Postmenopausal Breast Cancer in Women, Clinical and Epidemiological Factors Related to the Molecular Subtype: A Retrospective Cohort Study in a Single Institution for 13 Years. Follow-Up Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Fleming, T.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of Worldwide Burden of Cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chollet-Hinton, L.; Anders, C.K.; Tse, C.-K.; Bell, M.B.; Yang, Y.C.; Carey, L.A.; Olshan, A.F.; Troester, M.A. Breast Cancer Biologic and Etiologic Heterogeneity by Young Age and Menopausal Status in the Carolina Breast Cancer Study: A Case-Control Study. Breast Cancer Res. 2016, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K. Pathophysiology Made Incredibly Easy; Springhouse-Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1998. [Google Scholar]
- Murase, K.; Yanai, A.; Saito, M.; Imamura, M.; Miyagawa, Y.; Takatsuka, Y.; Inoue, N.; Ito, T.; Hirota, S.; Sasa, M.; et al. Biological Characteristics of Luminal Subtypes in Pre- and Postmenopausal Estrogen Receptor-Positive and HER2-Negative Breast Cancers. Breast Cancer 2012, 21, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Osako, T.; Okumura, Y.; Hayashi, M.; Toyozumi, Y.; Arima, N. Ki-67 as a Prognostic Marker According to Breast Cancer Subtype and a Predictor of Recurrence Time in Primary Breast Cancer. Exp. Ther. Med. 2010, 1, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Surakasula, A.; Nagarjunapu, G.C.; Raghavaiah, K.V. A Comparative Study of Pre- and Post-Menopausal Breast Cancer: Risk Factors, Presentation, Characteristics and Management. J. Res. Pharm. Pract. 2014, 3, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Okeke, T.C.; Anyaehie, U.B.; Ezenyeaku, C.C. Premature Menopause. Ann. Med. Health Sci. Res. 2013, 3, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Greene, F.L.; American Joint Committee on Cancer. AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2020. [Google Scholar]
- Fleming, I.D.; Okeke, J.S.; Henson, D.E.; Hutter, R.V.P.; Kennedy, B.J.; Murphy, G.P.; American Joint Committee on Cancer (AJCC) (Eds.) AJCC Cancer Staging Manual, 5th ed.; J. B. Lippincott: Philadelphia, PA, USA, 1997. [Google Scholar]
- Curigliano, G.; Burstein, H.; Winer, E.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-Escalating and Escalating Treatments for Early-Stage Breast Cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Buzas, R.; Rogobete, A.F.; Popovici, S.E.; Mateescu, T.; Hoinoiu, T.; Sorop, V.B.; Bratu, T.; Ticelea, M.; Popoiu, C.M.; Sandesc, D. Nuclear Transcription Factor Kappa B (NF-кB) and Molecular Damage Mechanisms in Acute Cardiovascular Diseases. A Review. J. Cardiovasc. Emergencies 2018, 4, 65–72. [Google Scholar] [CrossRef]
- SSPR-2017. Available online: http://insp.gov.ro/sites/cnepss/wp-content/uploads/2018/11/SSPR-2017.pdf (accessed on 26 April 2020).
- Breast Cancer Incidence (Invasive) Statistics. Cancer Research UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-invasive (accessed on 14 May 2015).
- Mitchell, K.; Fritschi, L.; Reid, A.; McEvoy, S.; Ingram, D.S.; Jamrozik, K.; Clayforth, C.; Byrne, M. Rural–Urban Differences in the Presentation, Management and Survival of Breast Cancer in Western Australia. Breast 2006, 15, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Stamenić, V.; Strnad, M. Urban-Rural Differences in a Population-Based Breast Cancer Screening Program in Croatia. Croat. Med. J. 2011, 52, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.; Maringe, C.; Butler, J.M.; Rachet, B.; Barrett-Lee, P.; Bergh, J.P.W.V.D.; Boyages, J.; Christiansen, P.; Lee, M.; Warnberg, F.; et al. Breast Cancer Survival and Stage at Diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000–2007: A Population-Based Study. Br. J. Cancer 2013, 108, 1195–12081. [Google Scholar] [CrossRef] [PubMed]
- Hoinoiu, T.; Grujic, D.; Prilipceanu, G.; Folescu, R.; Hoinoiu, B.; Bratu, T.; Poroch, V.; Grujic, L. The Use of Collagen-Glycosaminoglycan Biodegradable Matrix (Integra®) in the Management of Neck Postburn Hypertrophic Scars and Contractures. Appl. Sci. 2020, 10, 3731. [Google Scholar] [CrossRef]
- Collins, L.C.; Marotti, J.D.; Gelber, S.; Cole, K.; Ruddy, K.; Kereakoglow, S.; Brachtel, E.F.; Schapira, L.; Come, S.E.; Winer, E.P.; et al. Pathologic Features and Molecular Phenotype by Patient Age in a Large Cohort of Young Women with Breast Cancer. Breast Cancer Res. Treat. 2011, 131, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Sohn, V.Y.; Arthurs, Z.M.; Sebesta, J.A.; Brown, T.A. Primary Tumor Location Impacts Breast Cancer Survival. Am. J. Surg. 2008, 195, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Kroman, N.; Wohlfahrt, J.; Mouridsen, H.T.; Melbye, M. Influence of Tumor Location on Breast Cancer Prognosis. Int. J. Cancer 2003, 105, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.O.; Deal, A.M.; Anders, C.K.; Prat, A.; Perou, C.M.; Carey, L.A.; Muss, H.B. Age-Specific Changes in Intrinsic Breast Cancer Subtypes: A Focus on Older Women. Oncologist 2014, 19, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Su, K.; Zeng, J. Clinicopathological Classification and Traditional Prognostic Indicators of Breast Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8500–8505. [Google Scholar] [PubMed]
| Age at Diagnosis | Patients N = 721 | Percent % |
|---|---|---|
| 0–40 years | 7 | 1.0 |
| 41–50 years | 50 | 6.9 |
| 51–60 years | 257 | 35.6 |
| 61–70 years | 257 | 35.6 |
| 71–80 years | 129 | 17.9 |
| 81–90 years | 21 | 2.9 |
| Total | 721 | 100.0 |
| Molecular Subtype | |||||||
|---|---|---|---|---|---|---|---|
| Age at Diagnosis | Luminal A | Luminal B | Luminal HER-Positive | Non-Luminal HER-Positive | Triple Negative | Total | |
| 30–40 years | N | 2 | 1 | 3 | 0 | 0 | 6 |
| % | 33.3% | 16.7% | 50.0% | 0.0% | 0.0% | 100.0% | |
| 41–50 years | N | 12 | 21 | 9 | 3 | 2 | 47 |
| % | 25.5% | 44.7% | 19.1% | 6.4% | 4.3% | 100.0% | |
| 51–60 years | N | 76 | 108 | 28 | 12 | 26 | 250 |
| % | 30.4% | 43.2% | 11.2% | 4.8% | 10.4% | 100.0% | |
| 61–70 years | N | 105 | 96 | 29 | 8 | 16 | 254 |
| % | 41.3% | 37.8% | 11.4% | 3.1% | 6.3% | 100.0% | |
| 71–80 years | N | 54 | 48 | 5 | 7 | 7 | 121 |
| % | 44.6% | 39.7% | 4.1% | 5.8% | 5.8% | 100.0% | |
| 81–90 years | N | 6 | 7 | 3 | 1 | 1 | 18 |
| % | 33.3% | 38.9% | 16.7% | 5.6% | 5.6% | 100.0% | |
| Total | N | 255 | 281 | 77 | 31 | 52 | 696 |
| % | 36.6% | 40.4% | 11.1% | 4.5% | 7.5% | 100.0% | |
| Correlations | |||
|---|---|---|---|
| Age at Diagnosis | Molecular Subtype | ||
| Age at Diagnosis | Pearson Correlation | 1 | −0.114 * |
| Sig. (2-tailed) | 0.003 | ||
| N | 721 | 696 | |
| Luminal | Pearson Correlation | −0.114 ** | 1 |
| Sig. (2-tailed) | 0.003 | ||
| N | 696 | 696 | |
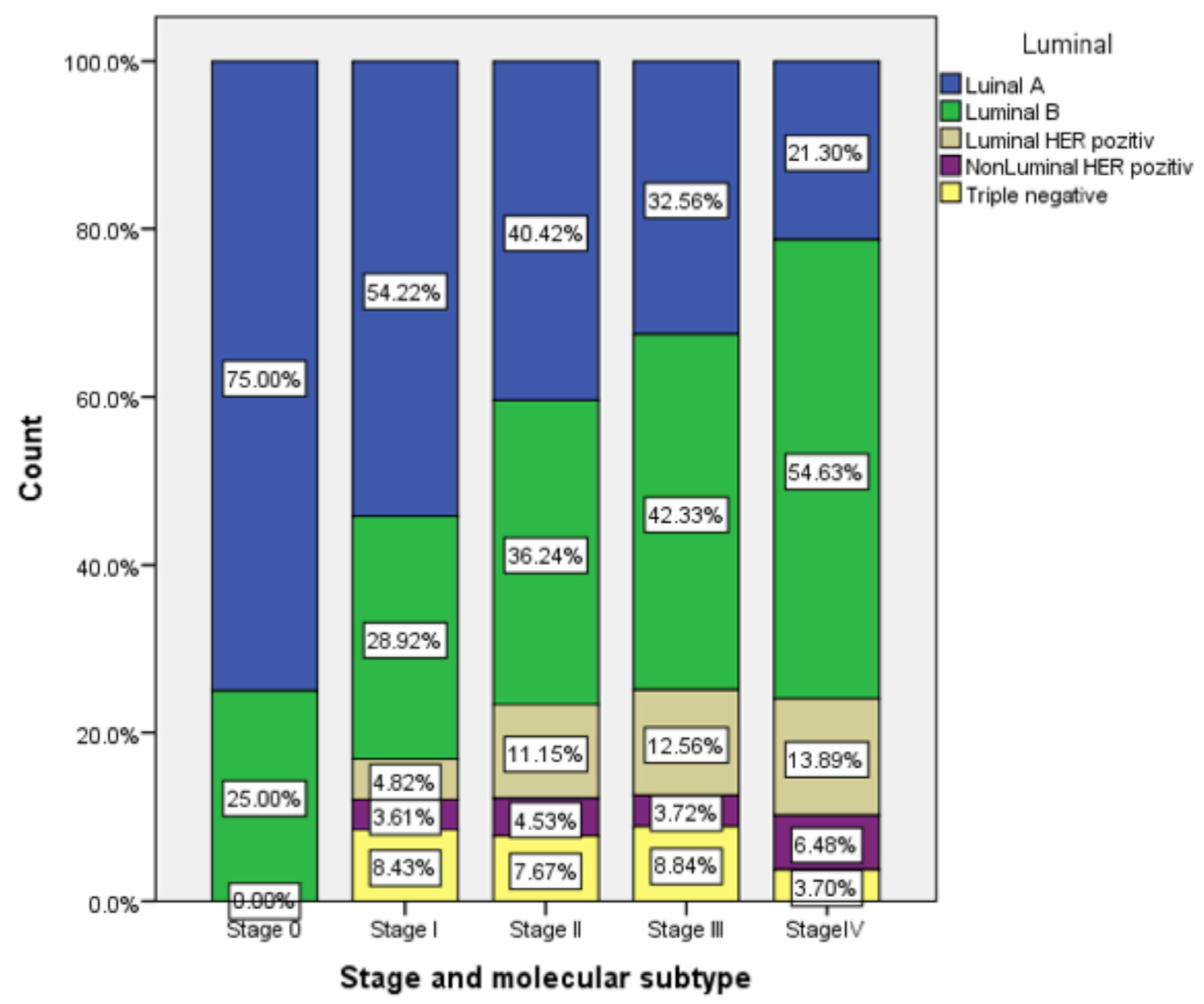
| Stage | Age at Diagnosis (years) | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 30–40 | 41–50 | 51–60 | 61–70 | 71–80 | 81–90 | |||
| 0 | N | 0 | 0 | 1 | 2 | 1 | 0 | 4 |
| % | 0.0% | 0.0% | 25.0% | 50.0% | 25.0% | 0.0% | 100.0% | |
| I | N | 0 | 2 | 28 | 38 | 13 | 2 | 83 |
| % | 0.0% | 2.4% | 33.7% | 45.8% | 15.7% | 2.4% | 100.0% | |
| II | N | 2 | 23 | 105 | 103 | 53 | 5 | 291 |
| % | 0.7% | 7.9% | 36.1% | 35.4% | 18.2% | 1.7% | 100.0% | |
| III | N | 3 | 14 | 81 | 69 | 41 | 10 | 218 |
| % | 1.4% | 6.4% | 37.2% | 31.7% | 18.8% | 4.6% | 100.0% | |
| III | N | 3 | 14 | 81 | 69 | 41 | 10 | 218 |
| % | 1.4% | 6.4% | 37.2% | 31.7% | 18.8% | 4.6% | 100.0% | |
| IV | N | 1 | 10 | 37 | 42 | 17 | 3 | 110 |
| % | 0.9% | 9.1% | 33.6% | 38.2% | 15.5% | 2.7% | 100.0% | |
| Total | N | 6 | 49 | 252 | 254 | 125 | 20 | 706 |
| % | 0.8% | 6.9% | 35.7% | 36.0% | 17.7% | 2.8% | 100.0% | |
| Correlations | |||
|---|---|---|---|
| Patients N | Age at Diagnosis | ||
| Stage | Pearson Correlation | 1 | −0.037 |
| Sig. (2-tailed) | - | 0.322 | |
| N | 706 | 706 | |
| Age at Diagnosis | Pearson Correlation | −0.037 | 1 |
| Sig. (2-tailed) | 0.322 | ||
| N | 706 | 721 | |
| Correlations | |||
|---|---|---|---|
| Stage of the Disease | Patients, N | Percent, % | |
| Stage | 0 | 4 | 0.6 |
| I | 83 | 11.5 | |
| II A | 171 | 23.7 | |
| II B | 120 | 16.6 | |
| III A | 87 | 12.1 | |
| III B | 111 | 15.4 | |
| III C | 20 | 2.8 | |
| IV | 110 | 15.3 | |
| Total | 706 | 98 | |
| Not evaluable | 15 | 2.0 | |
| Total | 721 | 100.0 | |
| Molecular Subtype | Patients, N | Percent, % | |
|---|---|---|---|
| Stage | Luminal A | 255 | 35.4 |
| Luminal B | 281 | 39.0 | |
| Luminal HER-positive | 77 | 10.7 | |
| Non-Luminal HER-positive | 31 | 4.3 | |
| Triple negative | 52 | 7.2 | |
| Total | 696 | 96.5 | |
| Not Evaluable | 25 | 3.5 | |
| Total | 721 | 100.0 | |
| Tumor Location, Breast Quadrant ** | Patients, N | Percent, % | |
|---|---|---|---|
| LOQ | 74 | 10.3 | |
| UOQ | 352 | 48.8 | |
| CQ- | 94 | 13.0 | |
| LIQ | 56 | 7.8 | |
| UIQ | 119 | 16.5 | |
| BB | 7 | 1.0 | |
| WB | 11 | 1.5 | |
| Total | 713 | 98.9 | |
| Not evaluable | 8 | 1.1 | |
| Total | 721 | 100.0 | |
| Correlations | |||
|---|---|---|---|
| Molecular Subtype | Stage of the Disease | ||
| Luminal | Pearson Correlation | 1 | 0.089 |
| Sig. (2-tailed) | 0.019 | ||
| N | 696 | 689 | |
| Stage of the disease | Pearson Correlation | 0.089 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprean, C.M.; Negru, S.M.; Popovici, D.I.; Saftescu, S.; Han, R.-A.; Dragomir, G.-M.; Hoinoiu, T.; Dema, A. Postmenopausal Breast Cancer in Women, Clinical and Epidemiological Factors Related to the Molecular Subtype: A Retrospective Cohort Study in a Single Institution for 13 Years. Follow-Up Data. Int. J. Environ. Res. Public Health 2020, 17, 8722. https://doi.org/10.3390/ijerph17238722
Oprean CM, Negru SM, Popovici DI, Saftescu S, Han R-A, Dragomir G-M, Hoinoiu T, Dema A. Postmenopausal Breast Cancer in Women, Clinical and Epidemiological Factors Related to the Molecular Subtype: A Retrospective Cohort Study in a Single Institution for 13 Years. Follow-Up Data. International Journal of Environmental Research and Public Health. 2020; 17(23):8722. https://doi.org/10.3390/ijerph17238722
Chicago/Turabian StyleOprean, Cristina Marinela, Serban Mircea Negru, Dorel Ionel Popovici, Sorin Saftescu, Robert-Alexandru Han, Gabriel-Mugurel Dragomir, Teodora Hoinoiu, and Alis Dema. 2020. "Postmenopausal Breast Cancer in Women, Clinical and Epidemiological Factors Related to the Molecular Subtype: A Retrospective Cohort Study in a Single Institution for 13 Years. Follow-Up Data" International Journal of Environmental Research and Public Health 17, no. 23: 8722. https://doi.org/10.3390/ijerph17238722
APA StyleOprean, C. M., Negru, S. M., Popovici, D. I., Saftescu, S., Han, R.-A., Dragomir, G.-M., Hoinoiu, T., & Dema, A. (2020). Postmenopausal Breast Cancer in Women, Clinical and Epidemiological Factors Related to the Molecular Subtype: A Retrospective Cohort Study in a Single Institution for 13 Years. Follow-Up Data. International Journal of Environmental Research and Public Health, 17(23), 8722. https://doi.org/10.3390/ijerph17238722





