |||
|---|---|---|---|---|
| Normal Particles | Extra-fine Particles | Total ICS Users | ||
| Initial Cohort | 15,388 | 14,048 | 380 | 14,428 |
| ACT Control Categories † | 1313 | 2881 | 118 | 3355 |
| Uncontrolled | 181 | 998 | 34 | 1199 |
| Well Controlled | 1132 | 1883 | 84 | 2156 |
| PFT Control Categories # | 547 | 1614 | 27 | 1814 |
| Uncontrolled | 210 | 891 | 8 | 989 |
| Well Controlled | 337 | 723 | 19 | 825 |
| Mean Value * | Standard Deviation | Number of Patients | |
|---|---|---|---|
| ACT Scores (Total) | 20.87 | 4.44 | 4668 |
| All ICS users | 20.19 | 4.67 | 3355 |
| Normal particle size | 20.30 | 4.62 | 2881 |
| Extra-fine particle size | 21.21 | 3.52 | 118 |
| Non-users | 22.61 | 3.17 | 1313 |
| FEV1% Predicted (Total) | 78.01 | 20.76 | 2361 |
| All ICS users | 76.12 | 20.91 | 1814 |
| Normal particle size | 75.95 | 21.00 | 1614 |
| Extra-fine particle size | 81.74 | 20.56 | 27 |
| Non-users | 83.78 | 19.36 | 547 |
| Unadjusted Odds Ratio | 95% CI | Adjusted Odds Ratio * | 95% CI | |
|---|---|---|---|---|
| Uncontrolled vs. Controlled Asthma: | ||||
| As determined by ACT score | 2.00 | 1.67–2.40 | 1.60 | 1.32–1.94 |
| As determined by PFT results | 1.66 | 1.39–1.99 | 1.45 | 1.19–1.77 |
| ICS Users and Categories: | ||||
| ICS users (any particle size) vs. non-users | 1.69 | 1.58–1.81 | 1.58 | 1.47–1.70 |
| Normal size particle ICS users vs. non-users | 1.62 | 1.51–1.74 | 1.56 | 1.45–1.69 |
| Extra-fine particle ICS users vs. non-users | 1.05 | 0.75–1.46 | 1.11 | 0.78–1.58 |
| Compared to Extra-Fine Size ICS Users: | ||||
| Normal size ICS users | 1.55 | 1.11–2.16 | 1.40 | 0.99–1.98 |
| Normal size ICS with BMI ≥25 only | 1.70 | 1.16–2.51 | 1.70 # | 1.15–2.50 |
| Normal size ICS, males only | 1.68 | 0.97–2.88 | 1.46 + | 0.82–2.57 |
| Normal size ICS, males with BMI ≥25 only | 2.44 | 1.21–4.90 | 2.45 † | 1.22–4.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henao, M.P.; Kraschnewski, J.L.; Bolton, M.D.; Ishmael, F.; Craig, T. Effects of Inhaled Corticosteroids and Particle Size on Risk of Obstructive Sleep Apnea: A Large Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 7287. https://doi.org/10.3390/ijerph17197287
Henao MP, Kraschnewski JL, Bolton MD, Ishmael F, Craig T. Effects of Inhaled Corticosteroids and Particle Size on Risk of Obstructive Sleep Apnea: A Large Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2020; 17(19):7287. https://doi.org/10.3390/ijerph17197287
Chicago/Turabian StyleHenao, Maria Paula, Jennifer L. Kraschnewski, Matthew D. Bolton, Faoud Ishmael, and Timothy Craig. 2020. "Effects of Inhaled Corticosteroids and Particle Size on Risk of Obstructive Sleep Apnea: A Large Retrospective Cohort Study" International Journal of Environmental Research and Public Health 17, no. 19: 7287. https://doi.org/10.3390/ijerph17197287
APA StyleHenao, M. P., Kraschnewski, J. L., Bolton, M. D., Ishmael, F., & Craig, T. (2020). Effects of Inhaled Corticosteroids and Particle Size on Risk of Obstructive Sleep Apnea: A Large Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 17(19), 7287. https://doi.org/10.3390/ijerph17197287






