Cell Viability and Immune Response to Low Concentrations of Nickel and Cadmium: An In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Enzyme Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
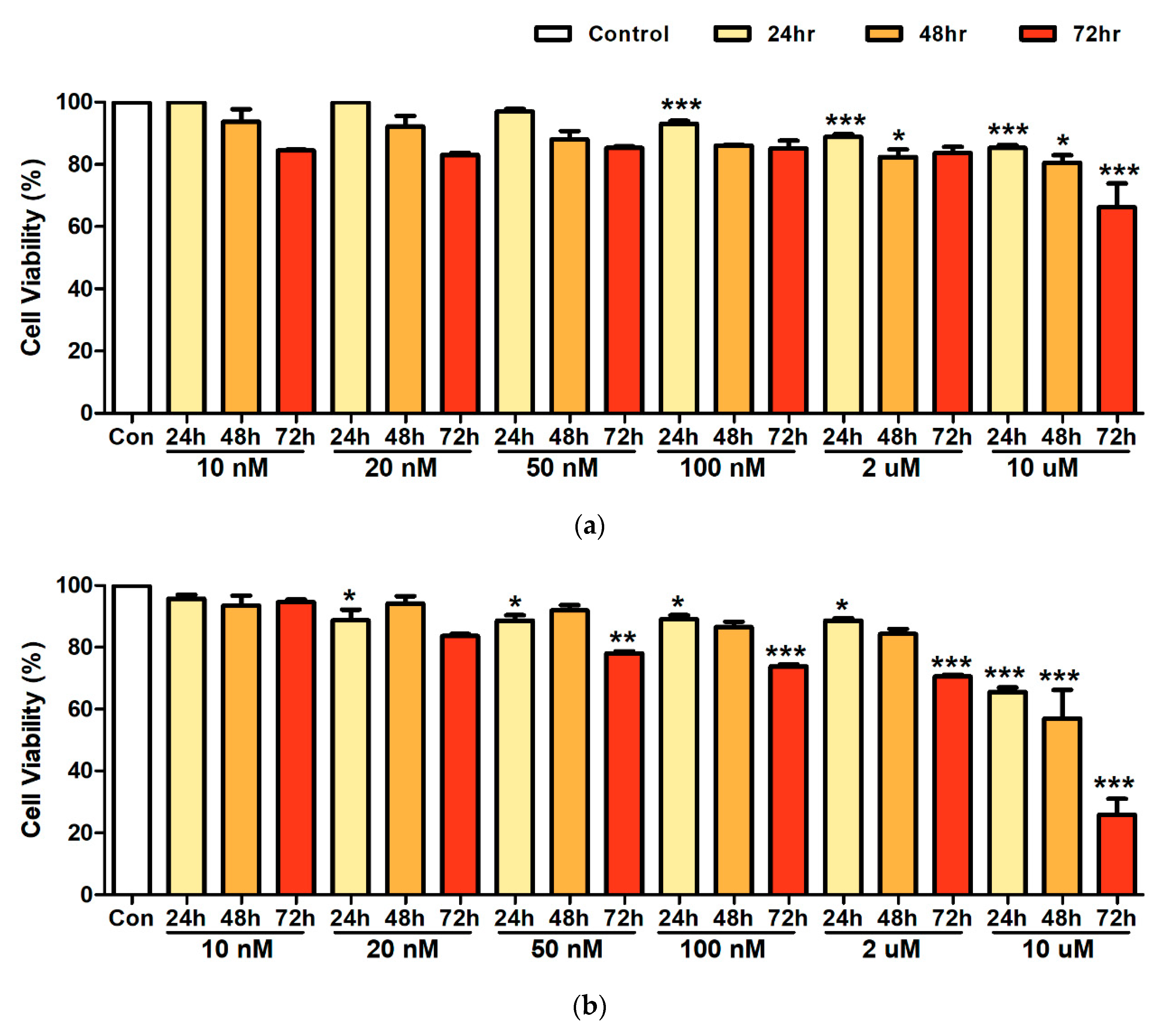
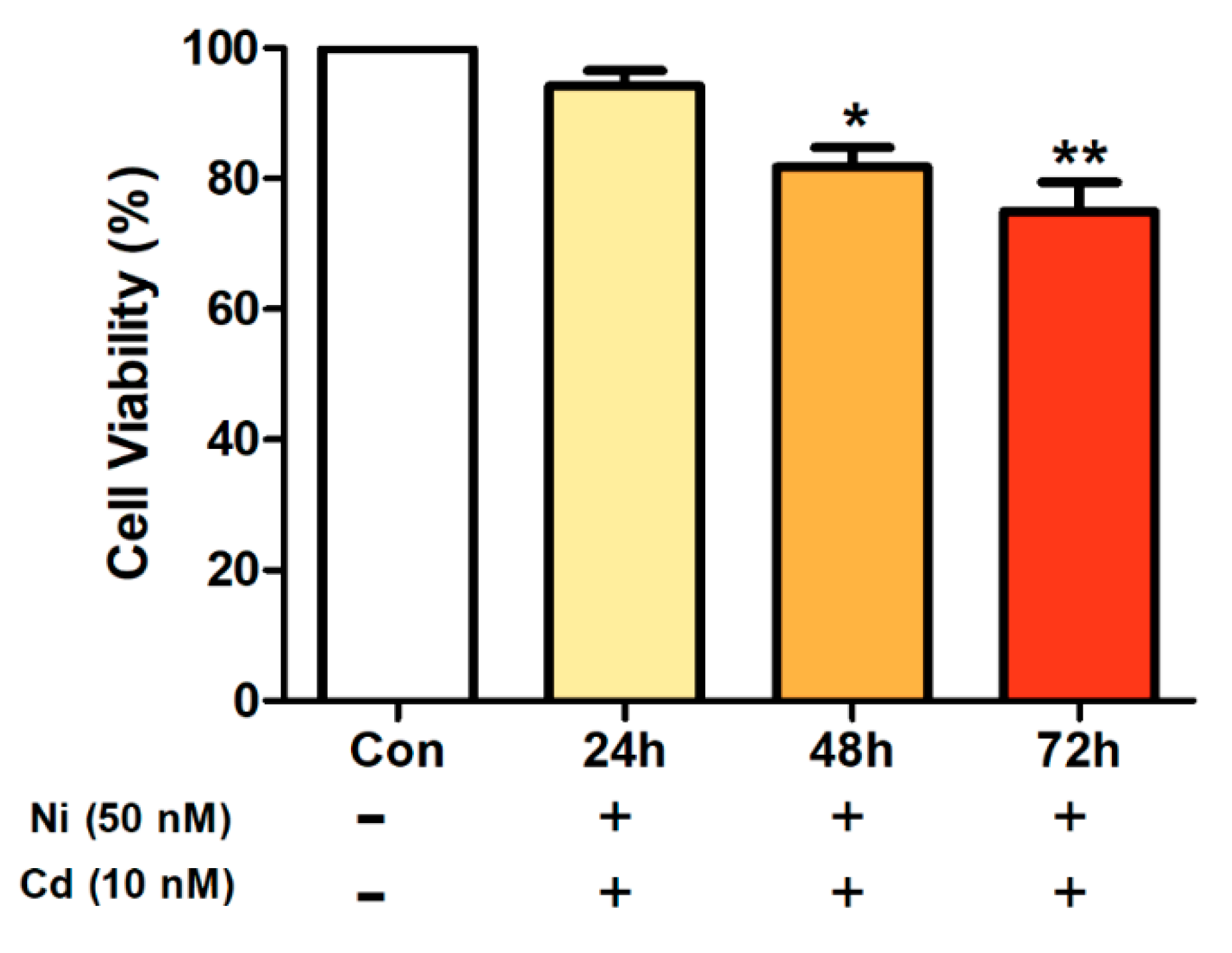
3.1. Cell Viability after Heavy Metal Exposure
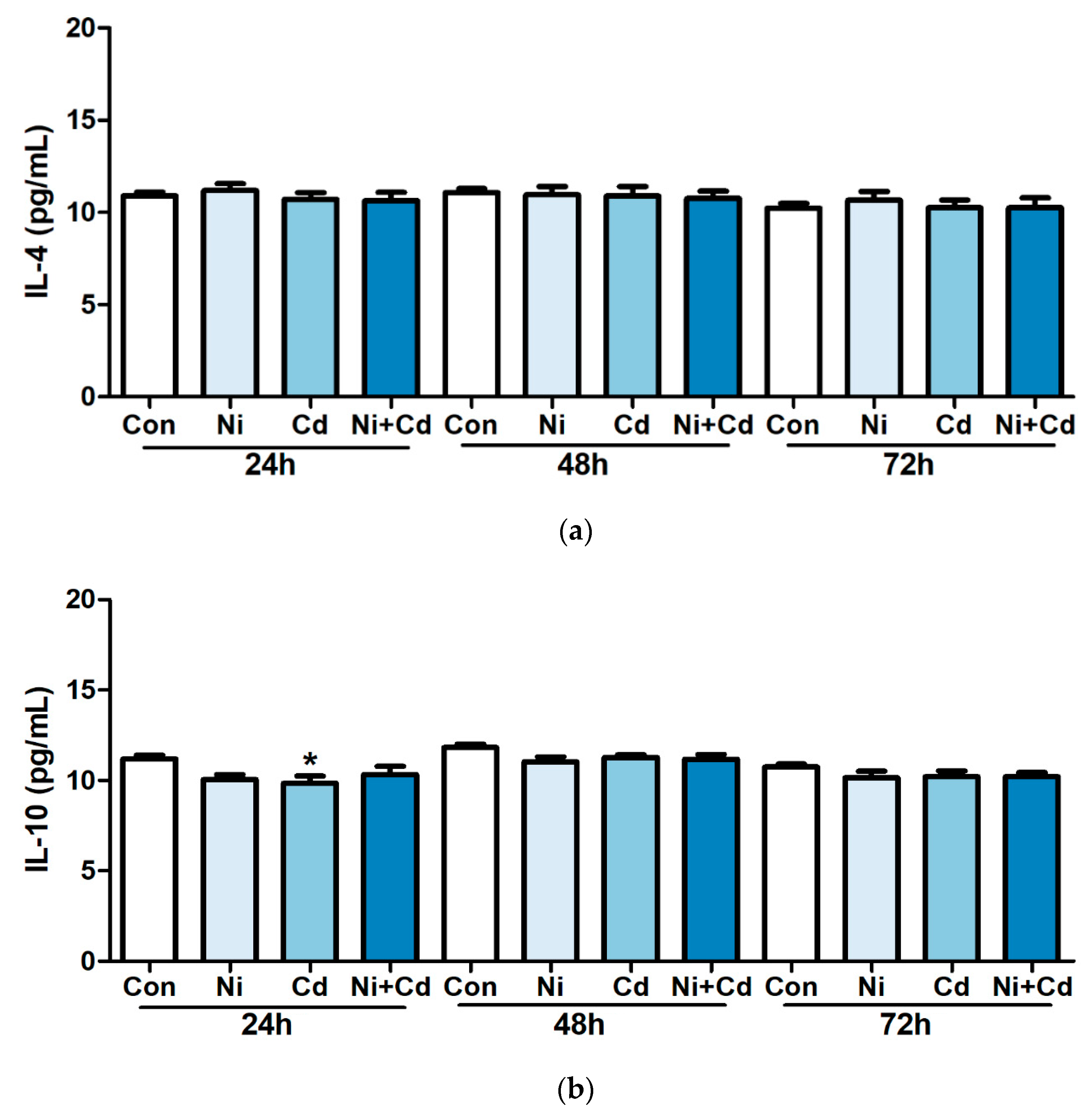
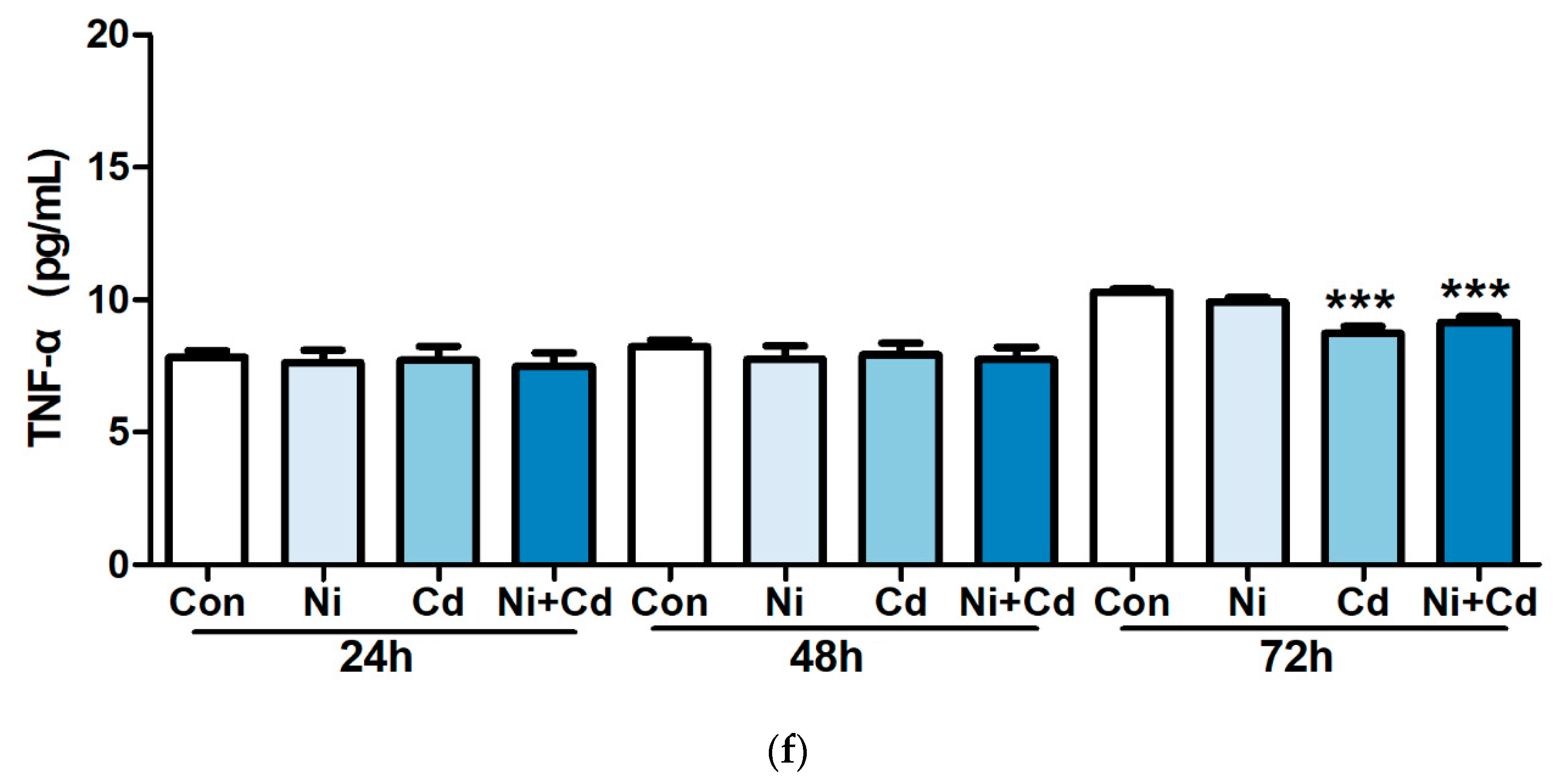
3.2. Effects of Heavy Metal Exposure on Cytokines Concentration
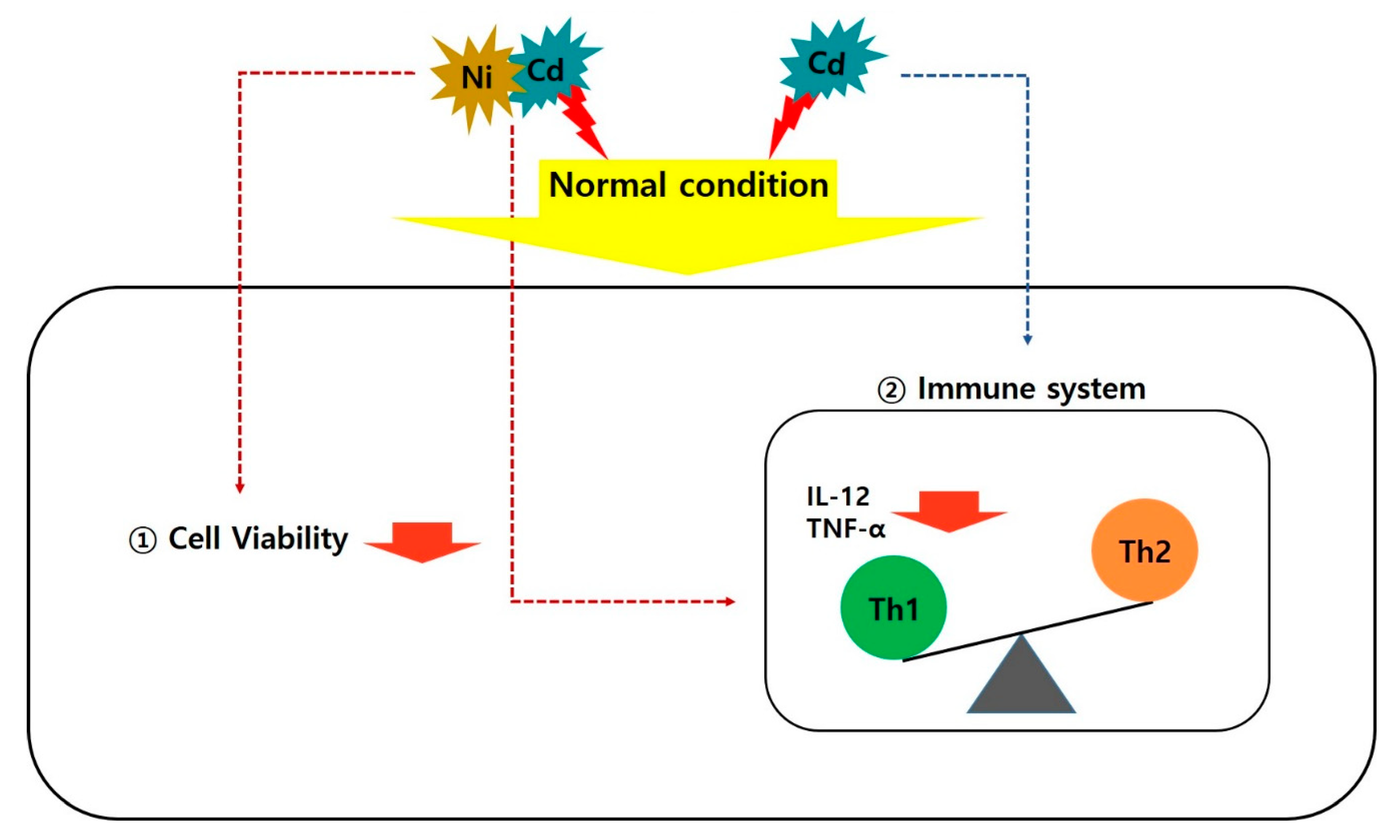
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Beckett, W.S.; Nordberg, G.F.; Clarkson, T.W. Ch 3 Routes of exposure, dose, and metabolism of metals. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 42–55. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Kang, S.K.; Kim, E.A. Occupational diseases in Korea. J. Korean Med. Sci. 2010, 25, S4–S12. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Ahn, J.; Lee, B.K.; Park, J.; Kim, Y. Environmental exposures to lead, mercury, and cadmium among South Korean teenagers (KNHANES 2010-2013): Body burden and risk factors. Environ. Res. 2017, 156, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.S.; Ha, E.; Shin, J.Y.; Park, H.; Hong, Y.C.; Ha, M.; Kim, S.; Lee, S.J.; Lee, K.Y.; Kim, J.H.; et al. Blood heavy metal concentrations in pregnant Korean women and their children up to age 5years: Mothers’ and Children’s Environmental Health (MOCEH) birth cohort study. Sci. Total Environ. 2017, 605–606, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Cadmium toxicity and treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity—An overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef]
- D’Antò, V.; Valletta, R.; Amato, M.; Schweikl, H.; Simeone, M.; Paduano, S.; Rengo, S.; Spagnuolo, G. Effect of nickel chloride on cell proliferation. Open Dent. J. 2012, 6, 177–181. [Google Scholar] [CrossRef]
- Bechara, R.; Antonios, D.; Azouri, H.; Pallardy, M. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and Jak-STAT pathways. J. Investig. Dermatol. 2017, 137, 2140–2148. [Google Scholar] [CrossRef]
- Wang, Y.F.; Shyu, H.W.; Chang, Y.C.; Tseng, W.C.; Huang, Y.L.; Lin, K.H.; Chou, M.C.; Liu, H.L.; Chen, C.Y. Nickel (II)-induced cytotoxicity and apoptosis in human proximal tubule cells through a ROS- and mitochondria-mediated pathway. Toxicol. Appl. Pharmacol. 2012, 259, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Panjehpour, M.; Bayesteh, M. The cytotoxic effects of cadmium chloride on the human lung carcinoma (Calu-6) cell line. Res. Pharm. Sci. 2009, 3, 49–53. [Google Scholar]
- Olszowski, T.; Baranowska-Bosiacka, I.; Gutowska, I.; Piotrowska, K.; Mierzejewska, K.; Korbecki, J.; Kurzawski, M.; Tarnowski, M.; Chlubek, D. The effects of cadmium at low environmental concentrations on THP-1 macrophage apoptosis. Int. J. Mol. Sci. 2015, 16, 21410–21427. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, H.; Heo, H.R.; Kim, J.W.; Kim, H.R.; Hong, Y.; Yang, S.R.; Han, S.S.; Lee, S.J.; Kim, W.J.; et al. Cadmium-induced ER stress and inflammation are mediated through C/EBP-DDIT3 signaling in human bronchial epithelial cells. Exp. Mol. Med. 2017, 49, e372. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Wu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Nickel carcinogenesis mechanism: DNA damage. Int. J. Mol. Sci. 2019, 20, 4690. [Google Scholar] [CrossRef]
- Kalaivani, P.; Saranya, S.; Poornima, P.; Prabhakaran, R.; Dallemer, F.; Vijaya Padma, V.; Natarajan, K. Biological evaluation of new nickel(II) metallates: Synthesis, DNA/protein binding and mitochondrial mediated apoptosis in human lung cancer cells (A549) via ROS hypergeneration and depletion of cellular antioxidant pool. Eur. J. Med. Chem. 2014, 82, 584–599. [Google Scholar] [CrossRef]
- Palusińska, M.; Barabasz, A.; Kozak, K.; Papierniak, A.; Maślińska, K.; Antosiewicz, D.M. Zn/Cd status-dependent accumulation of Zn and Cd in root parts in tobacco is accompanied by specific expression of ZIP genes. BMC Plant Biol. 2020, 20, 37. [Google Scholar] [CrossRef]
- Hu, K.H.; Li, W.X.; Sun, M.Y.; Zhang, S.B.; Fan, C.X.; Wu, Q.; Zhu, W.; Xu, X. Cadmium induced apoptosis in MG63 cells by increasing ROS, activation of p38 MAPK and inhibition of ERK 1/2 pathways. Cell. Physiol. Biochem. 2015, 36, 642–654. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef]
- Abouhamed, M.; Wolff, N.A.; Lee, W.K.; Smith, C.P.; Thévenod, F. Knockdown of endosomal/lysosomal divalent metal transporter 1 by RNA interference prevents cadmium-metallothionein-1 cytotoxicity in renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 2007, 293, F705–F712. [Google Scholar] [CrossRef]
- Urani, C.; Melchioretto, P.; Fabbri, M.; Bowe, G.; Maserati, E.; Gribaldo, L. Cadmium impairs p53 activity in HepG2 cells. ISRN Toxicol. 2014, 2014, 976428. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jeong, J. Synergistic cellular responses to heavy metal exposure: A minireview. Biochim. Biophys. Acta Gen. Sub. 2018, 1862, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, G.F.; Jin, T.; Hong, F.; Zhang, A.; Buchet, J.P.; Bernard, A. Biomarkers of cadmium and arsenic interactions. Toxicol. Appl. Pharmacol. 2005, 206, 191–197. [Google Scholar] [CrossRef]
- Pabis, K.; Gundacker, C.; Giacconi, R.; Basso, A.; Costarelli, L.; Piacenza, F.; Strizzi, S.; Provinciali, M.; Malavolta, M. Zinc supplementation can reduce accumulation of cadmium in aged metallothionein transgenic mice. Chemosphere 2018, 211, 855–860. [Google Scholar] [CrossRef]
- Vance, T.M.; Chun, O.K. Zinc intake is associated with lower cadmium burden in U.S. adults. J. Nutr. 2015, 145, 2741–2748. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Puddu, P.; Fantuzzi, L.; Borghi, P.; Varano, B.; Rainaldi, G.; Guillemard, E.; Malorni, W.; Nicaise, P.; Wolf, S.F.; Belardelli, F.; et al. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J. Immunol. 1997, 159, 3490–3497. [Google Scholar]
- Hemdan, N.Y. The role of interleukin-12 in the heavy metal-elicited immunomodulation: Relevance of various evaluation methods. J. Occup. Med. Toxicol. 2008, 3, 25. [Google Scholar] [CrossRef]
- Del Vecchio, M.; Bajetta, E.; Canova, S.; Lotze, M.T.; Wesa, A.; Parmiani, G.; Anichini, A. Interleukin-12: Biological properties and clinical application. Clin. Cancer Res. 2007, 13, 4677–4685. [Google Scholar] [CrossRef]
- Li, W.T.; Wang, L.Y.; Chang, H.W.; Yang, W.C.; Lo, C.; Pang, V.F.; Chen, M.H.; Jeng, C.R. Th2 cytokine bias induced by silver nanoparticles in peripheral blood mononuclear cells of common bottlenose dolphins (Tursiops truncatus). PeerJ 2018, 6, e5432. [Google Scholar] [CrossRef]
- Berger, A. Th1 and Th2 responses: What are they? BMJ 2000, 321, 424. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Mehrotra, S.; Agarwal, S.S. The paradigm of Th1 and Th2 cytokines: Its relevance to autoimmunity and allergy. Immunol. Res. 1999, 20, 147–161. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.; Park, S.; Sung, J.H. Cell Viability and Immune Response to Low Concentrations of Nickel and Cadmium: An In Vitro Model. Int. J. Environ. Res. Public Health 2020, 17, 9218. https://doi.org/10.3390/ijerph17249218
Kim A, Park S, Sung JH. Cell Viability and Immune Response to Low Concentrations of Nickel and Cadmium: An In Vitro Model. International Journal of Environmental Research and Public Health. 2020; 17(24):9218. https://doi.org/10.3390/ijerph17249218
Chicago/Turabian StyleKim, Ahra, SangJin Park, and Joo Hyun Sung. 2020. "Cell Viability and Immune Response to Low Concentrations of Nickel and Cadmium: An In Vitro Model" International Journal of Environmental Research and Public Health 17, no. 24: 9218. https://doi.org/10.3390/ijerph17249218
APA StyleKim, A., Park, S., & Sung, J. H. (2020). Cell Viability and Immune Response to Low Concentrations of Nickel and Cadmium: An In Vitro Model. International Journal of Environmental Research and Public Health, 17(24), 9218. https://doi.org/10.3390/ijerph17249218



