Supplementation with a Bioactive Melon Concentrate in Humans and Animals: Prevention of Oxidative Damages and Fatigue in the Context of a Moderate or Eccentric Physical Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the Melon Concentrate
2.2. Preclinical Study Design
2.2.1. Animals and Experimental Design
2.2.2. Histology of the Gastrocnemius Muscle
2.2.3. Gastrocnemius Western Blot Analysis
2.2.4. Plasma Immunoassay Measurements
2.3. Clinical Study
2.3.1. Methods: Tools for the Physical Evaluation
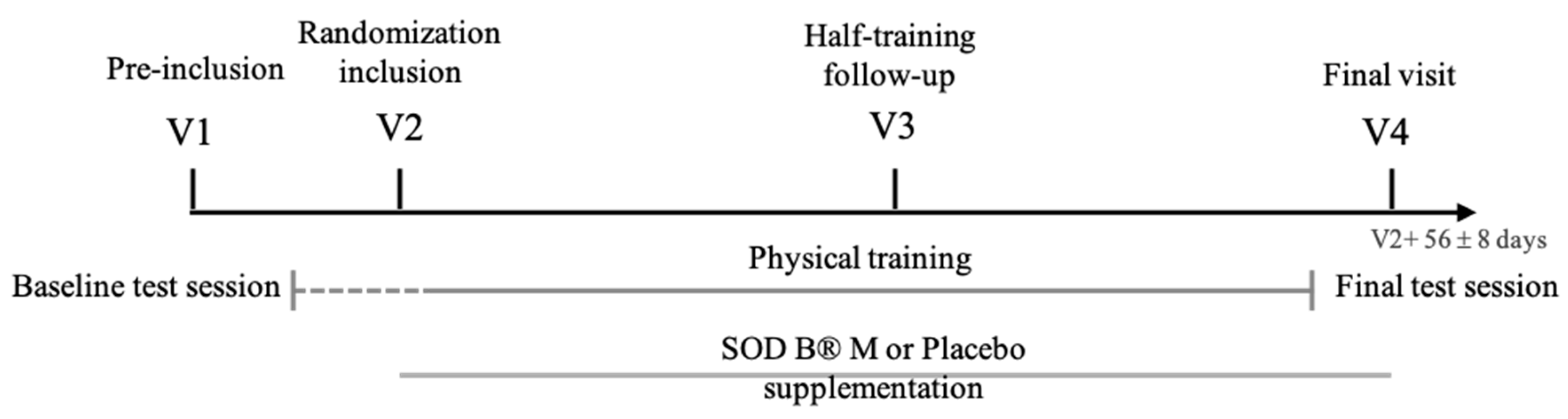
2.3.2. Study Design
2.4. Statistical Analyses
3. Results
3.1. Preclinical Study
3.1.1. Melon Concentrate Supplementation Reduced Myocytes Damages Induced by Eccentric Exercise
3.1.2. Melon Concentrate Supplementation Reduced Muscular Fibrosis Induced by Eccentric Exercise
3.1.3. Melon Concentrate Supplementation Induced a Rearrangement of Myosin Fibers
3.1.4. Melon Concentrate Supplementation Induced an Increase in PGC-1α Plasma Level
3.1.5. Melon Concentrate Supplementation Induced a Decrease of TNF-α Plasma Level
3.2. Clinical Study
3.2.1. Study Population
3.2.2. Physical Condition Improvement
3.2.3. Physical Performance Improvement
3.2.4. Quality of Life: Prevost Subjective Fatigue Scale and SF 36®; Health Survey
3.2.5. Melon Concentrate Supplementation Decreased Oxidative Stress
3.2.6. Melon Concentrate Supplementation Tended to Decrease CRP Plasma Level
3.2.7. Melon Concentrate Supplementation Modified the Platelet Level and Induced an Increase in Magnesium Plasma Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fentem, P.H. ABC of sports medicine. Benefits of exercise in health and disease. BMJ 1994, 308, 1291–1295. [Google Scholar] [CrossRef] [Green Version]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Schulenkorf, N.; Siefken, K. Managing sport-for-development and healthy lifestyles: The sport-for-health model. Sport Manag. Rev. 2019, 22, 96–107. [Google Scholar] [CrossRef]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [Green Version]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, M.B. Invited Review: Redox modulation of skeletal muscle contraction: What we know and what we don’t. J. Appl. Physiol. 2001, 90, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betteridge, D.J. What is oxidative stress? Metab. Clin. Exp. 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Carillon, J.; Rouanet, J.M.; Cristol, J.P.; Brion, R. Superoxide dismutase administration, a potential therapy against oxidative stress related diseases: Several routes of supplementation and proposal of an original mechanism of action. Pharm. Res. 2013, 30, 2718–2728. [Google Scholar] [CrossRef] [PubMed]
- Notin, C.; Vallon, L.; Desbordes, F.; Leleu, C. Oral supplementation with superoxide dismutase in Standardbred trotters in training: A double-blind placebo-controlled study. Equine Vet. J. Suppl. 2010, 42, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Sacy, A.; Le Treut, Y.; Lennen, C.; Lindinger, M. Maintien de l’intégrité musculaire et articulaire: Les bénéfices de la Superoxyde Dismutase végétale; 43ème Journée de la Recherche Équine; Inst. Français Cheval. L’Equitation: Saumur, France, 2017; pp. 132–135. [Google Scholar]
- Chabi, B.; Pauly, M.; Carillon, J.; Carnac, G.; Favier, F.B.; Fouret, G.; Bonafos, B.; Vanterpool, F.; Vernus, B.; Coudray, C.; et al. Protective effect of myostatin gene deletion on aging-related muscle metabolic decline. Exp. Gerontol. 2016, 78, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Carillon, J.; Gauthier, A.; Barial, S.; Tournier, M.; Gayrard, N.; Lajoix, A.D.; Jover, B. Relaxin and atrial natriuretic peptide pathways participate in the anti-fibrotic effect of a melon concentrate in spontaneously hypertensive rats. Food Nutr. Res. 2016, 60, 30985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carillon, J.; Rugale, C.; Rouanet, J.M.; Cristol, J.P.; Lacan, D.; Jover, B. Endogenous antioxidant defense induction by melon superoxide dismutase reduces cardiac hypertrophy in spontaneously hypertensive rats. Int. J. Food Sci. Nutr. 2014, 65, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Carillon, J.; Knabe, L.; Montalban, A.; Stevant, M.; Keophiphath, M.; Lacan, D.; Cristol, J.P.; Rouanet, J.M. Curative diet supplementation with a melon superoxide dismutase reduces adipose tissue in obese hamsters by improving insulin sensitivity. Mol. Nutr. Food Res. 2014, 58, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Carillon, J.; Romain, C.; Bardy, G.; Fouret, G.; Feillet-Coudray, C.; Gaillet, S.; Lacan, D.; Cristol, J.P.; Rouanet, J.M. Cafeteria diet induces obesity and insulin resistance associated with oxidative stress but not with inflammation: Improvement by dietary supplementation with a melon superoxide dismutase. Free Radic. Biol. Med. 2013, 65, 254–261. [Google Scholar] [CrossRef]
- Milesi, M.A.; Lacan, D.; Brosse, H.; Desor, D.; Notin, C. Effect of an oral supplementation with a proprietary melon juice concentrate (Extramel) on stress and fatigue in healthy people: A pilot, double-blind, placebo-controlled clinical trial. Nutr. J. 2009, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Hody, S.; Croisier, J.-L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric Muscle Contractions: Risks and Benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef]
- Nikolaidis, M.G. The Effects of Resistance Exercise on Muscle Damage, Position Sense, and Blood Redox Status in Young and Elderly Individuals. Geriatrics 2017, 2, 20. [Google Scholar] [CrossRef]
- Proske, U.; Allen, T.J. Damage to Skeletal Muscle from Eccentric Exercise. Exerc. Sport Sci. Rev. 2005, 33, 98–104. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef]
- Armstrong, R.B.; Ogilvie, R.W.; Schwane, J.A. Eccentric exercise-induced injury to rat skeletal muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 54, 80–93. [Google Scholar] [CrossRef]
- Carillon, J.; Del Rio, D.; Teissedre, P.L.; Cristol, J.P.; Lacan, D.; Rouanet, J.M. Antioxidant capacity and angiotensin I converting enzyme inhibitory activity of a melon concentrate rich in superoxide dismutase. Food Chem. 2012, 135, 1298–1302. [Google Scholar] [CrossRef]
- Chiang, J.; Shen, Y.-C.; Wang, Y.-H.; Hou, Y.-C.; Chen, C.-C.; Liao, J.-F.; Yu, M.-C.; Juan, C.-W.; Liou, K.-T. Honokiol protects rats against eccentric exercise-induced skeletal muscle damage by inhibiting NF-κB induced oxidative stress and inflammation. Eur. J. Pharmacol. 2009, 610, 119–127. [Google Scholar] [CrossRef]
- Ruffier, J.E. Considérations sur l’indice de résistance du coeur à l’effort. Med. Sport 1951, 3, 7–12. [Google Scholar]
- Dickson, J. L’utilisation de l’indice cardiaque de Ruffier dans le contrôle médico-sportif. Med. Sport 1950, 2, 65–80. [Google Scholar]
- Cooper, K.H. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA 1968, 203, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Bolonchuk, W.W. The Accuracy of the Six Minute Run Test to Measure Cardiorespiratory Fitness; North Dakota University: Grand Forks, ND, USA, 1975. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Prévost, P. Le surentraînement. Sport Prépa. Phys. 2002, 4, 1–3. [Google Scholar]
- Tendeng, M.S. Etude comparative des qualités physiques et medico-physiques d’étudiants de deuxième année après un an et trois mois de formation à l’INSEP. Mémoire de maîtrise es-sciences et technique, Cheikh Anta Diop University, Dakar, Senegal, 2007. [Google Scholar]
- Kim, J.; Lee, J. Role of transforming growth factor-beta in muscle damage and regeneration: Focused on eccentric muscle contraction. J. Exerc. Rehabil. 2017, 13, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Li, Y.; Foster, W.; Fukushima, K.; Badlani, N.; Adachi, N.; Usas, A.; Fu, F.H.; Huard, J. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve 2003, 28, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J. Cellular mechanism of eccentric-induced muscle injury and its relationship with sarcomere heterogeneity. J. Exerc. Rehabil. 2014, 10, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saovieng, S.; Wu, J.; Huang, C.Y.; Kao, C.L.; Higgins, M.F.; Chuanchaiyakul, R.; Kuo, C.H. Deep Ocean Minerals Minimize Eccentric Exercise-Induced Inflammatory Response of Rat Skeletal Muscle. Front. Physiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.H.; Huang, C.Y.; Lee, S.D.; Hsu, M.F.; Wang, R.Y.; Kao, C.L.; Kuo, C.H. Decreased eccentric exercise-induced macrophage infiltration in skeletal muscle after supplementation with a class of ginseng-derived steroids. PLoS ONE 2014, 9, e114649. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Q.; Wang, S.-C.; Yu, X.-K.; Chao, W.-W. Response of macrophages in rat skeletal muscle after eccentric exercise. Chin. J. Traumatol. Zhonghua Chuang Shang Za Zhi 2018, 21, 88–95. [Google Scholar] [CrossRef]
- Egoumenides, L.; Gauthier, A.; Barial, S.; Saby, M.; Orechenkoff, C.; Simoneau, G.; Carillon, J. A Specific Melon Concentrate Exhibits Photoprotective Effects from Antioxidant Activity in Healthy Adults. Nutrients 2018, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.; Khaledi, N.; Hedayati, M. Effect of acute and chronic eccentric exercise on FOXO1 mRNA expression as fiber type transition factor in rat skeletal muscles. Gene 2016, 584, 180–184. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Mortensen, O.H.; Frandsen, L.; Schjerling, P.; Nishimura, E.; Grunnet, N. PGC-1alpha and PGC-1beta have both similar and distinct effects on myofiber switching toward an oxidative phenotype. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E807–E816. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Puhke, R.; Aunola, S.; Ailanto, P.; Alev, K.; Venojärvi, M.; Rusko, H.; Seene, T. Adaptive changes of Myosin isoforms in response to long-term strength and power training in middle-aged men. J. Sports Sci. Med. 2006, 5, 349–358. [Google Scholar] [PubMed]
- Windisch, A.; Gundersen, K.; Szabolcs, M.J.; Gruber, H.; Lømo, T. Fast to slow transformation of denervated and electrically stimulated rat muscle. J. Physiol. 1998, 510, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Kiilerich, K.; Pilegaard, H. PGC-1alpha-mediated adaptations in skeletal muscle. Pflug. Arch. Eur. J. Physiol. 2010, 460, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 2008, 18, 426–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannavino, J.; Brocca, L.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J. Physiol. 2014, 592, 4575–4589. [Google Scholar] [CrossRef]
- Tiraby, C.; Langin, D. PGC-1alpha, a transcriptional coactivator involved in metabolism. Med. Sci. 2005, 21, 49–54. [Google Scholar]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Carillon, J.; Fouret, G.; Feillet-Coudray, C.; Lacan, D.; Cristol, J.P.; Rouanet, J.M. Short-term assessment of toxicological aspects, oxidative and inflammatory response to dietary melon superoxide dismutase in rats. Food Chem. Toxicol. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 55, 323–328. [Google Scholar] [CrossRef]
- Rossi, R.; Pastorelli, G.; Corino, C. Application of KRL test to assess total antioxidant activity in pigs: Sensitivity to dietary antioxidants. Res. Vet. Sci. 2013, 94, 372–377. [Google Scholar] [CrossRef]
- Kasapis, C.; Thompson, P.D. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers: A Systematic Review. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laires, M.J.; Monteiro, C. Exercise, magnesium and immune function. Magnes. Res. 2008, 21, 92–96. [Google Scholar] [PubMed]
- Nica, A.S.; Caramoci, A.; Vasilescu, M.; Ionescu, A.M.D.P.; Mazilu, V. Magnesium supplementation in top athletes-effects and recommendations. Med. Sport. J. Rom. Sports Med. Soc. 2015, XI, 2482–2494. [Google Scholar]
- Volpe, S.L. Magnesium and Athletic Performance. ACSM’s Health Fit. J. 2008, 12, 33–35. [Google Scholar] [CrossRef]
- Ryan, M.F. The role of magnesium in clinical biochemistry: An overview. Ann. Clin. Biochem. 1991, 28, 19–26. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar]
- Zhang, Y.; Xun, P.; Wang, R.; Mao, L.; He, K. Can Magnesium Enhance Exercise Performance? Nutrients 2017, 9, 946. [Google Scholar] [CrossRef] [Green Version]
- Carillon, J.; Notin, C.; Schmitt, K.; Simoneau, G.; Lacan, D. Dietary supplementation with a superoxide dismutase-melon concentrate reduces stress, physical and mental fatigue in healthy people: A randomised, double-blind, placebo-controlled trial. Nutrients 2014, 6, 2348–2359. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Gross, M.; Melendez, A. Sedentarism, active lifestyle and sport: Impact on health and obesity prevention. Nutr. Hosp. 2013, 28, 89–98. [Google Scholar]







| Placebo | Verum | ||
|---|---|---|---|
| Evolution V1–V4 | HR (b/min) | −3.3 ± 8.1 | −2.9 ± 12.2 |
| MHR (b/min) | −4.33 ± 10.09 | −2.35 ± 20.40 | |
| MAS (km/h) | 1.75 ± 1.14 *** | 1.66 ± 1.24 *** | |
| VO2max | 6.14 ± 3.98 *** | 5.80 ± 4.34 *** | |
| Prevost score | −0.8 ± 3.8 | −1.4 ± 3.2 t | |
| SF 36®;-Physical score | 1.97 ± 3.69 | 4.8 ± 1.68 *** | |
| SF 36®;-Mental Score | 13.04 ± 3.16 *** | 8.4 ± 3.14 ** | |
| Evolution V1–V3 | Ruffier index | −3.00 ± 2.42 *** | −2.78 ± 2.64 *** |
| Evolution V3–V4 | Ruffier index | −0.87 ± 2.42 | −1.1 ± 3.05 |
| Evolution V1–V4 | Ruffier index | −3.87 ± 2.32 *** | −3.93 ± 2.63 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saby, M.; Gauthier, A.; Barial, S.; Egoumenides, L.; Jover, B. Supplementation with a Bioactive Melon Concentrate in Humans and Animals: Prevention of Oxidative Damages and Fatigue in the Context of a Moderate or Eccentric Physical Activity. Int. J. Environ. Res. Public Health 2020, 17, 1142. https://doi.org/10.3390/ijerph17041142
Saby M, Gauthier A, Barial S, Egoumenides L, Jover B. Supplementation with a Bioactive Melon Concentrate in Humans and Animals: Prevention of Oxidative Damages and Fatigue in the Context of a Moderate or Eccentric Physical Activity. International Journal of Environmental Research and Public Health. 2020; 17(4):1142. https://doi.org/10.3390/ijerph17041142
Chicago/Turabian StyleSaby, Marion, Audrey Gauthier, Sandy Barial, Laure Egoumenides, and Bernard Jover. 2020. "Supplementation with a Bioactive Melon Concentrate in Humans and Animals: Prevention of Oxidative Damages and Fatigue in the Context of a Moderate or Eccentric Physical Activity" International Journal of Environmental Research and Public Health 17, no. 4: 1142. https://doi.org/10.3390/ijerph17041142
APA StyleSaby, M., Gauthier, A., Barial, S., Egoumenides, L., & Jover, B. (2020). Supplementation with a Bioactive Melon Concentrate in Humans and Animals: Prevention of Oxidative Damages and Fatigue in the Context of a Moderate or Eccentric Physical Activity. International Journal of Environmental Research and Public Health, 17(4), 1142. https://doi.org/10.3390/ijerph17041142




