Nitrogen Removal Efficiency for Pharmaceutical Wastewater with a Single-Stage Anaerobic Ammonium Oxidation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor and Operation Conditions
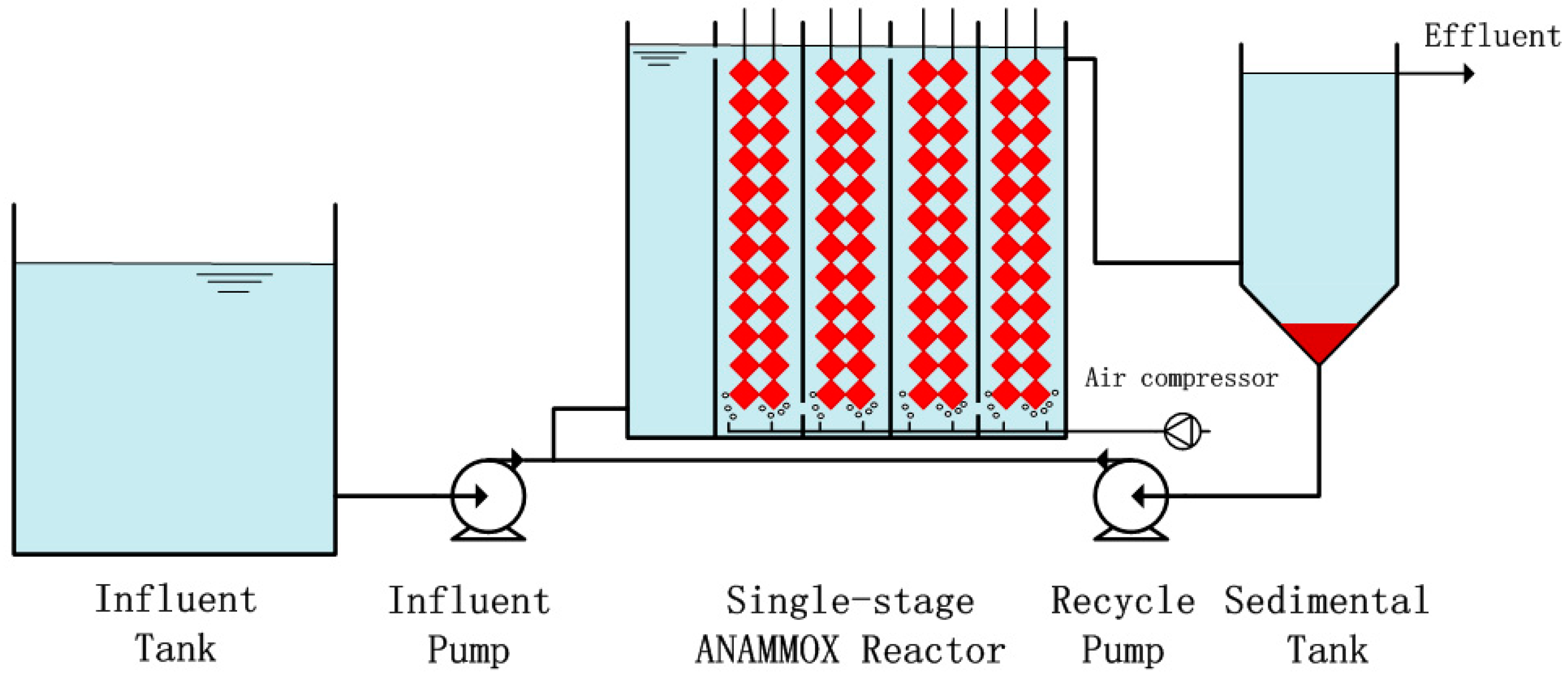
2.2. Seed Sludge and Experimental Setup
2.3. Batch Test
2.4. Chemical Analysis
3. Results and Discussion
3.1. Partial Nitrification Period
3.2. Batch Test
3.3. Single–Stage ANAMMOX Period
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuenen, J.G. ANAMMOX bacteria: From discovery to application. Nat. Rev. Microbiol. 2008, 6, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, H.; Salzgeber, D.; Eugster, J.; Joss, A. ANAMMOX brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal. Water Sci. Technol. 2008, 57, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Kuenen, J.G.; Jetten, M.S.M. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 1999, 65, 3248–3250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yang, J.; Furukawa, K. Stable and high-rate nitrogen removal from reject water by partial nitrification and subsequent anammox. J. Biosci. Bioeng. 2010, 110, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sliekers, A.O.; Third, K.A.; Abma, W.; Kuenen, J.G.; Jetten, M.S.M. CANON and ANAMMOX in a gas-lift reactor. FEMS Microbiol. Lett. 2003, 218, 339–344. [Google Scholar] [CrossRef]
- Third, K.A.; Sliekers, A.O.; Kuenen, J.G.; Jetten, M.S.M. The CANON system (completely autotrophic nitrogen-removal over nitrite) under ammonium limitation: Interaction and competition between three groups of bacteria. Syst. Appl. Microbiol. 2001, 24, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.; Bollmann, O.; Sliekers, M.; Jetten, M.; Schmid, M.; Strous, I.; Schmidt, L.H.; Larsen, L.P.; Revsbech, N.P. Kinetics, diffusional limitation and microscale distribution of chemistry and organisms in a CANON reactor. FEMS Microbiol. Ecol. 2005, 51, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.; Wagner, M.; Fuerst, J.; van Loosdrecht, M. Microbiology and application of the anaerobic ammonium oxidation (‘ANAMMOX’) process. Curr. Opin. Biotechnol. 2001, 12, 283–288. [Google Scholar] [CrossRef]
- Daverey, A.; Su, S.-H.; Huang, Y.-T.; Chen, S.-S.; Sung, S.; Lin, J.-G. Partial nitrification and anammox process: A method for high strength optoelectronic industrial wastewater treatment. Water Res. 2013, 47, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Cordola, M.; Kleerebezem, R.; Caffaz, S.; Lubello, C.; van Loosdrecht, M.C.M. Inhibition effect of swine wastewater heavy metals and antibiotics on ANAMMOX activity. Water Sci. Technol. 2012, 66, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Yang, S.; Liu, H.; Bao, S.; Hu, K.; Furukawa, K. Feasibility of a membrane-aerated biofilm reactor to achieve single-stage autotrophic nitrogen removal based on ANAMMOX. Chemosphere 2007, 69, 776–784. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Ballinger, S.; Head, I.; Curtis, T.; Godley, A. The effect of C/N ratio on ammonia oxidising bacteria community structure in a laboratory nitrification-denitrification reactor. Water Sci. Technol. 2005, 46, 543–550. [Google Scholar] [CrossRef]
- Jia, Y.; Lv, S.; Yang, T.; Zhang, L.; Jiang, G. Effect of COD/NH4+-N and influent pH on the simultaneous nitrification and denitrification by using a sequencing batch reactor. In Proceedings of the 2010 International Conference on Digital Manufacturing and Automation (ICDMA), Changsha, China, 18–20 December 2010; Volume 1, pp. 604–607. [Google Scholar]
- Zhang, X.; Chen, Y.; Qiu, J.; Zhao, J. Simultaneous Nitrification and Denitrification in SBBR: Its Performances Affected by Aeration Amount and COD. Environ. Sci. Technol. 2012, 35, 129–133. [Google Scholar]
- Sliekers, A.O.; Derwort, N.; Gomez, J.L.; Strous, M.; Kuenen, J.G.; Jetten, M.S.M. Completely autotrophic ammonia removal over nitrite in one reactor. Water Res. 2002, 36, 2475–2482. [Google Scholar] [CrossRef]
- Wett, B. Development and implementation of a robust deammonification process. Water Sci. Technol 2007, 56, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Salzgeber, D.; Eugster, J.; König, R.; Rottermann, K.; Burger, S.; Fabijan, P.; Leumann, S.; Mohn, J.; Siegrist, H. Full-scale nitrogen removal from digester liqiud with partial nitritation and anammox in one SBR. Environ. Sci. Technol. 2009, 43, 5301–5306. [Google Scholar] [CrossRef] [PubMed]
- Abma, W.R.; Driessen, W.; Haartrhuis, R.; van Loosdrecht, M.C.M. Upgrading of sewage treatment plant by sustainable and cost-effective separate treatment of industrial wastewater. Water Sci. Technol. 2010, 61, 1715–1722. [Google Scholar]
- Lackner, S.; Lindenblatt, C.; Horm, H. ‘Swinging ORP’ as operation strategy for stable reject water treatment by nitritation–anammox in sequencing batch reactors. Chem. Eng. J. 2012, 180, 190–196. [Google Scholar] [CrossRef]
- Qiao, S.; Tian, T.; Duan, X.; Zhou, J.; Cheng, Y. Novel single-stage autotrophic nitrogen removal via co-immobilizing partial nitrifying and anammox biomass. Chem. Eng. J. 2013, 23, 19–26. [Google Scholar] [CrossRef]




| Stage | Time (Day) | Purpose | COD (mg/L) | NH4+–N (mg/L) | Influent |
|---|---|---|---|---|---|
| 1–1 | 1–7 | Starting up for nitrification | 782 ± 162 | 143 ± 66 | All aerobic |
| 1–2 | 8–26 | Increasing influent nitrogen load | 1577 ± 296 | 332 ± 61 | Aerobic and anaerobic effluent (1:1) |
| 1–3 | 27–32 | Inhibition for AOB | 2447 ± 422 | 510 ± 55 | Aerobic and anaerobic effluent (1:2) |
| 1–4 | 33–48 | Activity recovery for AOB | 767 ± 242 | 515 ± 189 | Aerobic and anaerobic effluent (2:1) |
| 2–1 | 49–52 | Starting up for single–stage ANAMMOX | 782 ± 162 | 143 ± 66 | All aerobic |
| 2–2 | 53–92 | Increasing influent nitrogen load | 522 ± 88 | 287 ± 146 | 4–6 dilution times of the anaerobic effluent |
| 2–3 | 93–121 | steady operation | 844 ± 62 | 542 ± 106 | 2–3 dilution times of the anaerobic effluent |
| Reference | Type of Influent | Influent COD (mg/L) | Influent NH4+–N (mg/L) | TN Removal Load (kg/m3/d) | TN Removal Rate (%) |
|---|---|---|---|---|---|
| [17] | Sludge digestion liquid | 500 | 500 | 0.68 | 84% |
| [18] | Sludge digestion liquid | 100 | 500 | 0.4 | 90% |
| [19] | Synthetic wastewater | − | 300 | 0.73 | 73% |
| [20] | Reject water | 1000 | 1000 | 0.4 | 90% |
| [21] | Synthetic wastewater | 30–180 | 600 | 1.69 | 77% |
| This study | Pharmaceutical wastewater | 400–1000 | 600 | 1.0 | 75.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, L.; Yao, H.; Li, H.; Fan, L.; Jia, F. Nitrogen Removal Efficiency for Pharmaceutical Wastewater with a Single-Stage Anaerobic Ammonium Oxidation Process. Int. J. Environ. Res. Public Health 2020, 17, 7972. https://doi.org/10.3390/ijerph17217972
Zuo L, Yao H, Li H, Fan L, Jia F. Nitrogen Removal Efficiency for Pharmaceutical Wastewater with a Single-Stage Anaerobic Ammonium Oxidation Process. International Journal of Environmental Research and Public Health. 2020; 17(21):7972. https://doi.org/10.3390/ijerph17217972
Chicago/Turabian StyleZuo, Lushen, Hong Yao, Huayu Li, Liru Fan, and Fangxu Jia. 2020. "Nitrogen Removal Efficiency for Pharmaceutical Wastewater with a Single-Stage Anaerobic Ammonium Oxidation Process" International Journal of Environmental Research and Public Health 17, no. 21: 7972. https://doi.org/10.3390/ijerph17217972




