Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery
Abstract
:1. Introduction
2. Conventional Process Using Coagulants and Flocculants
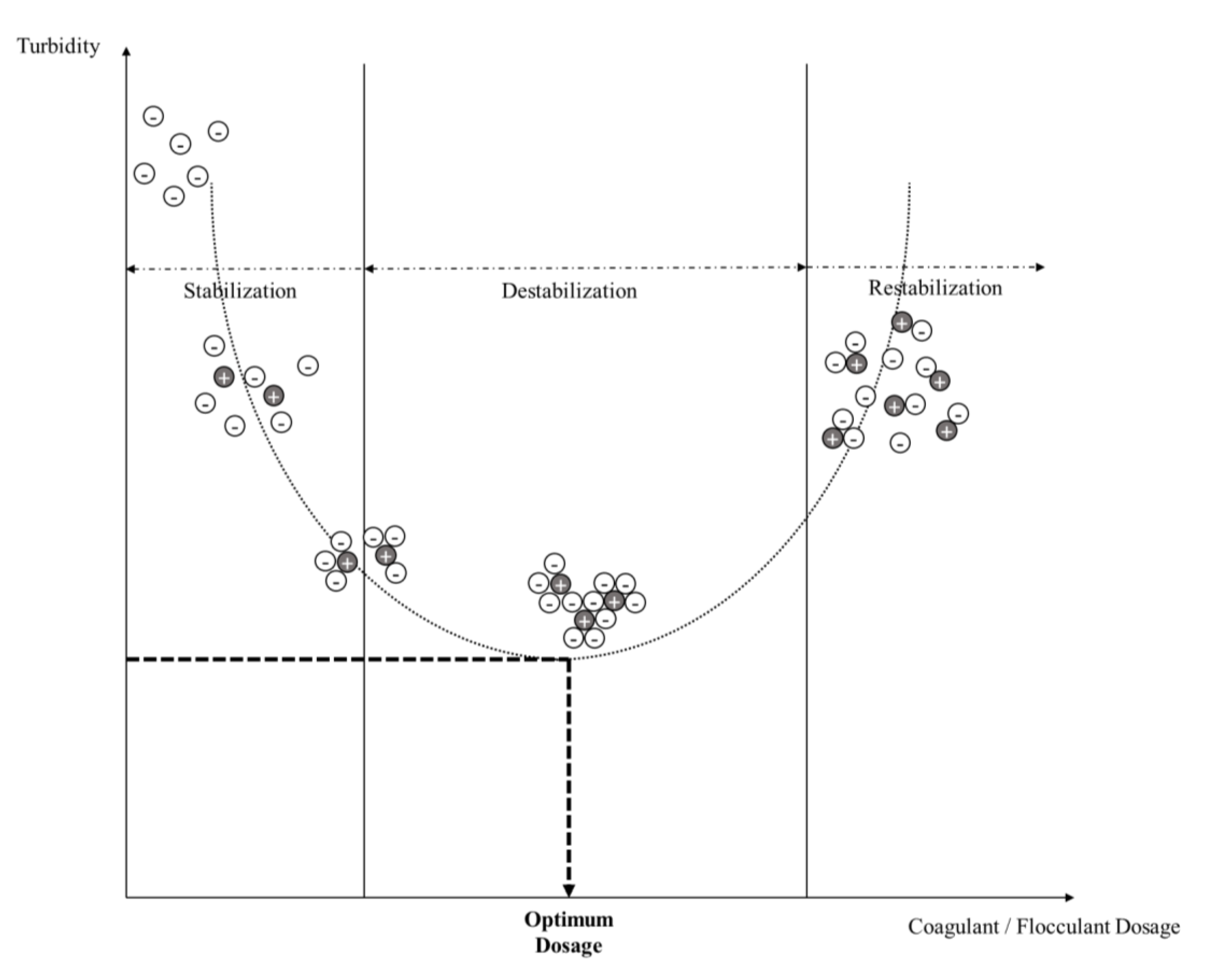
2.1. Fundamentals of the Coagulation–Flocculation Process
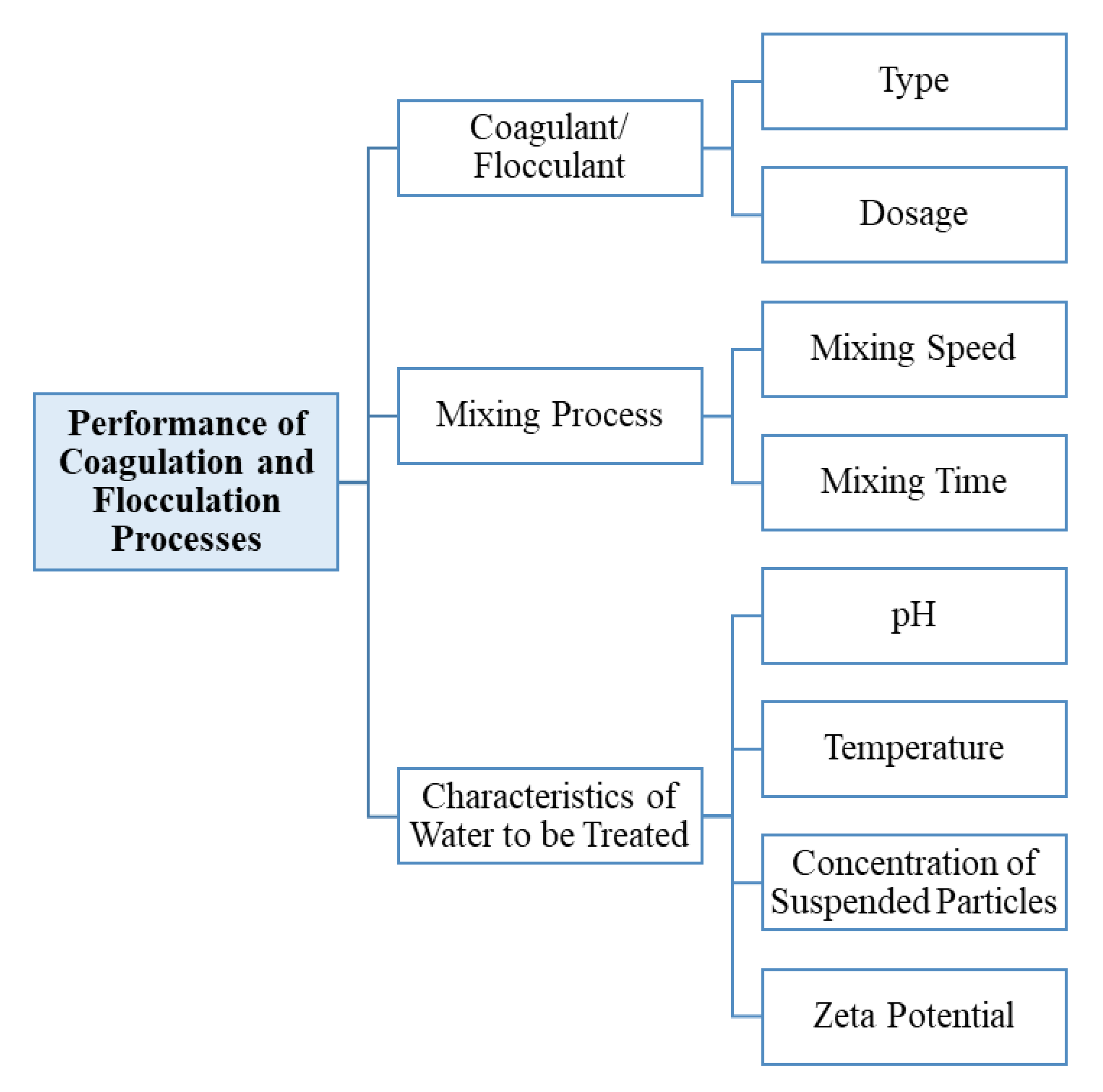
2.2. Factors Affecting the Coagulation–Flocculation Process
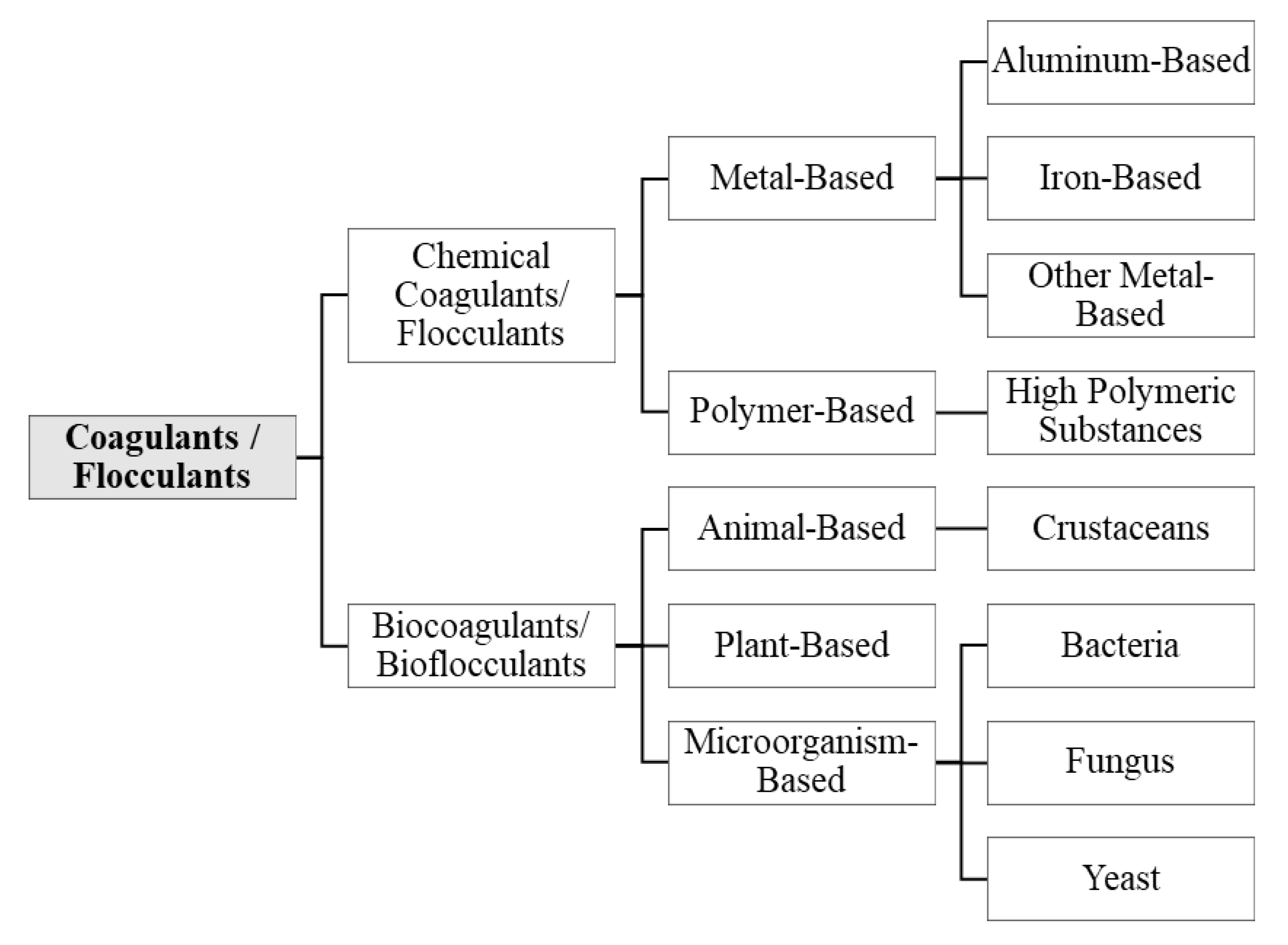
2.3. Types of Coagulants and Flocculants and Their Main Applications
2.4. Criteria for Effective Coagulants/Flocculants
3. Environmental and Health Impacts of Chemical Coagulants/Flocculants
3.1. Toxicity and Health Risk Potential of Conventional Coagulants and Flocculants
3.2. Environmental Pollution Caused by Chemical Coagulants/Flocculants
4. Characterization of Biocoagulants and Bioflocculants
4.1. Origin of Biocoagulants and Bioflocculants
4.2. Chemical Characteristics
4.3. Working Mechanism of Natural Coagulants/Flocculants
- Polymer bridging: Colloid particles will attach to a part of a long-chain polymer, while the other free part of the chain will form a loop and a tail. The molecules will continue to form a larger molecule when the free tail attaches with another free colloid, increasing the particle size. The correct dosage of coagulants to provide a free surface for the process is important [92,110].
- Charge neutralization: Colloid particles are normally negative in charge and cannot form a larger particle because they repel one another. Thus, the addition of cationic biocoagulants will produce carboxylate and H+ ions to neutralize the suspension near to zero zeta potential and make the formation of a large floc possible. A low dosage of coagulants will be needed for the treatment if they have a high charge density [92,93].
4.4. Ethical Utilization and Toxicity
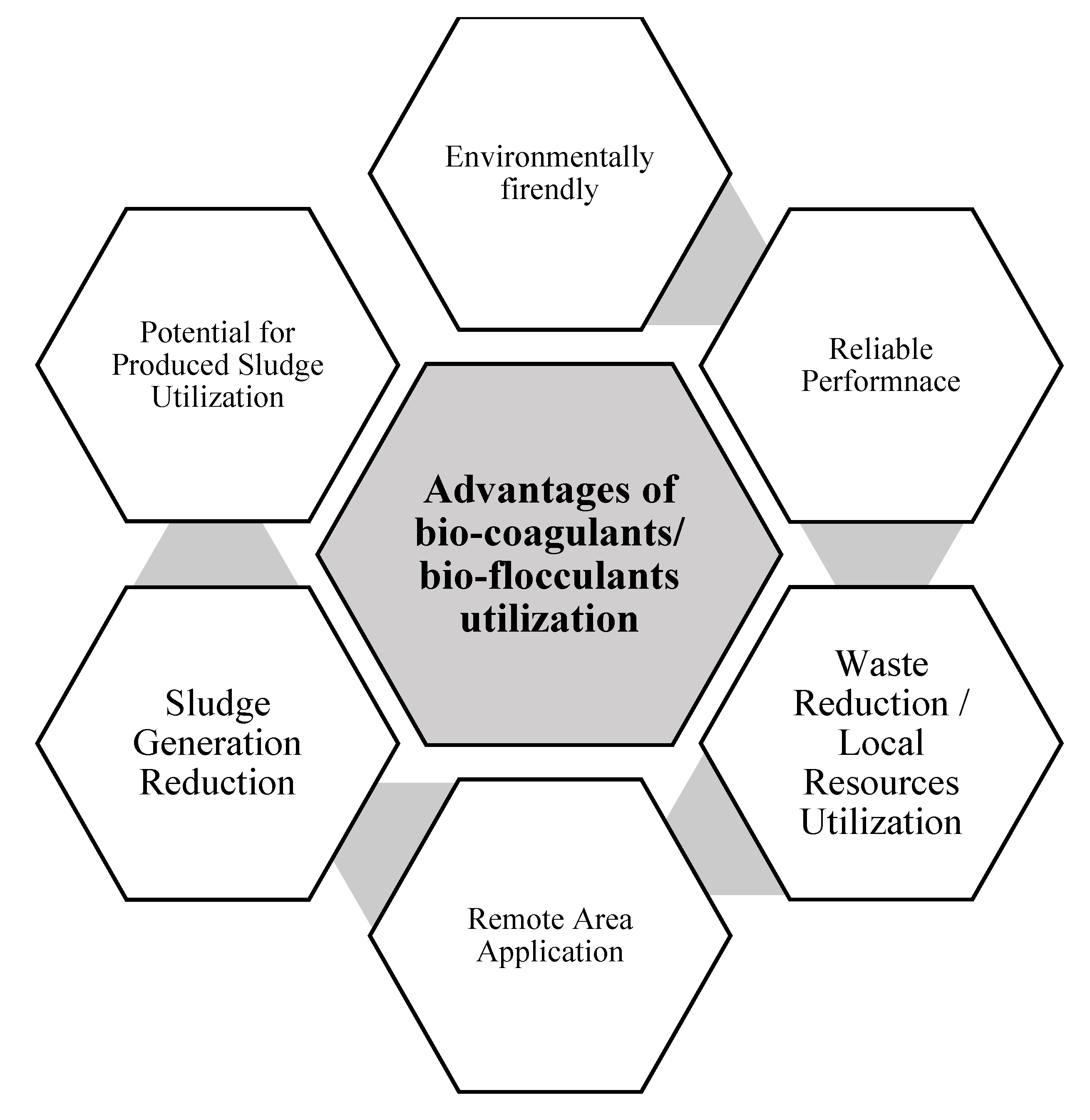
5. Advantages of the Utilization of Biocoagulants/Bioflocculants
5.1. Environmentally Friendly Technology
5.2. Reliable Performance
5.3. Waste Reduction/Local Resource Utilization
5.4. Remote Area Application
5.5. Sludge Generation Reduction
5.6. Potential of Produced Sludge Utilization
6. Application of Biocoagulants/Bioflocculants to Drinking Water and Wastewater Treatment
7. Comparative Evaluation of Chemical Coagulants vs. Biocoagulants
7.1. Public Acceptance
7.2. Availability and Handling of Materials
7.3. Generation and Handling of Sludge
7.4. Operational Costs
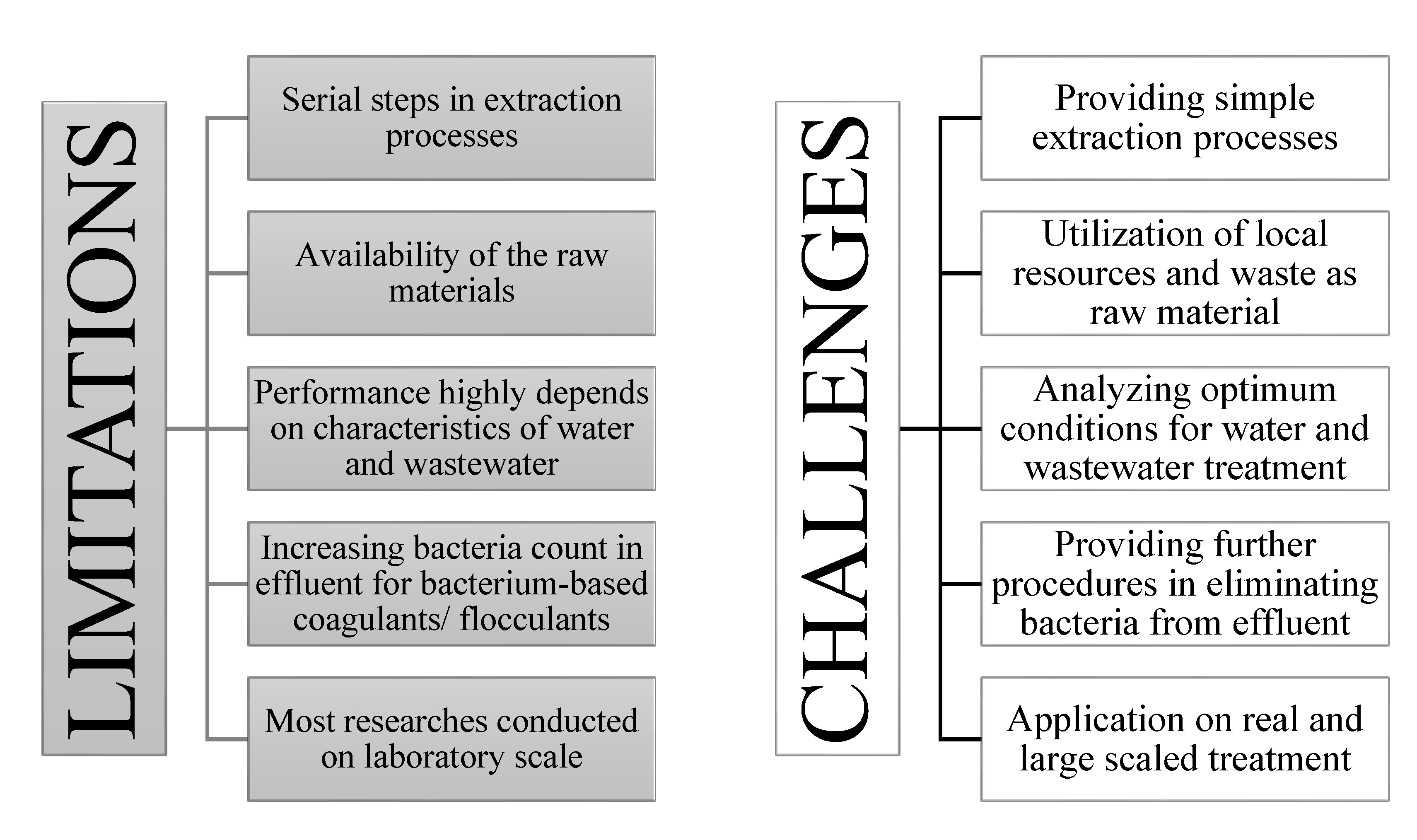
8. Limitations and Future Challenges
8.1. Limitations of Current Studies
8.2. Challenges for Future Studies
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madaki, Y.S.; Seng, L. Palm Oil Mill Effluent (Pome) from Malaysia Palm Oil Mills: Waste or Resource. Int. J. Sci. Environ. Technol. 2013, 2, 1138–1155. [Google Scholar]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour. Technol. 2016. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003. [Google Scholar] [CrossRef]
- Edzwald, J.K. Coagulation in drinking water treatment: Particles, organics and coagulants. Water Sci. Technol. 1993, 27, 21–35. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Sibrell, P.L.; Ogden, S.R.; Summerfelt, S.T. Evaluation of chemical coagulation-flocculation aids for the removal of suspended solids and phosphorus from intensive recirculating aquaculture effluent discharge. Aquac. Eng. 2003, 29, 23–42. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.; Koohestanian, A.; Abbasian, Z. The Separation Method for Removing of Colloidal Particles from Raw Water. J. Agric. Environ. Sci. 2008, 4, 266–273. [Google Scholar]
- Lapointe, M.; Barbeau, B. Understanding the roles and characterizing the intrinsic properties of synthetic vs. natural polymers to improve clarification through interparticle Bridging: A review. Sep. Purif. Technol. 2020, 231. [Google Scholar] [CrossRef]
- Moran, S. Engineering science of water treatment unit operations. In An Applied Guide to Water and Effluent Treatment Plant Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–51. [Google Scholar]
- Aragonés-Beltrán, P.; Mendoza-Roca, J.A.; Bes-Piá, A.; García-Melón, M.; Parra-Ruiz, E. Application of multicriteria decision analysis to jar-test results for chemicals selection in the physical–chemical treatment of textile wastewater. J. Hazard. Mater. 2009, 164, 288–295. [Google Scholar] [CrossRef]
- Weydts, D.; De Smet, D.; Vanneste, M. Processes for reducing the environmental impact of fabric finishing. In Sustainable Apparel; Elsevier: Amsterdam, The Netherlands, 2015; pp. 35–48. [Google Scholar]
- Barakwan, R.A.; Trihadiningrum, Y.; Bagastyo, A.Y. Characterization of alum sludge from Surabaya Water Treatment Plant, Indonesia. J. Ecol. Eng. 2019, 20, 7–13. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Ismail, N.I.; Abdullah, S.R.S. Future challenges in diesel biodegradation by bacteria isolates: A review. J. Clean. Prod. 2020, 251, 119716. [Google Scholar] [CrossRef]
- Cardwell, A.S.; Adams, W.J.; Gensemer, R.W.; Nordheim, E.; Santore, R.C.; Ryan, A.C.; Stubblefield, W.A. Chronic toxicity of aluminum, at a pH of 6, to freshwater organisms: Empirical data for the development of international regulatory standards/criteria. Environ. Toxicol. Chem. 2018, 37, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Tomljenovic, L. Aluminum and Alzheimer’s disease: After a century of controversy, is there a plausible link? J. Alzheimer’s Dis. 2011, 23, 567–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetteh, E.K.; Rathilal, S. Application of Organic Coagulants in Water and Wastewater Treatment. Org. Polym. 2019. [Google Scholar] [CrossRef] [Green Version]
- Fort, D.J.; Stover, E.L. Impact of toxicities and potential interactions of flocculants and coagulant aids on whole effluent toxicity testing. Water Environ. Res. 1995, 67, 921–925. [Google Scholar] [CrossRef]
- Rahmani, R.; Shahmoradi, B.; Maleki, A. Bioassay testing the toxicity of nano-structure polymer (PAMAM G2) as coagulant aid in water treatment. Res. J. Environ. Toxicol. 2015, 9, 261–267. [Google Scholar] [CrossRef]
- Al-Mutairi, N.Z. Coagulant toxicity and effectiveness in a slaughterhouse wastewater treatment plant. Ecotoxicol. Environ. Saf. 2006, 65, 74–83. [Google Scholar] [CrossRef]
- Niquette, P.; Monette, F.; Azzouz, A.; Hausler, R. Impacts of substituting aluminum-based coagulants in drinking water treatment. Water Qual. Res. J. Can. 2004, 39, 303–310. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Q. Toxic effects of Al-based coagulants on Brassica chinensis and Raphanus sativus growing in acid and neutral conditions. Environ. Toxicol. 2005, 20, 179–187. [Google Scholar] [CrossRef]
- Ismail, N.I.; Abdullah, S.R.S.; Idris, M.; Kurniawan, S.B.; Effendi Halmi, M.I.; AL Sbani, N.H.; Jehawi, O.H.; Hasan, H.A. Applying rhizobacteria consortium for the enhancement of Scirpus grossus growth and phytoaccumulation of Fe and Al in pilot constructed wetlands. J. Environ. Manag. 2020, 267. [Google Scholar] [CrossRef]
- Kluczka, J.; Zołotajkin, M.; Ciba, J.; Staroń, M. Assessment of aluminum bioavailability in alum sludge for agricultural utilization. Environ. Monit. Assess. 2017, 189, 422. [Google Scholar] [CrossRef] [Green Version]
- Mortula, M.; Bard, S.M.; Walsh, M.E.; Gagnon, G.A. Aluminum toxicity and ecological risk assessment of dried alum residual into surface water disposal. Can. J. Civ. Eng. 2009, 36, 127–136. [Google Scholar] [CrossRef]
- Barakwan, R.A.; Hardina, T.T.; Trihadiningrum, Y.; Bagastyo, A.Y. Recovery of alum from Surabaya water treatment sludge using electrolysis with carbon-silver electrodes. J. Ecol. Eng. 2019, 20, 126–133. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3′R’ concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Exley, C. Aluminum Should Now Be Considered a Primary Etiological Factor in Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2017, 1, 23–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, O.; Abidin, Z.Z.; Idris, A.; Kamarudin, S.; Al-Qubaisi, M.S. A novel biocoagulant agent from mushroom chitosan as water and wastewater therapy. Environ. Sci. Pollut. Res. 2017, 24, 20104–20112. [Google Scholar] [CrossRef]
- Oladoja, N.A. Headway on natural polymeric coagulants in water and wastewater treatment operations. J. Water Process Eng. 2015, 6, 174–192. [Google Scholar] [CrossRef]
- Chethana, M.; Sorokhaibam, L.G.; Bhandari, V.M.; Raja, S.; Ranade, V.V. Green Approach to Dye Wastewater Treatment Using Biocoagulants. ACS Sustain. Chem. Eng. 2016. [Google Scholar] [CrossRef]
- Braga, W.L.M.; Roberto, J.A.; Vaz, C.; Samanamud, G.R.L.; Loures, C.C.A.; França, A.B.; Lofrano, R.C.Z.; Naves, L.L.R.; José Henrique De Freitas Gomes, J.H.; Naves, F.L. Extraction and optimization of tannin from the flower of Musa sp. applied to the treatment of iron ore dump. J. Environ. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Yongabi, K.A.; Lewis, D.M.; Harris, P.L. A Moringa Oleifera Disinfectant-Sand Filter Integration: A Review of an Alternative Sustainable Technology for Household Water Treatment. Environ. Sci. Eng. 2011, 5, 1100–1108. [Google Scholar]
- Lichtfouse, E.; Morin-Crini, N.; Fourmentin, M.; Zemmouri, H.; do Carmo Nascimento, I.O.; Queiroz, L.M.; Tadza, M.Y.M.; Picos-Corrales, L.A.; Pei, H.; Wilson, L.D.; et al. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019, 17, 1603–1621. [Google Scholar] [CrossRef] [Green Version]
- Ogunlade, A.O.; Oyetayo, V.O.; Ojokoh, A.O. Effect of different biocoagulants on the microbial quality and mineral composition of west African cheese produced from sheep milk. Food Res. 2019. [Google Scholar] [CrossRef]
- Yin, C.Y. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Othman, N.; Asharuddin, S. Applications of Natural Coagulants to Treat Wastewater—A Review. In Proceedings of the MATEC Web of Conferences, Wuhan, China, 20–21 December 2016. [Google Scholar]
- Okaiyeto, K.; Nwodo, U.U.; Okoli, S.A.; Mabinya, L.V.; Okoh, A.I. Implications for public health demands alternatives to inorganic and synthetic flocculants: Bioflocculants as important candidates. Microbiologyopen 2016, 5, 177–211. [Google Scholar] [CrossRef]
- BinAhmed, S.; Ayoub, G.; Al-Hindi, M.; Azizi, F. The effect of fast mixing conditions on the coagulation–flocculation process of highly turbid suspensions using liquid bittern coagulant. Desalin. Water Treat. 2015, 53, 3388–3396. [Google Scholar] [CrossRef]
- Yunos, F.H.M.; Nasir, N.M.; Wan Jusoh, H.H.; Khatoon, H.; Lam, S.S.; Jusoh, A. Harvesting of microalgae (Chlorella sp.) from aquaculture bioflocs using an environmental-friendly chitosan-based bio-coagulant. Int. Biodeterior. Biodegrad. 2017, 124, 243–249. [Google Scholar] [CrossRef]
- Suopajärvi, T. Functionalized Nanocelluloses in Wastewater Treatment Applications. Ph.D. Thesis, Universitatis Ouluensis, Oulu, Finland, 2015. [Google Scholar]
- Akers, R.J. Flocculation. Chem. Eng. 1975. [Google Scholar] [CrossRef]
- Henderson, J.M.; Wheatley, A.D. Factors affecting the efficient flocculation of tailings by polyacrylamides. Coal Prep. 1987, 4, 1–49. [Google Scholar] [CrossRef]
- Lim, H.K.; Ismail, N.; Abustan, I.; Murshed, M.F.; Ahmad, A. Treatment of landfill leachate by using lateritic soil as a natural coagulant. J. Environ. Manag. 2012. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, C.S.B.; Fradin, E.; Gregory, J. Temperature effects on flocculation, using different coagulants. Water Sci. Technol. 2004, 50, 171–175. [Google Scholar] [CrossRef]
- Asrafuzzaman, M.; Fakhruddin, A.N.M.; Hossain, M.A. Reduction of Turbidity of Water Using Locally Available Natural Coagulants. ISRN Microbiol. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratby, J. Coagulation and flocculation in water and wastewater treatment. Water 2016, 15. [Google Scholar] [CrossRef]
- Brandt, M.J.; Johnson, K.M.; Elphinston, A.J.; Ratnayaka, D.D. Storage, Clarification and Chemical Treatment. Twort’s Water Supply 2017, 323–366. [Google Scholar] [CrossRef]
- Abiola, O.N. Polymers for Coagulation and Flocculation in Water Treatment; Springer International Publishing: Gewerbestr, Switzerland, 2019; pp. 77–92. [Google Scholar]
- Maurya, S.; Daverey, A. Evaluation of plant-based natural coagulants for municipal wastewater treatment. 3 Biotech 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Ramanan, R.N. A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 2015, 12, 367–390. [Google Scholar] [CrossRef] [Green Version]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoli, A.S.; Okoh, A.I. Evaluation of flocculating performance of a thermostable bioflocculant produced by marine Bacillus sp. Environ. Technol. 2016, 37, 1829–1842. [Google Scholar] [CrossRef]
- Nagaraj, V.; Skillman, L.; Li, D.; Ho, G. Review—Bacteria and their extracellular polymeric substances causing biofouling on seawater reverse osmosis desalination membranes. J. Environ. Manag. 2018, 223, 586–599. [Google Scholar] [CrossRef]
- Al-Wasify, R.S.; Al-Sayed, A.S.A.; Saleh, S.M.; Aboelwafa, A.M. Bacterial exopolysaccharides as new natural coagulants for surface water treatment. Int. J. PharmTech Res. 2015, 8, 198–207. [Google Scholar]
- Ayekoe, C.Y.P.; Robert, D.; Lanciné, D.G. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in real treated water from Agbô River (Ivory-Coast). Catal. Today 2017, 281, 2–13. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F. The effect of tidal fluctuation on the accumulation of plastic debris in the Wonorejo River Estuary, Surabaya, Indonesia. Environ. Technol. Innov. 2019, 15, 100420. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S.P. A comprehensive study on coagulant performance and floc characterization of natural Cassia obtusifolia seed gum in treatment of raw pulp and paper mill effluent. Ind. Crops Prod. 2014. [Google Scholar] [CrossRef] [Green Version]
- Freitas, T.K.F.S.; Oliveira, V.M.; de Souza, M.T.F.; Geraldino, H.C.L.; Almeida, V.C.; Fávaro, S.L.; Garcia, J.C. Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A. esculentus) mucilage as natural coagulant. Ind. Crops Prod. 2015, 76, 538–544. [Google Scholar] [CrossRef]
- Hamawand, I.; Ghadouani, A.; Bundschuh, J.; Hamawand, S.; Al Juboori, R.A.; Chakrabarty, S.; Yusaf, T. A critical review on processes and energy profile of the Australian meat processing industry. Energies 2017, 10, 731. [Google Scholar] [CrossRef] [Green Version]
- Stuart, M.C.; de Vries, R.; Lyklema, H. Polyelectrolytes; Springer International Publishing: Gewerbestr, Switzerland, 2005; pp. 2.1–2.84. [Google Scholar]
- Gregory, J.; Barany, S. Adsorption and flocculation by polymers and polymer mixtures. Adv. Colloid Interface Sci. 2011, 169, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, M.; Barbeau, B. Substituting polyacrylamide with an activated starch polymer during ballasted flocculation. J. Water Process Eng. 2019, 28, 129–134. [Google Scholar] [CrossRef]
- Jørgensen, S.E.; Löffler, H.; Rast, W.; Straškraba, M. Chapter 4 Measures for improving water quality. In Developments in Water Science; Elsevier B.V.: Amsterdam, Netherlands, 2005; Volume 54, pp. 169–242. [Google Scholar]
- Takigami, H.; Taniguchi, N.; Shimizu, Y.; Matsui, S. Toxicity assays and their evaluation on organic polymer flocculants used for municipal sludge dewatering. Water Sci. Technol. 1998, 38, 207–215. [Google Scholar] [CrossRef]
- Exley, C.; Mold, M.J. Correction to: Aluminium in human brain tissue: How much is too much? J. Biol. Inorg. Chem. 2019, 24, 1283. [Google Scholar] [CrossRef] [Green Version]
- Mold, M.; Linhart, C.; Gómez-Ramírez, J.; Villegas-Lanau, A.; Exley, C. Aluminum and Amyloid-β in Familial Alzheimer’s Disease. J. Alzheimer’s Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Mold, M.; Umar, D.; King, A.; Exley, C. Aluminium in brain tissue in autism. J. Trace Elem. Med. Biol. 2018, 46, 76–82. [Google Scholar] [CrossRef]
- Gillete-Guyonnet, S.; Andrieu, S.; Nourhashemi, F.; De La Guéronnière, V.; Grandjean, H.; Vellas, B. Cognitive impairment and composition of drinking water in women: Findings of the EPIDOS study. Am. J. Clin. Nutr. 2005, 81, 897–902. [Google Scholar] [CrossRef] [Green Version]
- Graves, A.B.; Rosner, D.; Echeverria, D.; Mortimer, J.A.; Larson, E.B. Occupational exposures to solvents and aluminium and estimated risk of Alzheimer’s disease. Occup. Environ. Med. 1998, 55, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Exley, C.; Clarkson, E. Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers Dis. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeley, J.; Jarvis, P.; Judd, S.J. Coagulant recovery from water treatment residuals: A review of applicable technologies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2675–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.R.; Huang, C.; Lin, S. Reuse of fresh water sludge in cement making. Water Sci. Technol. 2004. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Imron, M.F. Seasonal variation of plastic debris accumulation in the estuary of Wonorejo River, Surabaya, Indonesia. Environ. Technol. Innov. 2019, 16, 100490. [Google Scholar] [CrossRef]
- Henriksson, P. Comparative Life Cycle Assessment of Sludge Treatment Systems: Is Recycling Aluminium Based Coagulant from Chemical Sludge the Way of the Future; KTH Royal Institute of Technology: Stockholm, Sweden, 2017. [Google Scholar]
- Fouad, M.M.; El-Gendy, A.S.; Razek, T.M.A. Evaluation of leached metals in recovered aluminum coagulants from water treatment slurry. Water Sci. Technol. 2017, 75, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 363–369. [Google Scholar] [CrossRef]
- EPA Final Aquatic Life Ambient Water Quality Criteria for Aluminum 2018. Available online: https://www.epa.gov/wqc/2018-final-aquatic-life-criteria-aluminum-freshwater (accessed on 13 March 2020).
- Skaar, E.P. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010, 6, e1000949. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Krewulak, K.D.; Vogel, H.J. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1781–1804. [Google Scholar] [CrossRef] [Green Version]
- Vose, P.B. Iron nutrition in plants: A world overview. J. Plant Nutr. 1982, 5, 233–249. [Google Scholar] [CrossRef]
- Lindsay, W.L. Soil and Plant Relationships Associated with Iron Deficiency with Emphasis on Nutrient Interactions. J. Plant Nutr. 1984, 7, 489–500. [Google Scholar] [CrossRef]
- Li, W.; Lan, P. The understanding of the plant iron deficiency responses in strategy I plants and the role of ethylene in this process by omic approaches. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokina, E.V.; Yudina, T.P.; Bubnov, I.A.; Danilov, V.S. Assessment of iron toxicity using a luminescent bacterial test with an Escherichia coli recombinant strain. Microbiology 2013, 82, 439–444. [Google Scholar] [CrossRef]
- Kalantari, N.F.; Ghaffari, S. Evaluation of toxicity of heavy metals for Escherichia coli growth. Iran. J. Environ. Health Sci. Eng. 2008, 5, 173–178. [Google Scholar]
- De Dorlodot, S.; Lutts, S.; Bertin, P. Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J. Plant Nutr. 2005, 28, 1–20. [Google Scholar] [CrossRef]
- Saaltink, R.M.; Dekker, S.C.; Eppinga, M.B.; Griffioen, J.; Wassen, M.J. Plant-specific effects of iron-toxicity in wetlands. Plant Soil 2017, 416, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Connolly, E.L.; Guerinot, M. Iron stress in plants. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]
- Mehraban, P.; Zadeh, A.A.; Sadeghipour, H.R. Iron toxicity in rice (Oryza sativa L.), under different potassium nutrition. Asian J. Plant Sci. 2008, 7, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.; Halmi, M.I.E.; Al Sbani, N.H.; Idris, M.; Hasan, H.A.; Hashim, M.H.; Abdullah, S.R.S.; Jehawi, O.H.; Sanusi, S.N.A.; Sheikh Abdullah, S.R.; et al. Accumulation of Fe-Al by Scirpus grossus Grown in Synthetic Bauxite Mining Wastewater and Identification of Resistant Rhizobacteria. Environ. Eng. Sci. 2017, 34, 367–375. [Google Scholar] [CrossRef]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Raghunandan, M.E.; Ramanan, R.N. Utilization of plant-based natural coagulants as future alternatives towards sustainable water clarification. J. Environ. Sci. 2014, 26, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Amran, A.H.; Syamimi Zaidi, N.; Muda, K.; Wai Loan, L. Effectiveness of Natural Coagulant in Coagulation Process: A Review. Int. J. Eng. Technol. 2018, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Muruganandam, L.; Kumar, M.P.S.; Jena, A.; Gulla, S.; Godhwani, B. Treatment of waste water by coagulation and flocculation using biomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263. [Google Scholar] [CrossRef]
- Manholer, D.D.; De Souza, M.T.F.; Ambrosio, E.; De Souza Freitas, T.K.F.; Geraldino, H.C.L.; Garcia, J.C. Coagulation/flocculation of textile effluent using a natural coagulant extracted from Dillenia indica. Water Sci. Technol. 2019, 80, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Hosny, R.; Fathy, M.; Ramzi, M.; Abdel Moghny, T.; Desouky, S.E.M.; Shama, S.A. Treatment of the oily produced water (OPW) using coagulant mixtures. Egypt. J. Pet. 2016, 25, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Menkiti, M.C.; Ndaji, C.R.; Ezemagu, I.G.; Uddameri, V. Application of Periwinkle Shell Coagulant (PSC) for the Remediation of Petroleum Produced Water (PPW) by Coag-Flocculation. J. Dispers. Sci. Technol. 2016, 37, 760–774. [Google Scholar] [CrossRef]
- Zemmouri, H.; Drouiche, M.; Sayeh, A.; Lounici, H.; Mameri, N. Coagulation flocculation test of Keddara’s water dam using chitosan and sulfate aluminium. Procedia Eng. 2012, 33, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Subudhi, S.; Bisht, V.; Batta, N.; Pathak, M.; Devi, A.; Lal, B. Purification and characterization of exopolysaccharide bioflocculant produced by heavy metal resistant Achromobacter xylosoxidans. Carbohydr. Polym. 2016, 137, 441–451. [Google Scholar] [CrossRef]
- Deng, S.B.; Bai, R.B.; Hu, X.M.; Luo, Q. Characteristics of a bioflocculant produced by Bacillus mucilaginosus and its use in starch wastewater treatment. Appl. Microbiol. Biotechnol. 2003, 60, 588–593. [Google Scholar] [CrossRef]
- Tawila, Z.M.A.; Ismail, S.; Dadrasnia, A.; Usman, M.M. Production and characterization of a bioflocculant produced by bacillus salmalaya 139si-7 and its applications in wastewater treatment. Molecules 2018, 23, 2689. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhong, C.; Chen, H.; Yao, J.; Tan, L.; Zhang, Y.; Zhou, J. Production of bioflocculants prepared from formaldehyde wastewater for the potential removal of arsenic. J. Environ. Manag. 2016, 172, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.A.; Ezril Hafiz, R.; Muhamad, M.H.; Sheikh Abdullah, S.R.; Hasan, H.A.; Ezril Hafiz, R.; Muhamad, M.H.; Sheikh Abdullah, S.R.; Hassimi, A.H.; Ezril Hafiz, R.; et al. Bioflocculant production using palm oil mill and sago mill effluent as a fermentation feedstock: Characterization and mechanism of flocculation. J. Environ. Manag. 2020, 260. [Google Scholar] [CrossRef]
- Teh, C.Y.; Wu, T.Y.; Juan, J.C. Potential use of rice starch in coagulation-flocculation process of agro-industrial wastewater: Treatment performance and flocs characterization. Ecol. Eng. 2014, 71, 509–519. [Google Scholar] [CrossRef]
- Chua, S.C.; Malek, M.A.; Chong, F.K.; Sujarwo, W.; Ho, Y.C. Red lentil (Lens culinaris) extract as a novel natural coagulant for turbidity reduction: An evaluation, characterization and performance optimization study. Water 2019, 11, 1686. [Google Scholar] [CrossRef] [Green Version]
- Othman, N.; Abd-Rahim, N.S.; Tuan-Besar, S.N.F.; Mohd-Asharuddin, S.; Kumar, V. A Potential Agriculture Waste Material as Coagulant Aid: Cassava Peel. IOP Conf. Ser. Mater. Sci. Eng. 2018, 311. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Dual starch–polyacrylamide polymer system for improved flocculation. Water Res. 2017, 124, 202–209. [Google Scholar] [CrossRef]
- Magalhães, E.R.B.; Fonseca de Menezes, N.N.; Silva, F.L.; Alves Garrido, J.W.; Angélica dos Santos Bezerra Sousa, M.; Santos, E.S. dos Effect of oil extraction on the composition, structure, and coagulant effect of Moringa oleifera seeds. J. Clean. Prod. 2021, 279, 123902. [Google Scholar] [CrossRef]
- Nonfodji, O.M.; Fatombi, J.K.; Ahoyo, T.A.; Osseni, S.A.; Aminou, T. Performance of Moringa oleifera seeds protein and Moringa oleifera seeds protein-polyaluminum chloride composite coagulant in removing organic matter and antibiotic resistant bacteria from hospital wastewater. J. Water Process Eng. 2020, 33. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Karthikeyan, P.; Sirajudheen, P.; Meenakshi, S. Optimization of sustainable chitosan/Moringa. oleifera as coagulant aid for the treatment of synthetic turbid water—A systemic study. Environ. Chem. Ecotoxicol. 2020, 2, 132–140. [Google Scholar] [CrossRef]
- Bolto, B.; Gregory, J. Organic polyelectrolytes in water treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef] [PubMed]
- Dorea, C.C. Use of Moringa spp. seeds for coagulation: A review of a sustainable option. Water Sci. Technol. Water Supply 2006, 6, 219–227. [Google Scholar] [CrossRef]
- Jadhav, M.V.; Mahajan, Y.S. Investigation of the performance of chitosan as a coagulant for flocculation of local clay suspensions of different turbidities. KSCE J. Civ. Eng. 2013, 17, 328–334. [Google Scholar] [CrossRef]
- Gunaratna, K.R.; Garcia, B.; Andersson, S.; Dalhammar, G. Screening and evaluation of natural coagulants for water treatment. Water Sci. Technol. Water Supply 2007, 7, 19–25. [Google Scholar] [CrossRef]
- Ramavandi, B. Treatment of water turbidity and bacteria by using a coagulant extracted from Plantago ovata. Water Resour. Ind. 2014, 6, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Othman, Z.; Ahmad, A.L. Pretreatment of palm oil mill effluent (POME) using Moringa oleifera seeds as natural coagulant. J. Hazard. Mater. 2007. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.L.; Mohammad, A.W.; Benamor, A.; Hilal, N. Chitosan as natural coagulant in hybrid coagulation-nanofiltration membrane process for water treatment. J. Environ. Chem. Eng. 2016. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, A.M. Using a Natural Coagulant for Treating Wastewater. Eighth Int. Water Technol. Conf. 2004. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Kazi, T.; Virupakshi, A.; Scholar, M.T. Treatment of Tannery Wastewater Using Natural Coagulants. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 4061–4068. [Google Scholar]
- Freitas, T.K.F.S.; Almeida, C.A.; Manholer, D.D.; Geraldino, H.C.L.; de Souza, M.T.F.; Garcia, J.C. Review of Utilization Plant-Based Coagulants as Alternatives to Textile Wastewater Treatment; Springer: Singapore, 2018. [Google Scholar]
- Ali, M.; Mustafa, A.; Saleem, M. Comparative Study between Indigenous Natural Coagulants and Alum for Microalgae Harvesting. Arab. J. Sci. Eng. 2019, 44, 6453–6463. [Google Scholar] [CrossRef]
- Irhayyim, O.A. Performance of Chitosan from Mushroom as Biocoagulant Agent for Kaolin and Palm Oil Mill Effluent Wastewater. Master’s Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2017. [Google Scholar]
- Sha’arani, S.; Azizan, S.N.F.; Md Akhir, F.N.; Muhammad Yuzir, M.A.; Othman, N.; Zakaria, Z.; Mohd Noor, M.J.M.; Hara, H. Removal efficiency of Gram-positive and Gram-negative bacteria using a natural coagulant during coagulation, flocculation, and sedimentation processes. Water Sci. Technol. 2019, 80, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Megersa, M.; Beyene, A.; Ambelu, A.; Asnake, D.; Bekele, T.; Firdissa, B.; Alebachew, Z.; Triest, L. A Preliminary Evaluation of Locally Used Plant Coagulants for Household Water Treatment. Water Conserv. Sci. Eng. 2016, 1, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Aviso, K.B.; Tan, R.R.; Culaba, A.B.; Cruz, J.B. Fuzzy input-output model for optimizing eco-industrial supply chains under water footprint constraints. J. Clean. Prod. 2011. [Google Scholar] [CrossRef]
- Khadidi, M.H.J.; Hamid, E.A. A New Flocculant-Coagulant with Potential Use for Industrial Wastewater Treatment. In Proceedings of the 2nd International Conference on Environment, Energy and Biotechnology, Kuala Lumpur, Malaysia, 8–9 June 2013. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Muda, K.; Abdul Rahman, M.A.; Sgawi, M.S.; Amran, A.H. Effectiveness of Local Waste Materials as Organic-Based Coagulant in Treating Water. IOP Conf. Ser. Mater. Sci. Eng. 2019, 636. [Google Scholar] [CrossRef]
- Saranya, P.; Ramesh, S.T.; Gandhimathi, R. Effectiveness of natural coagulants from non-plant-based sources for water and wastewater treatment—A review. Desalin. Water Treat. 2014, 52, 6030–6039. [Google Scholar] [CrossRef]
- Mumbi, A.W.; Fengting, L.; Karanja, A. Sustainable treatment of drinking water using natural coagulants in developing countries: A case of informal settlements in Kenya. Water Util. J. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer International Publishing: Gewerbestr, Switzerland, 2016; ISBN 9789401772761. [Google Scholar]
- Birima, A.H.; Hammad, H.A.; Desa, M.N.M.; Muda, Z.C. Extraction of natural coagulant from peanut seeds for treatment of turbid water. IOP Conf. Ser. Earth Environ. Sci. 2013, 16. [Google Scholar] [CrossRef]
- Kaushal, R.; Goyal, H. Treatment of Waste Water Using Natural Coagulants. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Kristianto, H. The Potency of Indonesia Native Plants as Natural Coagulant: A Mini Review. Water Conserv. Sci. Eng. 2017, 2, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Mathuram, M.; Meera, R.; Vijayaraghavan, G. Application of Locally Sourced Plants as Natural Coagulants For Dye Removal from Wastewater: A Review. J. Mater. Environ. Sci. 2018, 2508, 2058–2070. [Google Scholar]
- Kristianto, H.; Rahman, H.; Prasetyo, S.; Sugih, A.K. Removal of Congo red aqueous solution using Leucaena leucocephala seed’s extract as natural coagulant. Appl. Water Sci. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Theregowda, R.B.; González-Mejía, A.M.; Ma, X.; Garland, J. Nutrient Recovery from Municipal Wastewater for Sustainable Food Production Systems: An Alternative to Traditional Fertilizers. Environ. Eng. Sci. 2019, 36, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Kadir, A.A.; Abdullah, S.R.S.; Othman, B.A.; Hasan, H.A.; Othman, A.R.; Imron, M.F.; Ismail, N.; Kurniawan, S.B. Dual function of Lemna minor and Azolla pinnata as phytoremediator for Palm Oil Mill Effluent and as feedstock. Chemosphere 2020, 259, 127468. [Google Scholar] [CrossRef]
- Nash, D.A.H.; Abdullah, S.R.S.; Hasan, H.A.; Idris, M.; Muhammad, N.F.; Al-Baldawi, I.A.; Ismail, N.I. Phytoremediation of nutrients and organic carbon from sago mill effluent using water hyacinth (Eichhornia crassipes). J. Eng. Technol. Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Said, N.S.M.; Abdullah, S.R.S.; Ismail, N.; Hasan, H.A.; Othman, A.R. Phytoremediation of real coffee industry effluent through a continuous two-stage constructed wetland system. Environ. Technol. Innov. 2020, 17, 100502. [Google Scholar] [CrossRef]
- You, X.; Valderrama, C.; Cortina, J.L. Nutrients recovery from treated secondary mainstream in an urban wastewater treatment plant: A financial assessment case study. Sci. Total Environ. 2019, 656, 902–909. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. The Possibility of Organo-Mineral Fertilizer Production from Sewage Sludge. Waste Biomass Valorization 2017, 8, 1781–1791. [Google Scholar] [CrossRef] [Green Version]
- Mtshali, J.S.; Tiruneh, A.T.; Fadiran, A.O. Sewage sludge, Nutrient value, Organic fertilizer, Soil amendment, Sludge reuse, Nitrogen, Phosphorus; Sewage sludge, Nutrient value, Organic fertilizer, Soil amendment, Sludge reuse, Nitrogen, Phosphorus. Resour. Environ. 2014, 4, 190–199. [Google Scholar] [CrossRef]
- Kirchmann, H.; Börjesson, G.; Kätterer, T.; Cohen, Y. From agricultural use of sewage sludge to nutrient extraction: A soil science outlook. Ambio 2017, 46, 143–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, S.H.A.; Lananan, F.; Din, W.N.S.; Lam, S.S.; Khatoon, H.; Endut, A.; Jusoh, A. Harvesting microalgae, Chlorella sp. by bio-flocculation of Moringa oleifera seed derivatives from aquaculture wastewater phytoremediation. Int. Biodeterior. Biodegrad. 2014, 95, 270–275. [Google Scholar] [CrossRef]
- Omotade, I.; Alatise, M.; Olanrewaju, O.O. Growth and yield performance of hot pepper using aquaculture wastewater. Agric. Eng. Int. CIGR J. 2019, 21, 18–25. [Google Scholar]
- Antov, M.G.; Šćiban, M.B.; Prodanović, J.M.; Kukić, D.V.; Vasić, V.M.; Đorđević, T.R.; Milošević, M.M. Common oak (Quercus robur) acorn as a source of natural coagulants for water turbidity removal. Ind. Crops Prod. 2018, 117, 340–346. [Google Scholar] [CrossRef]
- Idris, J.; Som, A.M.; Musa, M.; Ku Hamid, K.H.; Husen, R.; Muhd Rodhi, M.N. Dragon fruit foliage plant-based coagulant for treatment of concentrated latex effluent: Comparison of treatment with ferric sulfate. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Hassan, K.F.; Obeid, S.H. Efficiency of fungi suspension spores as Biocoagulants for suspended solid sedimentation in wastewater. Int. J. Sci. Eng. Res. 2016, 7, 578–585. [Google Scholar]
- Pu, S.; Ma, H.; Deng, D.; Xue, S.; Zhu, R.; Zhou, Y.; Xiong, X. Isolation, identification, and characterization of an Aspergillus niger bioflocculant-producing strain using potato starch wastewater as nutrilite and its application. PLoS ONE 2018, 13, e0190236. [Google Scholar] [CrossRef]
- Nasir, N.M.; Mohd Yunos, F.H.; Wan Jusoh, H.H.; Mohammad, A.; Lam, S.S.; Jusoh, A. Advances in water and wastewater treatment harvesting of Chlorella sp. microalgae using Aspergillus niger as bio-flocculant for aquaculture wastewater treatment. J. Environ. Manag. 2019, 249. [Google Scholar] [CrossRef]
- Menkiti, M.C.; Ejimofor, M.I. Experimental and artificial neural network application on the optimization of paint effluent (PE) coagulation using novel Achatinoidea shell extract (ASE). J. Water Process Eng. 2016, 10, 172–187. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Aliu, Y.D. Snail shell as coagulant aid in the alum precipitation of malachite green from aqua system. J. Hazard. Mater. 2009, 164, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Devrimci, H.A.; Yuksel, A.M.; Sanin, F.D. Algal alginate: A potential coagulant for drinking water treatment. Desalination 2012, 299, 16–21. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Jiang, J.H.; Liu, W.J.; Wang, J.Y. A novel bioflocculant produced by a salt-tolerant, alkaliphilic and biofilm-forming strain Bacillus agaradhaerens C9 and its application in harvesting Chlorella minutissima UTEX2341. Biochem. Eng. J. 2015, 93, 166–172. [Google Scholar] [CrossRef]
- Ambarsari, L.; Artika, I.; Susanti, H.E. Characterization of Bioflocculant Producing-Bacteria Isolated from Tapioca Waste Water. HAYATI J. Biosci. 2011, 18, 193–196. [Google Scholar] [CrossRef] [Green Version]
- Keogh, M.B.; Elmusharaf, K.; Borde, P.; Mc Guigan, K.G. Evaluation of the natural coagulant Moringa oleifera as a pretreatment for SODIS in contaminated turbid water. Sol. Energy 2017, 158, 448–454. [Google Scholar] [CrossRef]
- Rasool, M.A.; Tavakoli, B.; Chaibakhsh, N.; Pendashteh, A.R.; Mirroshandel, A.S. Use of a plant-based coagulant in coagulation-ozonation combined treatment of leachate from a waste dumping site. Ecol. Eng. 2016, 90, 431–437. [Google Scholar] [CrossRef]
- National Research Council; Division on Earth and Life Studies; Water Science and Technology Board; Committee on the Assessment of Water Reuse as an Approach to Meeting Future Water Supply Needs. Water Reuse: Potential for Expanding the Nation’s Water Supply Through Reuse of Municipal Wastewater; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Hasan, H.A.; Abdullah, S.R.S.; Kofli, N.T.; Kamarudin, S.K. Isotherm equilibria of Mn2+ biosorption in drinking water treatment by locally isolated Bacillus species and sewage activated sludge. J. Environ. Manag. 2012, 111, 34–43. [Google Scholar] [CrossRef]
- Aguilar, M.I.; Sáez, J.; Lloréns, M.; Soler, A.; Ortuño, J.F.; Meseguer, V.; Fuentes, A. Improvement of coagulation-flocculation process using anionic polyacrylamide as coagulant aid. Chemosphere 2005. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Murcia, M.D.; Gómez, M.; Gómez, E.; García-Izquierdo, C.; Solano, C. Possible Uses for Sludge from Drinking Water Treatment Plants. J. Environ. Eng. 2017. [Google Scholar] [CrossRef]
- Chew; Chia; Yen; Nomanbhay; Ho; Show Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [CrossRef] [Green Version]
- Zannikos, F.; Kalligeros, S.; Anastopoulos, G.; Lois, E. Converting Biomass and Waste Plastic to Solid Fuel Briquettes. J. Renew. Energy 2013, 2013, 360368. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, S.; Gonzalez, P.; Mena, C.; Guerrero, L.; Borja, R. Influence of the food to microorganisms (F/M) ratio and temperature on batch anaerobic digestion processes with and without zeolite addition. J. Environ. Sci. Health Part A 2012, 47, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.; Jarvis, P.; Judd, S.J. An economic assessment of coagulant recovery from water treatment residuals. Desalination 2012. [Google Scholar] [CrossRef]
- Phillip, M.J. Combating Water Scarcity in Southern Africa: Case Studies from Namibia; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 9789400770973. [Google Scholar]
- Aziz, H.A.; Yii, Y.C.; Syed, Z.S.F.F.; Ramli, S.F.; Akinbile, C.O. Effects of using Tamarindus indica Seeds as a natural coagulant aid in landfill leachate treatment. Glob. NEST J. 2018, 20, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Kangama, A.; Zeng, D.; Tian, X.; Fang, J. Application of Chitosan Composite Flocculant in Tap Water Treatment. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Sutherland, J.P.; Folkard, G.K.; Mtawali, M.A.; Grant, W.D. Moringa oleifera as a natural coagulant. In Proceedings of the Affordable Water Supply and Sanitation of the 20th WEDC Conference, Colombo, Sri Lanka, 22–26 August 1994. [Google Scholar]
- Marobhe, N.J.M. Water Supply in Tanzania and Performance of Local Plant Materials in Purification of Turbid Water; Royal Institute of Technology (KTH): Stockholm, Sweden, 2008. [Google Scholar]
- Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Microalgal fatty acids-From harvesting until extraction. In Microalgae-Based Biofuels and Bioproducts from Feedstock Cultivation to End-Products; Elsevier B.V.: Amsterdam, Netherlands, 2017; pp. 369–400. [Google Scholar] [CrossRef]
- Dodbiba, G.; Ponou, J.; Fujita, T. Biosorption of Heavy Metals. In Microbiology for Minerals, Metals, Materials and the Environment; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 2001, 35, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Ali, E.N.; Muyibi, S.A.; Salleh, H.M.; Alam, M.Z.; Salleh, M.R.M. Production of Natural Coagulant from Moringa Oleifera Seed for Application in Treatment of Low Turbidity Water. J. Water Resour. Prot. 2010, 2, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Varun, T.K.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, V.B.; Gautam, M.; Kumar, N. Extraction of chitosan and its oligomers from shrimp shell waste, their characterization and antimicrobial effect. Vet. World 2017, 10, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Feria-Diaz, J.J.; Tavera-Quiroz, M.J.; Vergara-Suarez, O. Efficiency of Chitosan as a Coagulant for Wastewater from Slaughterhouses. Indian J. Sci. Technol. 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Castro, L.; Zhang, R.; Muñoz, J.A.; González, F.; Blázquez, M.L.; Sand, W.; Ballester, A. Characterization of exopolymeric substances (EPS) produced by Aeromonas hydrophila under reducing conditions. Biofouling 2014, 30, 501–511. [Google Scholar] [CrossRef]
- Aguilera, A.; Souza-Egipsy, V.; San Martín-Úriz, P.; Amils, R. Extraction of extracellular polymeric substances from extreme acidic microbial biofilms. Appl. Microbiol. Biotechnol. 2008, 78, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Jachlewski, S.; Jachlewski, W.D.; Linne, U.; Bräsen, C.; Wingender, J.; Siebers, B. Isolation of extracellular polymeric substances from biofilms of the thermoacidophilic archaeon Sulfolobus acidocaldarius. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghebremichael, K.A.; Gunaratna, K.R.; Henriksson, H.; Brumer, H.; Dalhammar, G. A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res. 2005, 39, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Badrus, Z. Potential of Natural Flocculant in Coagulation-Flocculation Wastewater Treatment Process. E3S Web Conf. 2018, 73. [Google Scholar] [CrossRef] [Green Version]
- Bulson, P.C.; Johnstone, D.L.; Gibbons, H.L.; Funk, W.H. Removal and inactivation of bacteria during alum treatment of a lake. Appl. Environ. Microbiol. 1984. [Google Scholar] [CrossRef] [Green Version]
- Grehs, B.W.N.; Lopes, A.R.; Moreira, N.F.F.; Fernandes, T.; Linton, M.A.O.; Silva, A.M.T.; Manaia, C.M.; Carissimi, E.; Nunes, O.C. Removal of microorganisms and antibiotic resistance genes from treated urban wastewater: A comparison between aluminium sulphate and tannin coagulants. Water Res. 2019, 166. [Google Scholar] [CrossRef]
- Unnisa, S.A.; Bi, S.Z. Carica papaya seeds effectiveness as coagulant and solar disinfection in removal of turbidity and coliforms. Appl. Water Sci. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Vaningelgem, F.; Zamfir, M.; Mozzi, F.; Adriany, T.; Vancanneyt, M.; Swings, J.; De Vuyst, L. Biodiversity of Exopolysaccharides Produced by Streptococcus thermophilus Strains Is Reflected in Their Production and Their Molecular and Functional Characteristics. Appl. Environ. Microbiol. 2004, 70, 900–912. [Google Scholar] [CrossRef] [Green Version]
- Moghannem, S.A.M.; Farag, M.M.S.; Shehab, A.M.; Azab, M.S. Exopolysaccharide production from Bacillus velezensis KY471306 using statistical experimental design. Braz. J. Microbiol. 2018, 49, 452–462. [Google Scholar] [CrossRef]
- Samer, M. Biological and Chemical Wastewater Treatment Processes. Wastewater Treat. Eng. 2015. [Google Scholar] [CrossRef] [Green Version]
- Arulanantham, R.; Pathmanathan, S.; Ravimannan, N.; Niranjan, K. Alternative culture media for bacterial growth using different formulation of protein sources. J. Nat. Prod. Plant Resour. 2012, 2, 697–700. [Google Scholar]
- Sari, G.L.; Trihadiningrum, Y.; Wulandari, D.A.; Pandebesie, E.S.; Warmadewanthi, I.D.A.A. Compost humic acid-like isolates from composting process as bio-based surfactant: Properties and feasibility to solubilize hydrocarbon from crude oil contaminated soil. J. Environ. Manag. 2018, 225, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Uthayasooriyan, M.; Pathmanathan, S.; Ravimannan, N.; Sathyaruban, S. Formulation of alternative culture media for bacterial and fungal growth. Der Pharm. Lett. 2016, 8, 431–436. [Google Scholar]
- Jehawi, O.H.; Abdullah, S.R.S.; Kurniawan, S.B.; Ismail, N.I.; Abu Hasan, H.; Idris, M.; Al Sbani, N.H.; Muhamad, M.H. Performance of pilot hybrid reed bed constructed wetland with aeration system on phosphorus and ammonia removal for domestic wastewater. Environ. Technol. Innov. 2020, 100891. [Google Scholar] [CrossRef]
- Al-Ajalin, F.A.H.; Idris, M.; Abdullah, S.R.S.; Kurniawan, S.B.; Imron, M.F. Effect of wastewater depth to the performance of short-term batching-experiments horizontal flow constructed wetland system in treating domestic wastewater. Environ. Technol. Innov. 2020, 20, 101106. [Google Scholar] [CrossRef]

| No | Type | Species | Chemical Compounds/Functional Groups | Source |
|---|---|---|---|---|
| 1 | Animal-based | Shellfish | Chitin and polysaccharides | [33] |
| 2 | Animal-based | Shrimp shell | Chitosan and carboxy methyl cellulose | [95] |
| 3 | Animal-based | Periwinkle shell | Alcohol, phenol, secondary amide group, amine group, alkyne group, and polysaccharides | [96] |
| 4 | Animal-based | Crab shell | Chitosan | [97] |
| 5 | Microorganism-based (bacteria) | Bacillus agaradhaerens C9 | Carboxyl, hydroxyl, amino, and glycoprotein groups | [98] |
| 6 | Microorganism-based (bacteria) | Bacillus mucilaginosus | Uronic acid, neutral sugar, amino sugar, carboxyl group, and hydroxyl group | [99] |
| 7 | Microorganism-based (bacteria) | Bacillus salmalaya 139SI-7 | Carboxyl group, hydroxyl group, amino group, polysaccharides, and proteins | [100] |
| 8 | Microorganism-based (bacteria) | Paenibacillus polymyxa | Polysaccharides and proteins | [101] |
| 9 | Microorganism-based (bacteria) | Bacillus licheniformis strain W7 | Polysaccharides, protein, hydroxyl group, carboxyl group, and amino group | [51] |
| 10 | Microorganism-based (bacteria) | Bacillus velezensis | Xylose and glucose | [102] |
| 11 | Plant-based | Rice starch | Cellulose, lignin, aldehydes, ketones, esters, and carboxylic acids | [103] |
| 12 | Plant-based | Lens culinaris | Hydroxyl and carboxyl groups | [104] |
| 13 | Plant-based | Cassava | Amino acids, carboxyl group, and hydroxyl group | [105] |
| 14 | Plant-based | Dillenia indica | Polysaccharides | [94] |
| 15 | Plant-based | Potato starch | Branched-structure polymers | [106] |
| 16 | Plant-based | Moringa oleifera seed | Cationic protein, starch, glucose, fatty acids, and phenolic compounds | [107] |
| 17 | Plant-based | Moringa oleifera seed | Alcoholic compound, polysaccharides, and amides | [108] |
| 18 | Plant-based | Moringa oleifera seed | Amines, carboxylate groups, and alcoholic compounds | [109] |
| No | Sludge Recovery/Utilization | Type of Wastewater | Summary of Findings | Country | Source |
|---|---|---|---|---|---|
| 1 | Algae biomass | Aquaculture | Chlorella sp. was recovered from aquaculture wastewater. The recovery process needs to be conducted with biodegradable coagulants in order to use the sludge further. Chitosan can perform 80% algae biomass recovery after coagulation–flocculation and sedimentation processes. Recovered biomass can be utilized as feed inside the biofloc cultivation pond system. | Malaysia | [39] |
| 2 | Algae biomass | Aquaculture | Derivates of Moringa oleifera showed potential to be used as bioflocculant to recover Chlorella sp. from aquaculture effluent. Biomass recovery efficiency using Moringa oleifera was significantly higher as compared to commercial chemical coagulant. | Malaysia | [145] |
| 3 | Nutrient into fertilizer | Domestic | Sludge of domestic wastewater contains high concentrations of nitrogen and phosphorus, which are essential nutrients for plant growth. Recovered sludge from domestic wastewater even contains trace elements, which might boost plant growth. Utilization of sludge from domestic wastewater needs to be accompanied by the utilization of biodegradable coagulants. | Eswatini | [143] |
| 4 | Nutrient into fertilizer | Domestic | Recovered domestic sewage sludge characterized as organic-rich solid may be very useful to provide readily bioavailable macro- and micro-components for plant growth. The result showed that the organic content in sludge can act as a soil conditioner. | Poland | [142] |
| 5 | Nutrients into fertilizer | Aquaculture | Recovered sludge from aquaculture effluent was used as biofertilizer for hot pepper plants. Positive effect on the plant height was obtained and considered to be comparable to that with the utilization of commercial fertilizer. | Nigeria | [146] |
| 6 | Nutrients into fertilizer | Domestic | Domestic wastewater contains high amounts of nutrients, which can be recovered as fertilizer. Recovered sludge as fertilizer has a characteristic of slow-release activity, which is useful for providing nutrients over a longer period of time. | Ohio | [137] |
| No. | Name | Type | Function | Treated Water | Summary | Country | Source |
|---|---|---|---|---|---|---|---|
| 1 | Achatinoidea shell | Animal-based | Biocoagulant | Paint industry wastewater | Achatinoidea shell could reduce total dissolved solid (TDS) by up to 13% for 35 min of settling time with a dosage of 4 g/L at pH 7.9. Optimum performance of 99.22% was obtained at pH 4, 4 g/L dosage, and 45 °C. | Texas | [153] |
| 2 | Crab shell | Animal-based | Biocoagulant | Lake water | The crab shell could aid alum as a biocoagulant to enhance turbidity removal (97%) with 0.2 mg/L dosage after 45 min of settling time. Crab shell could be used as a natural aid coagulant for drinking water treatment with the lowest risks of organic release. | Algeria | [97] |
| 3 | Crab shells | Animal-based | Biocoagulant | Drinking water | Combining crab shell as biocoagulant and alum could reduce turbidity of low-, medium-, and high-turbidity water by up to 74.8%, 96.7%, and 98.2%, respectively. This removal was higher than that using only alum as coagulant. This biocoagulant could reduce the alum dose by up to 75%, and the sludge by-product is readily biodegradable. The optimum pH and biocoagulant dose for removing turbidity were 7 and 1.5 mg/L, respectively. | India | [112] |
| 4 | Periwinkle shell | Animal-based | Biocoagulant | Petroleum wastewater | Varying the dosage of periwinkle shell and pH had a significant effect on the coagulation–flocculation efficiency. The optimum conditions were pH 4 and a 100 mg/L periwinkle shell dosage. The removal of particles was up to 83.57%. | Texas | [96] |
| 5 | Shrimp shells | Animal-based | Biocoagulant | Wastewater containing oil | The chitosan from shrimp shell as a biocoagulant could reduce oil by up to 96.35% at pH 4 over 60 min of contact time. The removal of oil by using chitosan was increased after adding carboxy methyl cellulose (CMC), with percentage efficiency of 99% at (90% chitosan and 10% CMC) with 30–60 min of contact time. | Egypt | [95] |
| 6 | Snail shell | Animal-based | Biocoagulant | Wastewater containing dye | The snail shell alone as biocoagulant could reduce malachite green (MG) dye by up to 60% with a dosage of 100 mg/L. The combination of snail shell and alum could enhance the removal of MG dye. The optimum pH for MG dye removal was found to range between 4 and 5. The optimum flocculation time was 30 min with an alum–snail shell dosage of 20–100 mg/L. The sludge produced from the alum–snail shell combination had better settling characteristics than the sludge obtained from the use of snail shell alone. | Nigeria | [154] |
| 7 | Alginate | Microorganism-based (algae) | Biocoagulant | Drinking water | Algal alginate has a high polysaccharide content that could perform as a biocoagulant. Alginate removed up to 98% of suspended solids from high-turbidity water. A low dosage of the coagulant (as low as 0.02 mg/L) still achieved high turbidity removal. | Turkey | [155] |
| 8 | Achromobacter xylosoxidans strain TERI L1 | Microorganism-based (bacteria) | Bioflocculant | Wastewater containing heavy metals | Achromobacter xylosoxidans strain TERI L1 could produce exopolysaccharide as a bioflocculant. The bioflocculant contained 75% total sugar, with 72.9% neutral sugar and 11.5% protein. Achromobacter xylosoxidans strain TERI L1 could flocculate Zn, Pb, Ni, Cd, and Cu by up to 90%. | India | [98] |
| 9 | Bacillus agaradhaerens C9 | Microorganism-based (bacteria) | Bioflocculant | Wastewater containing microalgae | A bioflocculant was extracted from Bacillus agaradhaerens C9 and contained 65.42% polysaccharides, 4.70% proteins, and 1.65% nucleic acids. The optimum conditions for producing bioflocculant from Bacillus agaradhaerens C9 were 10 g/L of glucose, 10 g/L of yeast extract, and an initial pH of 10.2. The flocculation rate for kaolin suspension was 95.29%, with optimum dosage, pH, and temperature of 1.5 mg/L, 6.53, and 29 °C, respectively. The bioflocculant had the potential to treat alkaline wastewater. | China | [156] |
| 10 | Bacillus licheniformis strain W7 | Microorganism-based (bacteria) | Bioflocculant | Synthetic wastewater containing kaolin and river water | A bioflocculant (MBF-W7) was produced using Bacillus licheniformis strain W7. The optimum conditions for flocculant production were a 5% (v/v) inoculum size with maltose and NH4NO3 as carbon and nitrogen sources. The pH and cultivation time were 6 and 72 h, respectively. The flocculation rate for kaolin clay suspension was 85.8%, observed at pH 3, and MBF-W7 of 0.2 mg/mL. MBF-W7 could remove turbidity and chemical oxygen demand (COD) by up to 86.9% and 75.3%, respectively, in Tyume River. | South Africa | [51] |
| 11 | Bacillus mucilaginosus | Microorganism-based (bacteria) | Bioflocculant | Starch wastewater | A bioflocculant (MBFA9) was produced from Bacillus mucilaginosus. The major component was a polysaccharide that contained uronic acid (19.1%), neutral sugar (47.4%), and amino sugar (2.7%). The flocculation rate for kaolin suspension was 99.6% with a 0.1 mL/L MBFA9 dosage. MBFA9 could reduce total suspended solid (TSS) and COD by up to 85.5% and 68.5%, respectively. | Singapore | [99] |
| 12 | Bacillus salmalaya 139SI-7 | Microorganism-based (bacteria) | Bioflocculant | Organic-rich wastewater | A bioflocculant (QZ-7) was synthesized using Bacillus salmalaya strain 139SI with flocculation activity of 83.3%. The optimum temperature, pH, and incubation time conditions for flocculant production were 35.5 °C, 7, and 72 h, respectively, with inoculum size of 5% (v/v), sucrose as carbon source, and yeast extract as nitrogen source. Bioflocculant QZ-7 could remove COD and BOD by 93% and 92.4%, respectively. | Malaysia | [100] |
| 13 | Bacillus velezensis | Microorganism-based (bacteria) | Bioflocculant | Lake water | This study investigated the effects of incubation time and temperature on the production of bioflocculants by using Bacillus velezensis grown in sago mill effluent (SME) and palm oil mill effluent (POME) as a fermentation feedstock. The highest bioflocculant yield (2.03 g/L) at a temperature of 40 °C was achieved in POME medium. The bioflocculant produced from a fermented SME medium (BioF-SME) showed the highest activity. Bioflocculants from POME and SME had performance comparable with alum’s in removing color and turbidity from lake water. | Malaysia | [102] |
| 14 | Chromobacterium violaceum and Citrobacter koseri | Microorganism-based (bacteria) | Bioflocculant | Tapioca wastewater | Chromobacterium violaceum and Citrobacter koseri were isolated from tapioca wastewater and had high flocculation activities of 68.92% and 71.38%, respectively. The optimum pH and temperature for Chromobacterium violaceum and Citrobacter koseri were 2–4 and 6–8 and 40 °C and 30 °C, respectively. | Indonesia | [157] |
| 15 | Paenibacillus polymyxa | Microorganism-based (bacteria) | Bioflocculant | Formaldehyde wastewater | A novel bioflocculant-producing bacterium (MBF-79) was isolated from formaldehyde wastewater sludge. The optimum inoculum size, pH, and formaldehyde concentration for bioflocculant production were 7.0%, 6, and 350 mg/L, respectively. The major components of MBF-79 were polysaccharide (71.2%) and protein (27.9%). The optimum MBF-79, pH, contact time for the removal of arsenate and arsenite by using MBF-79 were 120 mg/L, 7, and 60 min, respectively, with removal efficiencies of 98.9% and 84.6%, respectively. | China | [101] |
| 16 | Aspergillus niger | Microorganism-based (fungi) | Bioflocculant | Aquaculture wastewater | Aspergillus niger was applied to flocculate microalgae from aquaculture wastewater. More than 90% harvesting efficiency was obtained at pH 3.0 to 9.0 and a mixing rate of 100–150 rpm. | Malaysia | [152] |
| 17 | Aspergillus niger | Microorganism-based (fungi) | Bioflocculant | Potato starch wastewater | Two milliliters of the bioflocculant produced using A. niger was able to remove up to 91.15% of COD and 60.22% of turbidity within 20 min of treatment. Compared with the conventional coagulants (alum- and iron-based), this bioflocculant showed nearly identical performance with a lower material cost and a smaller yield of sludge. | Hong Kong | [151] |
| 18 | Penicillium sp. and Trichoderma sp. | Microorganism-based (fungi) | Biocoagulant | Domestic wastewater | Suspension of fungal spores was proven to reduce 84% (relative to alum efficiency) of turbidity from sewage at pH 7.8 with 60 min of treatment. | Iraq | [150] |
| 19 | Abelmoschus esculentus | Plant-based | Biocoagulant | Industrial textile wastewater | Abelmoschus esculentus as biocoagulant is more efficient for treating textile wastewater than chloride ferric. Abelmoschus esculentus can remove turbidity, COD, and color by up to 97.25%, 85.69%, and 93.57%, respectively, with optimum pH and concentration of biocoagulant of 6 and 3.2 mg/L, respectively. | Brazil | [57] |
| 20 | Dragon fruit foliage | Plant-based | Biocoagulant | Concentrated latex wastewater | Dragon fruit foliage as biocoagulant could reduce COD, SS, and turbidity from latex effluent by up to 94.7%, 88.9%, and 99.7%, respectively, at pH 10. The biocoagulant dosage range of 200–800 mg/L showed consistent removal of pollutants. The removal percentage for pollutants using ferric sulfate was higher than that using dragon fruit foliage. | Malaysia | [148] |
| 21 | Moringa oleifera | Plant-based | Biocoagulant | Synthetic turbid wastewater | Raw Moringa oleifera seed contains high amounts of oil, which can reduce the potential for coagulation activity. Oil extraction significantly increased the coagulation activity of Moringa oleifera seed. The utilization of this biocoagulant showed 82.43% oil and grease removal from water. | Brazil | [107] |
| 22 | Moringa oleifera | Plant-based | Biocoagulant | Hospital wastewater | Moringa oleifera extract contains dimeric protein. Utilization of this biocoagulant showed 65% removal of turbidity, 38% of COD, and up to 90% removal of Pseudomonas aeruginosa. | Benin | [108] |
| 23 | Moringa oleifera | Plant-based | Biocoagulant | Drinking water | Integrating seed powder of Moringa oleifera into solar water disinfection could reduce turbidity by up to 85% in 24 h and remove Escherichia coli in 6 h. | Ireland | [158] |
| 24 | Moringa oleifera | Plant-based | Biocoagulant | Freshwater containing microalgae | Moringa oleifera (MO) seed derivatives were used to harvest suspended microalgae, Chlorella sp. Flocculation efficiency of more than 95% was achieved with 20 min sedimentation. MO derivatives had better performance compared with aluminum sulfate at a low dosage of 10 mg L−1 and normal pH (6.9–7.5). | Malaysia | [145] |
| 25 | Ocimum basilicum L. | Plant-based | Biocoagulant | Leachate | Ocimum basilicum L. has potential as biocoagulant for leachate pretreatment. Combining O. basilicum and alum as coagulant could remove COD and color by up to 64.4% and 77.8%, respectively, with optimum conditions of 15 min of settling time, pH of 7, and alum/O. basilicum ratio of 1:1. Integrating biocoagulant of O. basilicum and ozonation could increase the percentage removal of COD and color by up to 92% and 87%, respectively. | Iran | [159] |
| 26 | Rice starch | Plant-based | Biocoagulant | Palm oil mill effluent (POME) | The floc shaped by rice starch was more stable than alum. The rice starch as biocoagulant could reduce TSS from POME by up to 84.1% with optimum conditions of dosage, pH, settling time, and slow stirring speed of 2 g/L, pH 3, 5 min, and 10 rpm, respectively. Combining rice starch (0.55 g/L) and alum (0.2 g/L) could increase the removal of TSS from POME by up to 88.4%. | Malaysia | [103] |
| No. | Treated Water | Type | Estimated Cost (USD per m3) | Source |
|---|---|---|---|---|
| 1 | Drinking Water | Chitosan | 0.0025 | [170] |
| 2 | Drinking Water | Moringa oleifera | 0.75 | [171] |
| 3 | Drinking Water | Parkinsonia aculeata | 2 | [172] |
| 4 | Wastewater | Moringa oleifera | 2 | [35] |
| 5 | Wastewater | Azadirachta indica | 6.8 | [121] |
| 6 | Wastewater | Moringa oleifera | 5.2 | [121] |
| 7 | Wastewater (Domestic) | Chitosan | 0.015 | [58] |
| 8 | Wastewater (Leachate) | Tamarindus indica | 19.50 | [169] |
| 9 | Wastewater (Palm Oil Mill Effluent) | Water-soluble chitosan from mushrooms | 1.25 | [122] |
| 10 | Wastewater (Palm Oil Mill Effluent) | Acid-soluble chitosan from mushrooms | 1 | [122] |
| 11 | Wastewater (Paper Mill Effluent) | Starch | 1–2.85 | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.'.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. https://doi.org/10.3390/ijerph17249312
Kurniawan SB, Abdullah SRS, Imron MF, Said NSM, Ismail N', Hasan HA, Othman AR, Purwanti IF. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. International Journal of Environmental Research and Public Health. 2020; 17(24):9312. https://doi.org/10.3390/ijerph17249312
Chicago/Turabian StyleKurniawan, Setyo Budi, Siti Rozaimah Sheikh Abdullah, Muhammad Fauzul Imron, Nor Sakinah Mohd Said, Nur 'Izzati Ismail, Hassimi Abu Hasan, Ahmad Razi Othman, and Ipung Fitri Purwanti. 2020. "Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery" International Journal of Environmental Research and Public Health 17, no. 24: 9312. https://doi.org/10.3390/ijerph17249312
APA StyleKurniawan, S. B., Abdullah, S. R. S., Imron, M. F., Said, N. S. M., Ismail, N. '., Hasan, H. A., Othman, A. R., & Purwanti, I. F. (2020). Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. International Journal of Environmental Research and Public Health, 17(24), 9312. https://doi.org/10.3390/ijerph17249312










