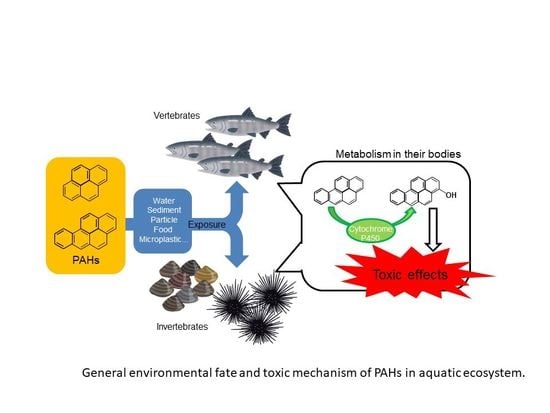
Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals
Abstract
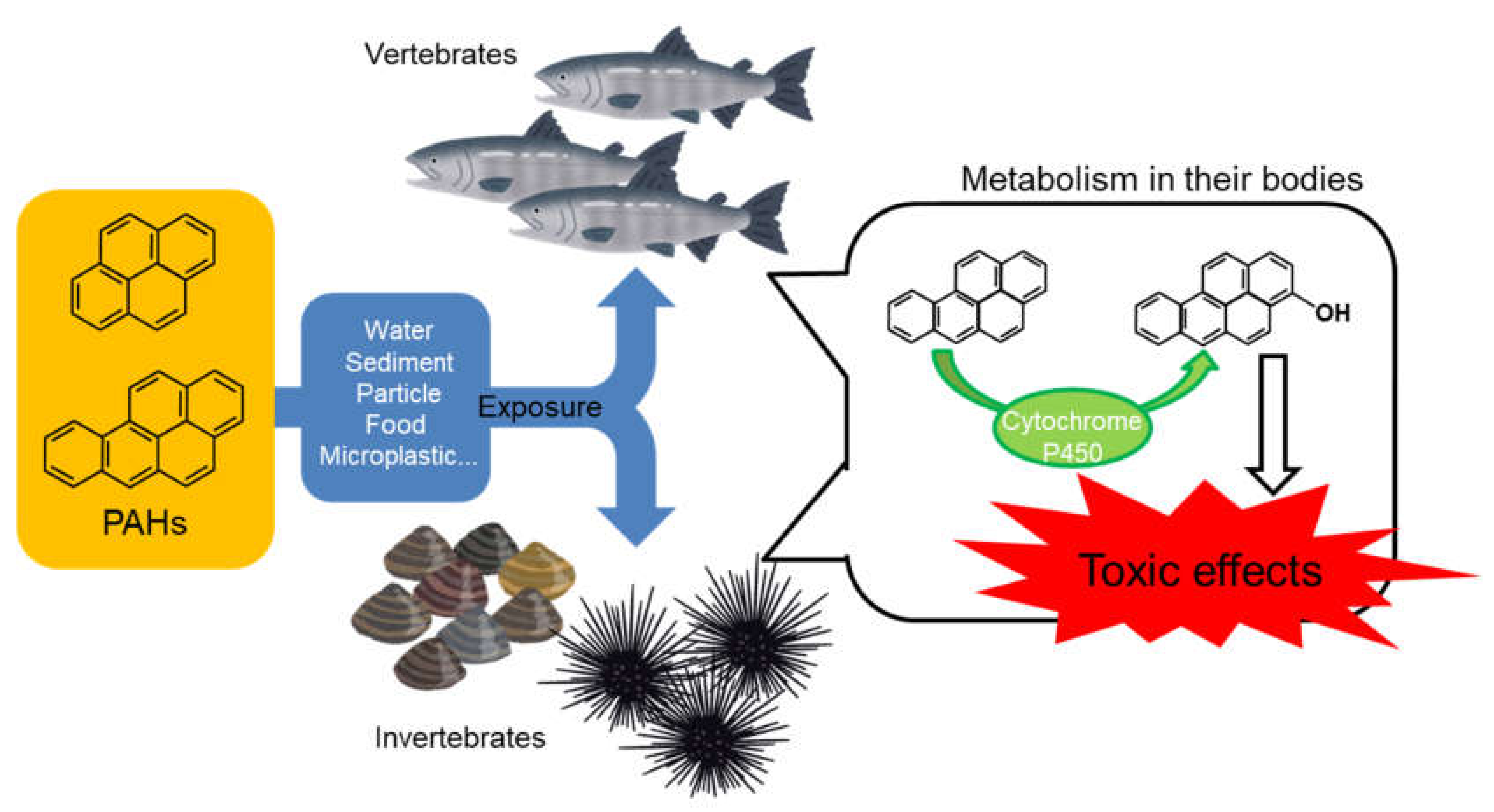
:1. Introduction
2. Toxicities of PAHs in Aquatic Animals
2.1. Carcinogenic Properties of PAHs in Mammals and Fish
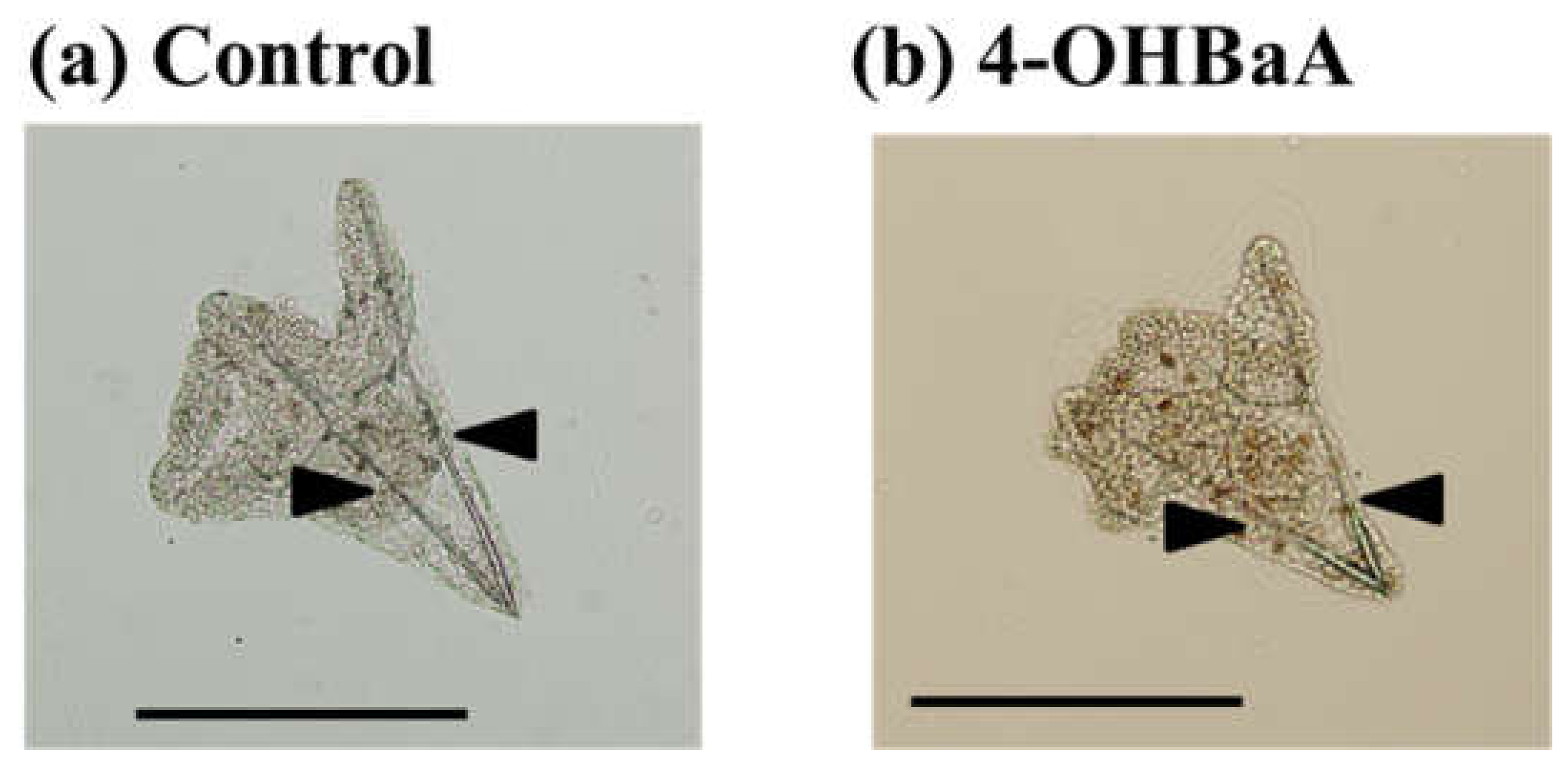
2.1.1. Toxicity of PAHs on the Early Development of Fish
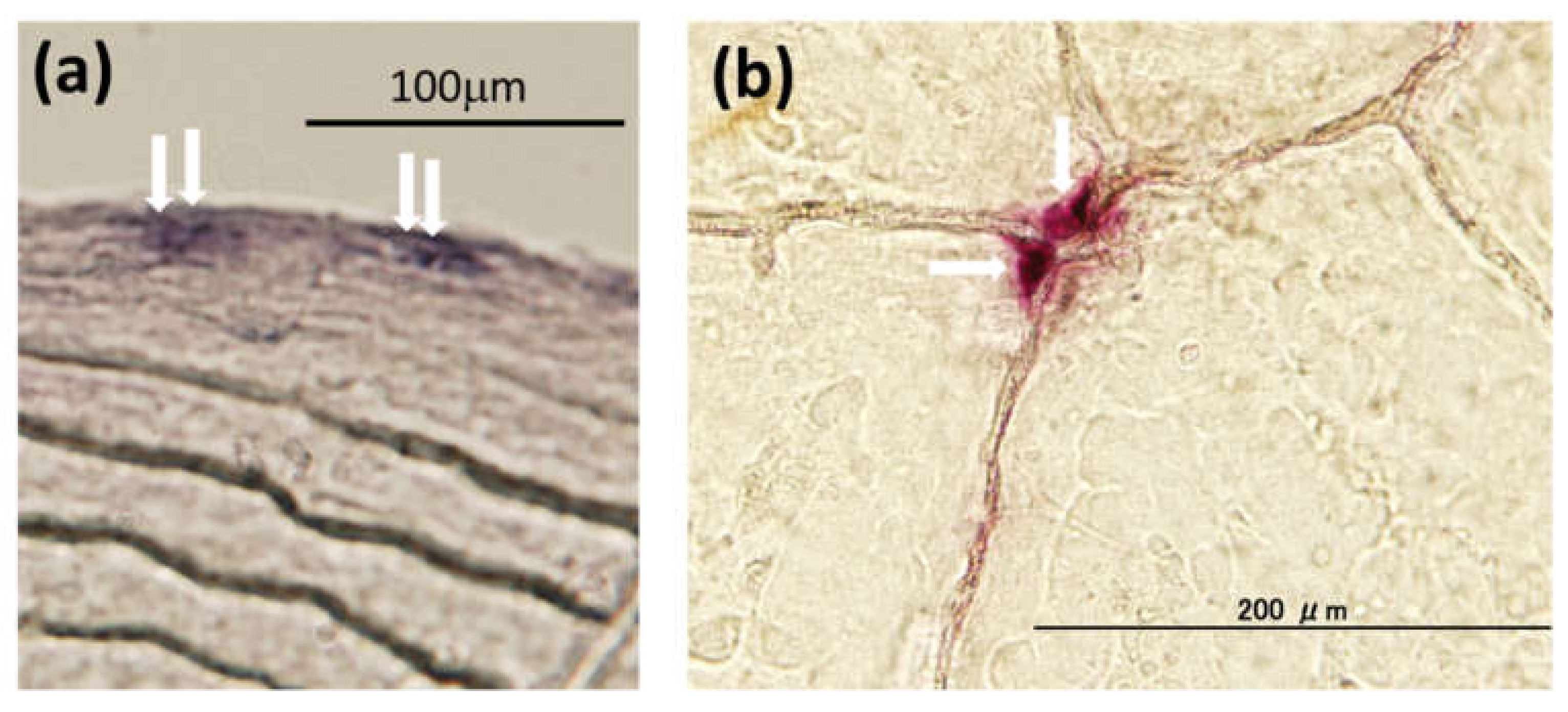
2.1.2. Toxicity of PAHs on the Bone Metabolism of Fish
2.1.3. Toxicity of PAHs on the Liver Metabolism of Fish
2.1.4. Toxicity and Endocrine-Disruptive Action of PAHs on Fish Reproduction
2.1.5. Possible Toxicity of PAHs Attached to Microplastics
2.2. Toxicities of PAHs in Invertebrates
2.2.1. Lethal Concentration 50% (LC50) in Invertebrates
2.2.2. Toxicity of OHPAHs to Sea Urchins
3. Bioaccumulation of PAHs
3.1. General Trend of the Bioaccumulation of PAHs in Aquatic Organisms
3.2. Bioaccumulation of PAHs in Fish
3.3. Bioaccumulation in Aquatic Invertebrates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Honda, K.; Mizukami, M.; Ueda, Y.; Hamada, N.; Seike, N. Residue level of polycyclic aromatic hydrocarbons in Japanese paddy soils from 1959 to 2002. Chemosphere 2007, 68, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Uehara, K.; Goto, Y.; Fukumura, M.; Shimasaki, H.; Takikawa, K.; Miyawaki, T. Polycyclic aromatic hydrocarbons in oysters and sediments from the Yatsushiro Sea, Japan: Comparison of potential risks among PAHs, dioxins and dioxin-like compounds in benthic organisms. Ecotoxicol. Environ. Saf. 2014, 99, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pies, C.; Hoffmann, B.; Petrowsky, J.; Yang, Y.; Ternes, T.A.; Hofmann, T. Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 2008, 72, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Slezakova, K.; Pires, J.C.M.; Castro, D.; Alvim-Ferraz, M.D.C.M.; Delerue-Matos, C.; Morais, S.; Pereira, M.D.C. PAH air pollution at a Portuguese urban area: Carcinogenic risks and sources identification. Environ. Sci. Pollut. Res. 2013, 20, 3932–3945. [Google Scholar] [CrossRef] [PubMed]
- Tamamura, S.; Sato, T.; Ota, Y.; Wang, X.; Tang, N.; Hayakawa, K. Long-range transport of polycyclic aromatic hydrocarbons (PAHs) from the eastern Asian continent to Kanazawa, Japan with Asian dust. Atmos. Environ. 2007, 41, 2580–2593. [Google Scholar] [CrossRef]
- Chizhova, T.; Hayakawa, K.; Tishchenko, P.; Nakase, H.; Koudryashova, Y. Distribution of PAHs in the northwestern part of the Japan Sea. Deep Sea Res. Part II 2013, 86, 19–24. [Google Scholar] [CrossRef]
- Honda, M.; Qiu, X.; Koyama, J.; Uno, S.; Undap, S.L.; Shimasaki, Y.; Oshima, Y. The wharf roach, Ligia sp., A novel indicator of polycyclic aromatic hydrocarbon contamination in coastal areas. Int. J. Environ. Res. 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Hu, G.; Sun, C.; Li, J.; Zhao, Y.; Wang, H.; Li, Y. POPs accumulated in fish and benthos bodies taken from Yangtze River in Jiangsu area. Ecotoxicology 2009, 18, 647–651. [Google Scholar] [CrossRef]
- Kannan, K.; Perrotta, E. Polycyclic aromatic hydrocarbons (PAHs) in livers of California sea otters. Chemosphere 2008, 71, 649–655. [Google Scholar] [CrossRef]
- Miki, S.; Uno, S.; Ito, K.; Koyama, J.; Tanaka, H. Distributions of polycyclic aromatic hydrocarbons and alkylated polycyclic aromatic hydrocarbons in Osaka Bay, Japan. Mar. Pollut. Bull. 2014, 85, 558–565. [Google Scholar] [CrossRef]
- Pereira, M.G.; Walker, L.A.; Wright, J.; Best, J.; Shore, R.F. Concentrations of polycyclic aromatic hydrocarbons (PAHs) in the eggs of predatory birds in Britain. Environ. Sci. Technol. 2009, 43, 9010–9015. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.; Koyama, J.; Kokushi, E.; Monteclaro, H.; Santander, S.; Cheikyula, J.O.; Miki, S.; Anasco, N.; Pahila, I.G.; Taberna, H.S., Jr.; et al. Monitoring of PAHs and alkylated PAHs in aquatic organisms after 1 month from the Solar I oil spill off the coast of Guimaras Island, Philippines. Environ. Monit. Assess. 2010, 165, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Hylland, K. Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. J. Toxicol. Environ. Health A 2006, 69, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K. Oil spills and polycyclic aromatic hydrocarbons. In Polycyclic Aromatic Hydrocarbons; Hayakawa, K., Ed.; Springer: Singapore, 2018; pp. 213–223. ISBN 978-981-10-6775-4. [Google Scholar] [CrossRef]
- Koyama, J.; Uno, S.; Kohno, K. Polycyclic aromatic hydrocarbon contamination and recovery characteristics in some organisms after the Nakhodka oil spill. Mar. Pollut. Bull. 2004, 49, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Uno, S.; Nagai, Y.; Anukorn, B. Early monitoring of spilled oil contamination in Rayong, Thailand. Jpn. J. Environ. Toxicol. 2016, 19, 25–33. [Google Scholar] [CrossRef]
- Ladwani, K.D.; Ladwani, K.D.; Ramteke, D.S. Assessment of poly aromatic hydrocarbon (PAH) dispersion in the near shore environment of Mumbai, India after a large scale oil spill. Bull. Environ. Contam. Toxicol. 2013, 90, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Tronczyński, J.; Munschy, C.; Héas-Moisan, K.; Guiot, N.; Truquet, I.; Olivier, N.; Men, S.; Furaut, A. Contamination of the Bay of Biscay by polycyclic aromatic hydrocarbons (PAHs) following the T/V “Erika” oil spill. Aquat. Living Resour. 2004, 17, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Uno, S.; Kokushi, E.; Añasco, N.C.; Iwai, T.; Ito, K.; Koyama, J. Oil spill off the coast of Guimaras Island, Philippines: Distributions and changes of polycyclic aromatic hydrocarbons in shellfish. Mar. Pollut. Bull. 2017, 124, 962–973. [Google Scholar] [CrossRef]
- McNutt, M.K.; Camilli, R.; Crone, T.J.; Guthrie, G.D.; Hsieh, P.A.; Ryerson, T.B.; Savas, O.; Shaffer, F. Review of flow rate estimates of the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. USA 2012, 109, 20260–20267. [Google Scholar] [CrossRef] [Green Version]
- Barron, M.G. Ecological impacts of the Deepwater Horizon oil spill: Implications for immunotoxicity. Toxicol. Pathol. 2012, 40, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Romero, I.C.; Sutton, T.; Carr, B.; Quintana-Rizzo, E.; Ross, S.W.; Hollander, D.J.; Torres, J.J. Decadal assessment of polycyclic aromatic hydrocarbons in mesopelagic fishes from the Gulf of Mexico reveals exposure to oil-derived sources. Environ. Sci. Technol. 2018, 52, 10985–10996. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.M.; Pulster, E.L.; Wetzel, D.L.; Murawski, S.A. PAH exposure in Gulf of Mexico demersal fishes, post-Deepwater Horizon. Environ. Sci. Technol. 2015, 49, 8786–8795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, J.F.; Brown, J.P.; Alexeeff, G.V.; Salmon, A.G. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul. Toxicol. Pharmacol. 1998, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.L.; Yadav, I.C.; Shihua, Q.; Dan, Y.; Zhang, G.; Raha, P. Environmental carcinogenic polycyclic aromatic hydrocarbons in soil from Himalayas, India: Implications for spatial distribution, sources apportionment and risk assessment. Chemosphere 2016, 144, 493–502. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Cheng, Y.-W.; Chen, Y.-W.; Lee, H. Correlation between the amounts of polycyclic aromatic hydrocarbons and mutagenicity of airborne particulate samples from Taichung City, Taiwan. Environ. Res. 1998, 78, 43–49. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Bekki, K.; Toriba, A.; Tang, N.; Kameda, T.; Hayakawa, K. Biological effects of polycyclic aromatic hydrocarbon derivatives. J. UOEH 2013, 35, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Ikenaka, Y.; Oguri, M.; Saengtienchai, A.; Nakayama, S.M.; Ijiri, S.; Ishizuka, M. Characterization of phase-II conjugation reaction of polycyclic aromatic hydrocarbons in fish species: Unique pyrene metabolism and species specificity observed in fish species. Environ. Toxicol. Pharmacol. 2013, 36, 567–578. [Google Scholar] [CrossRef]
- Jacob, J. The significance of polycyclic aromatic hydrocarbons as environmental carcinogens. 35 years research on PAH—A retrospective. Polycycl. Aromat. Compd. 2008, 28, 242–272. [Google Scholar] [CrossRef]
- Jørgensen, A.; Giessing, A.M.; Rasmussen, L.J.; Andersen, O. Biotransformation of polycyclic aromatic hydrocarbons in marine polychaetes. Mar. Environ. Res. 2008, 65, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Bekki, K.; Takigami, H.; Suzuki, G.; Tang, N.; Hayakawa, K. Evaluation of toxic activities of polycyclic aromatic hydrocarbon derivatives using in vitro bioassays. J. Health Sci. 2009, 55, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Cherr, G.N.; Fairbairn, E.; Whitehead, A. Impacts of petroleum-derived pollutants on fish development. Annu. Rev. Anim. Biosci. 2017, 5, 185–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannam, M.L.; Bamber, S.D.; Moody, A.J.; Galloway, T.S.; Jones, M.B. Immunotoxicity and oxidative stress in the Arctic scallop Chlamys islandica: Effects of acute oil exposure. Ecotoxicol. Environ. Saf. 2010, 73, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Shim, W.J.; Lee, J.; Kim, G.B. Temporal and geographical trends in the genotoxic effects of marine sediments after accidental oil spill on the blood cells of striped beakperch (Oplegnathus fasciatus). Mar. Pollut. Bull. 2011, 62, 2264–2268. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.Z.; Hogan, N.S.; Köllner, B.; Thorpe, K.L.; Phalen, L.J.; Wagner, B.D.; Van Den Heuvel, M.R. Immunotoxic effects of oil sands-derived naphthenic acids to rainbow trout. Aquat. Toxicol. 2013, 126, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High quantities of microplastic in Arctic deep-sea sediments from the hausgarten observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef] [Green Version]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef]
- Collignon, A.; Hecq, J.H.; Glagani, F.; Voisin, P.; Collard, F.; Goffart, A. Neustonic microplastic and zooplankton in the North Western Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Lin, X.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Zarfl, C.; Matthies, M. Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar. Pollut. Bull. 2010, 60, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Fisner, M.; Majer, A.; Taniguchi, S.; Bícego, M.; Turra, A.; Gorman, D. Colour spectrum and resin-type determine the concentration and composition of polycyclic aromatic hydrocarbons (PAHs) in plastic pellets. Mar. Pollut. Bull. 2017, 122, 323–330. [Google Scholar] [CrossRef]
- Camacho, M.; Herrera, A.; Gómez, M.; Acosta-Dacal, A.; Martínez, I.; Henríquez-Hernández, L.A.; Luzardo, O.P. Organic pollutants in marine plastic debris from Canary Islands beaches. Sci. Total Environ. 2019, 662, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Gorman, D.; Moreira, F.T.; Turra, A.; Fontenelle, F.R.; Combi, T.; Bícego, M.C.; de Castro Martins, C. Organic contamination of beached plastic pellets in the South Atlantic: Risk assessments can benefit by considering spatial gradients. Chemosphere 2019, 223, 608–615. [Google Scholar] [CrossRef]
- Menzie, C.A.; Potocki, B.B.; Santodonato, J. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 1992, 26, 1278–1284. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [Green Version]
- David, R.M.; Gooderham, N.J. Dose-dependent synergistic and antagonistic mutation responses of binary mixtures of the environmental carcinogen benzo[a]pyrene with food-derived carcinogens. Arch. Toxicol. 2018, 92, 3459–3469. [Google Scholar] [CrossRef]
- Rodgman, A.; Smith, C.J.; Perfetti, T.A. The composition of cigarette smoke: A retrospective, with emphasis on polycyclic components. Hum. Exp. Toxicol. 2000, 19, 573–595. [Google Scholar] [CrossRef]
- Denissenko, M.F.; Pao, A.; Tang, M.S.; Pfeifer, G.P. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science 1996, 274, 430–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, N.; Pink, M.; Rettenmeier, A.W.; Schmitz-Spanke, S. Review on proteomic analyses of benzo[a]pyrene toxicity. Proteomics 2012, 12, 1731–1755. [Google Scholar] [CrossRef] [PubMed]
- Bunton, T.E. Experimental chemical carcinogenesis in fish. Toxicol. Pathol. 1996, 24, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Bailey, G.S.; Hendricks, J.D.; Nixon, J.E.; Pawlowski, N.E. The sensitivity of rainbow trout and other fish species to carcinogens. Drug Metab. Rev. 1984, 15, 725–750. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, U.; Stein, J.E.; Nishimoto, M.; Reichert, W.L.; Collier, T.K. Chemical carcinogenesis in feral fish: Uptake, activation, and detoxication of organic xenobiotics. Environ. Health. Perspect. 1987, 71, 155–1570. [Google Scholar] [CrossRef] [PubMed]
- Incardona, J.P.; Scholz, N.L. The influence of heart developmental anatomy on cardiotoxicity-based adverse outcome pathways in fish. Aquat. Toxicol. 2016, 177, 515–525. [Google Scholar] [CrossRef]
- Farrell, A.P. From hagfish to tuna: A perspective on cardiac function in fish. Physiol. Zool. 1991, 64, 1137–1164. [Google Scholar] [CrossRef] [Green Version]
- Brette, F.; Machado, B.; Cros, C.; Incardona, J.P.; Scholz, N.L.; Block, B.A. Crude oil impairs cardiac excitation-contraction coupling in fish. Science 2014, 343, 772–776. [Google Scholar] [CrossRef]
- Magnuson, J.T.; Khursigara, A.J.; Allmon, E.B.; Esbaugh, A.J.; Roberts, A.P. Effects of Deepwater Horizon crude oil on ocular development in two estuarine fish species, red drum (Sciaenops ocellatus) and sheepshead minnow (Cyprinodon variegatus). Ecotoxicol. Environ. Saf. 2018, 166, 186–191. [Google Scholar] [CrossRef]
- Sørensen, L.; Sørhus, E.; Nordtug, T.; Incardona, J.P.; Linbo, T.L.; Giovanetti, L.; Karlsen, Ø.; Meier, S. Oil droplet fouling and differential toxicokinetics of polycyclic aromatic hydrocarbons in embryos of Atlantic haddock and cod. PLoS ONE 2017, 12, e0180048. [Google Scholar] [CrossRef]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the obvious: Environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tsutsumi, Y.; Yoshitake, S.; Qiu, X.; Xu, H.; Hashiguchi, Y.; Honda, M.; Tashiro, K.; Nakayama, K.; Hano, T.; et al. Alteration of development and gene expression induced by in ovo-nanoinjection of 3-hydroxybenzo[c]phenanthrene into Japanese medaka (Oryzias latipes) embryos. Aquatic. Toxicol. 2017, 182, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Nomura, M.; Nakagawa, T.; Oguri, S.; Kawanishi, T.; Toriba, A.; Kizu, R.; Sakaguchi, T.; Tamiya, E. Damage to and recovery of coastlines polluted with C-heavy oil spilled from the Nakhodka. Water Res. 2006, 40, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Porazinski, S.R.; Wang, H.; Furutani-Seiki, M. Microinjection of medaka embryos for use as a model genetic organism. J. Vis. Exp. 2010, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.L.; Lee, J.S.C.; Waldman, S.D.; Casper, R.F.; Grynpas, M.D. Polycyclic aromatic hydrocarbons present in cigarette smoke cause bone loss in an ovariectomized rat model. Bone 2002, 30, 917–923. [Google Scholar] [CrossRef]
- Andreou, V.; D’Addario, M.; Zohar, R.; Sukhu, B.; Casper, R.F.; Ellen, R.P.; Tenenbaum, H.C. Inhibition of osteogenesis in vitro by a cigarette smoke-associated hydrocarbon combined with Porphyromonas gingivalis lipopolysaccharide: Reversal by resveratrol. J. Periodontol. 2004, 75, 939–948. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Y.; Bian, S.; Zhao, C.; Jin, Y.; Yu, D.; Wu, X.; Zhang, D.; Cao, W.; Jing, F.; et al. Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in U.S. adults, NHANES 2005-2010. Environ. Pollut. 2018, 240, 209–218. [Google Scholar] [CrossRef]
- Barron, M.G.; Carls, M.G.; Heintz, R.; Rice, S.D. Evaluation of fish early life-stage toxicity models of chronic embryonic exposures to complex polycyclic aromatic hydrocarbon. Toxicol. Sci. 2004, 78, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Billiard, S.M.; Timme-Laragy, A.R.; Wassenberg, D.M.; Cockman, C.; Giulio, R.T.D. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006, 92, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Farwell, A.; Nero, V.; Croft, M.; Bal, P.; Dixon, D.G. Modified Japanese medaka embryo-larval bioassay for rapid determination of developmental abnormalities. Arch. Environ. Contam. Toxicol. 2006, 51, 600–607. [Google Scholar] [CrossRef]
- Bereiter-Hahn, J.; Zylberberg, L. Regeneration of teleost fish scale. Comp. Biochem. Physiol. Part A 1993, 105, 625–641. [Google Scholar] [CrossRef]
- Yoshikubo, H.; Suzuki, N.; Takemura, K.; Hoso, M.; Yashima, S.; Iwamuro, S.; Takagi, Y.; Tabata, M.J.; Hattori, A. Osteoblastic activity and estrogenic response in the regenerating scale of goldfish, a good model of osteogenesis. Life Sci. 2005, 76, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Kitamura, K.; Nemoto, T.; Shimizu, N.; Wada, S.; Kondo, T.; Tabata, M.J.; Sodeyama, F.; Ijiri, K.; Hattori, A. Effect of vibration on osteoblastic and osteoclastic activities: Analysis of bone metabolism using goldfish scale as a model for bone. Adv. Space Res. 2007, 40, 1711–1721. [Google Scholar] [CrossRef]
- Ohira, Y.; Shimizu, M.; Ura, K.; Takagi, Y. Scale regeneration and calcification in goldfish Carassius auratus: Quantitative and morphological process. Fish. Sci. 2007, 73, 46–54. [Google Scholar] [CrossRef]
- Ikegame, M.; Hattori, A.; Tabata, M.J.; Kitamura, K.; Tabuchi, Y.; Furusawa, Y.; Maruyama, Y.; Yamamoto, T.; Sekiguchi, T.; Matsuoka, R.; et al. Melatonin is a potential drug for the prevention of bone loss during space flight. J. Pineal Res. 2019, 67, e12594. [Google Scholar] [CrossRef]
- Zylberberg, L.; Bonaventure, J.; Cohen-Solal, L.; Hartmann, D.J.; Bereiter-Hahn, J. Organization and characterization of fibrillar collagens in fish scales in situ and in vitro. J. Cell Sci. 1992, 103, 273–285. [Google Scholar]
- Nishimoto, S.K.; Araki, N.; Robinson, F.D.; Waite, J.H. Discovery of bone γ−carboxyglutamic acid protein in mineralized scales. J. Biol. Chem. 1992, 267, 11600–11605. [Google Scholar]
- Redruello, B.; Estevao, M.D.; Rotllant, J.; Guerreiro, P.M.; Anjos, L.I.; Canario, A.V.M.; Power, D.M. Isolation and characterization of piscine osteonectin and down regulation of its expression by PTH-related protein. J. Bone Miner. Res. 2005, 20, 682–692. [Google Scholar] [CrossRef] [Green Version]
- Onozato, H.; Watabe, N. Studies on fish scale formation and resorption III: Fine structure and calcification of the fibrillary plates of the scales in Carassius auratus (Cypriniformes: Cyprinidae). Cell Tissue Res. 1979, 201, 409–422. [Google Scholar] [CrossRef]
- Suzuki, N.; Suzuki, T.; Kurokawa, T. Suppression of osteoclastic activities by calcitonin in the scales of goldfish (freshwater teleost) and nibbler fish (seawater teleost). Peptides 2000, 21, 115–124. [Google Scholar] [CrossRef]
- Suzuki, N.; Danks, J.A.; Maruyama, Y.; Ikegame, M.; Sasayama, Y.; Hattori, A.; Nakamura, M.; Tabata, M.J.; Yamamoto, T.; Furuya, R.; et al. Parathyroid hormone 1 (1-34) acts on the scales and involves calcium metabolism in goldfish. Bone 2011, 48, 1186–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Kitamura, K.; Hattori, A. Fish scale is a suitable model for analyzing determinants of skeletal fragility in type 2 diabetes. Endocrine 2016, 54, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hattori, A. Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J. Pineal Res. 2002, 33, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Vaes, G. Cellular biology and biochemical mechanism of bone resorption. Clin. Orthop. Relat. Res. 1988, 231, 239–271. [Google Scholar] [CrossRef]
- Dimai, H.P.; Linkhart, T.A.; Linkhart, S.G.; Donahue, L.R.; Beamer, W.G.; Rosen, C.J.; Farley, J.R.; Baylink, D.J. Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone 1998, 22, 211–216. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Suzuki, N.; Fujiwara, N.; Aoyama, M.; Kawada, T.; Sugase, K.; Murata, Y.; Sasayama, Y.; Ogasawara, M.; Satake, H. Calcitonin in a protochordate, Ciona intestinalis: The prototype of the vertebrate Calcitonin/Calcitonin gene related peptide superfamily. FEBS J. 2007, 276, 4437–4447. [Google Scholar] [CrossRef] [Green Version]
- Kase, Y.; Ikari, T.; Sekiguchi, T.; Sato, M.; Ogiso, S.; Kawada, T.; Matsubara, S.; Satake, H.; Sasayama, Y.; Endo, M.; et al. Sardine procalcitonin amino-terminal cleavage peptide has a different action from calcitonin and promotes osteoblastic activity in the scales of goldfish. Comp. Biochem. Physiol. Part A 2017, 211, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Yamamoto, M.; Watanabe, K.; Kambegawa, A.; Hattori, A. Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: The scale is a good model for the evaluation of heavy metals in bone metabolism. J. Bone Miner. Metab. 2004, 22, 439–446. [Google Scholar] [CrossRef]
- Suzuki, N.; Watanabe, K.; Sekimoto, A.; Urata, M.; Zanaty, M.I.; Sekiguchi, T.; Kitani, Y.; Matsubara, H.; Srivastav, A.K.; Hattori, A. Gadolinium at low concentration suppresses both osteoclastic and osteoblastic activities in the scales of goldfish. Am. J. Environ. Sci. 2019, 15, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Tabata, M.J.; Kambegawa, A.; Srivastav, A.K.; Shimada, A.; Takeda, H.; Kobayashi, M.; Wada, S.; Katsumata, T.; Hattori, A. Tributyltin inhibits osteoblastic activity and disrupts calcium metabolism through an increase in plasma calcium and calcitonin levels in teleosts. Life Sci. 2006, 78, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Brekke, C.; Solberg, A.H.S. Oil spill detection by satellite remote sensing. Remote Sens. Environ. 2005, 95, 1–13. [Google Scholar] [CrossRef]
- de Soysa, T.Y.; Ulrich, A.; Friedrich, T.; Pite, D.; Compton, S.L.; Ok, D.; Bernardos, R.L.; Downes, G.B.; Hsieh, S.; Stein, R.; et al. Macondo crude oil from the Deepwater Horizon oil spill disrupts specific developmental processes during zebrafish embryogenesis. BMC Biol. 2012, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Sato, M.; Nassar, F.H.; Abdel-gawad, F.K.; Bassem, S.M.; Yachiguchi, K.; Tabuchi, Y.; Endo, M.; Sekiguchi, T.; Urata, M.; et al. Seawater polluted with highly concentrated polycyclic aromatic hydrocarbons suppresses osteoblastic activity in the scales of goldfish, Carassius auratus. Zool. Sci. 2016, 33, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Rahmanpour, S.; Farzaneh Ghorghani, N.; Lotfi Ashtiyani, S.M. Polycyclic aromatic hydrocarbon (PAH) in four fish species from different trophic levels in the Persian Gulf. Environ. Monit. Assess. 2014, 186, 7047–7053. [Google Scholar] [CrossRef]
- Baumann, P.C. Epizootics of cancer in fish associated with genotoxins in sediment and water. Mutat. Res. 1998, 411, 227–233. [Google Scholar] [CrossRef]
- Frapiccini, E.; Annibaldi, A.; Betti, M.; Polidori, P.; Truzzi, C.; Marini, M. Polycyclic aromatic hydrocarbon (PAH) accumulation in different common sole (Solea solea) tissues from the North Adriatic Sea peculiar impacted area. Mar. Pollut. Bull. 2018, 137, 61–68. [Google Scholar] [CrossRef]
- Brinkmann, M.; Blenkle, H.; Salowsky, H.; Bluhm, K.; Schiwy, S.; Tiehm, A.; Hollert, H. Genotoxicity of heterocyclic PAHs in the micronucleus assay with the fish liver cell line RTL-W1. PLoS ONE 2014, 9, e85692. [Google Scholar] [CrossRef]
- Yadetie, F.; Zhang, X.; Hanna, E.M.; Aranguren-Abadía, L.; Eide, M.; Blaser, N.; Brun, M.; Jonassen, I.; Goksøyr, A.; Karlsen, O.A. RNA-Seq analysis of transcriptome responses in Atlantic cod (Gadus morhua) precision-cut liver slices exposed to benzo[a]pyrene and 17α-ethynylestradiol. Aquat. Toxicol. 2018, 201, 174–186. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Walle, T. Benzo[a]pyrene-induced cytochrome P450 1A and DNA binding in cultured trout hepatocytes—nhibition by plant polyphenols. Chem. Biol. Interact. 2007, 169, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Zha, J.; Hong, X.; Rao, H.; Yuan, L.; Wang, Z.; Kumaran, S.S. Benzo[a]pyrene-induced a mitochondria-independent apoptosis of liver in juvenile Chinese rare minnows (Gobiocypris rarus). Environ. Pollut. 2017, 231, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zanaty, M.I.; Sawada, N.; Kitani, Y.; Nassar, H.F.; Mahmoud, H.M.; Hayakawa, K.; Sekiguchi, T.; Ogiso, S.; Tabuchi, Y.; Urata, M.; et al. Influence of benz[a]anthracene on bone metabolism and on liver metabolism in nibbler fish, Girella punctate. Int. J. Environ. Res. Public Health. (in press).
- Souza, T.; Jennen, D.; van Delft, J.; van Herwijnen, M.; Kyrtoupolos, S.; Kleinjans, J. New insights into BaP-induced toxicity: Role of major metabolites in transcriptomics and contribution to hepatocarcinogenesis. Arch. Toxicol. 2016, 90, 1449–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Li, J.; Yu, L.; Wei, Y.; Miao, Z.; Chen, M.; Huang, R. Characterization of transcriptional responses mediated by benzo[a]pyrene stress in a new marine fish model of goby, Mugilogobius chulae. Genes Genom. 2019, 41, 113–123. [Google Scholar] [CrossRef]
- An, K.W.; Shin, H.S.; Choi, C.Y. Physiological responses and expression of metallothionein (MT) and superoxide dismutase (SOD) mRNAs in olive flounder, Paralichthys olivaceus exposed to benzo[a]pyrene. Comp. Biochem. Physiol. Part B 2008, 149, 534–539. [Google Scholar] [CrossRef]
- Colli-Dula, R.C.; Fang, X.; Moraga-Amador, D.; Albornoz-Abud, N.; Zamora-Bustillos, R.; Conesa, A.; Zapata-Perez, O.; Moreno, D.; Hernandez-Nuñez, E. Transcriptome analysis reveals novel insights into the response of low-dose benzo[a]pyrene exposure in male tilapia. Aquat. Toxicol. 2018, 201, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Onoda, Y.; Tachikawa, C.; Hosoi, S.; Yoshita, M.; Chung, S.W.; Kizu, R.; Toriba, A.; Kameda, T.; Tang, N. Estrogenic/antiestrogenic activities of polycyclic aromatic hydrocarbons and their monohydroxylated derivatives by yeast two-hybrid assay. J. Health Sci. 2007, 53, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Ebright, R.H.; Wong, J.R.; Chen, L.B. Binding of 2-hydroxybenzo[a]pyrene to estrogen receptors in rat cytosol. Cancer Res. 1986, 46, 2349–2351. [Google Scholar]
- Charles, G.D.; Bartels, M.J.; Zacharewski, T.R.; Gollapudi, B.B.; Freshour, N.L.; Carney, E.W. Activity of benzo[a]pyrene and its hydroxylated metabolites in an estrogen receptor-α reporter gene assay. Toxicol. Sci. 2000, 55, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Jaruchotikamol, A.; Jarukamjorn, K.; Sirisangtrakul, W.; Sakuma, T.; Kawasaki, Y.; Nemoto, N. Strong synergistic induction of CYP1A1 expression by andrographolide plus typical CYP1A inducers in mouse hepatocytes. Toxicol. Appl. Pharmacol. 2007, 224, 156–162. [Google Scholar] [CrossRef]
- Suzuki, N.; Ikari, T.; Sato, M.; Toriba, A.; Sekiguchi, T.; Kitani, Y.; Ogiso, S.; Yachiguchi, K.; Hattori, A.; Oshima, Y.; et al. Chapter 19. Toxicities of polycyclic aromatic hydrocarbons in fish and marine invertebrates. In Polycyclic Aromatic Hydrocarbons: Environmental Behavior and Toxicity in East Asia; Hayakawa, K., Ed.; Springer: Singapore, 2018; pp. 245–259. ISBN 978-981-10-6775-4. [Google Scholar] [CrossRef]
- Brown, D.R.; Clark, B.W.; Garner, L.V.T.; Di Giulio, R.T. Zebrafish cardiotoxicity: The effects of CYP1A inhibition and AHR2 knockdown following exposure to weak aryl hydrocarbon receptor agonists. Environ. Sci. Pollut. Res. Int. 2015, 22, 8329–8338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018, 235, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Guven, O.; Bach, L.; Munk, P.; Dinh, K.V.; Mariani, P.; Nielsen, T.G. Microplastic does not magnify the acute effect of PAH pyrene on predatory performance of a tropical fish (Lates calcarifer). Aquat. Toxicol. 2018, 198, 287–293. [Google Scholar] [CrossRef]
- Sese, B.; Grant, A.; Reid, B.J. Toxicity of polycyclic aromatic hydrocarbons to the nematode Caenorhabditis elegans. J. Toxicol. Environ. Health Part A 2009, 72, 1–13. [Google Scholar] [CrossRef]
- Atienzar, F.A.; Conradi, M.; Evenden, A.J.; Jha, A.N.; Depledge, M.H. Qualitative assessment of genotoxicity using random amplified polymorphic DNA: Comparison of genomic template stability with key fitness parameters in Daphnia magna exposed to benzo[a]pyrene. Environ. Toxicol. Chem. 1999, 18, 2275–2282. [Google Scholar] [CrossRef]
- Kagan, J.; Kagan, E.D.; Kagan, I.A.; Kagan, P.A.; Quigley, S. The phototoxicity of non-carcinogenic polycyclic aromatic hydrocarbons in aquatic organisms. Chemosphere 1985, 14, 1829–1834. [Google Scholar] [CrossRef]
- Eastmond, D.A.; Booth, G.M.; Lee, M.I. Toxicity, accumulation, and elimination of polycyclic aromatic sulfur heterocycles in Daphnia magna. Arch. Environ. Contam. Toxicol. 1984, 13, 105–111. [Google Scholar] [CrossRef]
- LeBlanc, G.A. Acute toxicity of priority pollutants to water flea (Daphnia magna). Bull. Environ. Contam. Toxicol. 1980, 24, 684–691. [Google Scholar] [CrossRef]
- Suedel, B.C.; Rodgers, J.H., Jr. Toxicity of fluoranthene to Daphnia magna, Hyalella azteca, Chironomus tentans, and Stylaria lacustris in water-only and whole sediment exposures. Bull. Environ. Contam. Toxicol. 1996, 57, 132–138. [Google Scholar] [CrossRef]
- Millemann, R.E.; Birge, W.J.; Black, J.A.; Cushman, R.M.; Daniels, K.L.; Franco, P.J.; Giddings, J.M. Comparative acute toxicity to aquatic organisms of components of coal-derived synthetic fuels. Trans. Am. Fish. Soc. 1984, 113, 74–85. [Google Scholar] [CrossRef]
- Nam, T.H.; Jeon, H.J.; Mo, H.H.; Cho, K.; Ok, Y.S.; Lee, S.E. Determination of biomarkers for polycyclic aromatic hydrocarbons (PAHs) toxicity to earthworm (Eisenia fetida). Environ. Geochem. Health. 2015, 37, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Dinnel, P.A.; Stober, Q.J.; DiJulio, D.H. Sea urchin sperm bioassay for sewage and chlorinated seawater and its relation to fish bioassays. Mar. Environ. Res. 1981, 5, 29–39. [Google Scholar] [CrossRef]
- Kobayashi, N. Marine pollution bioassay by sea urchin eggs, an attempt to enhance sensitivity. Public Seto Mar. Biol. Lab. 1990, 34, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Beiras, R.; Vazquez, E.; Bellas, J.; Lorenzo, J.; Fernandez, N.; Macho, G.; Marino, J.; Casas, L. Sea-urchin embryo bioassay for in situ evaluation of the biological quality of coastal seawater. Estuar. Coast. Shelf Sci. 2001, 52, 29–32. [Google Scholar] [CrossRef]
- Bellas, J.; Nieto, Ó.; Beiras, R. Integrative assessment of coastal pollution: Development and evaluation of sediment quality criteria from chemical contamination and ecotoxicological data. Cont. Shelf Res. 2011, 31, 448–456. [Google Scholar] [CrossRef]
- Alexander, F.J.; King, C.K.; Reichelt-Brushett, A.J.; Harrison, P.L. Fuel oil and dispersant toxicity to the Antarctic sea urchin (Sterechinus neumayeri). Environ. Toxicol. Chem. 2017, 36, 1563–1571. [Google Scholar] [CrossRef]
- Meador, J.P.; Stein, J.E.; Reichert, W.L.; Varanasi, U. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Rev. Environ. Contam. Toxicol. 1995, 143, 79–165. [Google Scholar] [CrossRef]
- Suzuki, N.; Ogiso, S.; Yachiguchi, K.; Kawabe, K.; Makino, F.; Toriba, A.; Kiyomoto, M.; Sekiguchi, T.; Tabuchi, Y.; Kondo, T.; et al. Monohydroxylated polycyclic aromatic hydrocarbons influence spicule formation in the early development of sea urchins (Hemicentrotus pulcherrimus). Comp. Biochem. Physiol. Part C 2015, 171, 55–60. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Yachiguchi, K.; Kiyomoto, M.; Ogiso, S.; Wada, S.; Tabuchi, Y.; Hong, C.S.; Srivastav, A.K.; Archer, S.D.J.; Pointing, S.B.; et al. Molecular mechanism of the suppression of larval skeleton by polycyclic aromatic hydrocarbons in early development of sea urchin Hemicentrotus pulcherrimus. Fish. Sci. 2018, 84, 1073–1079. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Bigalke, M.; Boamah, L.; Nyarko, E.; Saalia, F.K.; Wilcke, W. Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): Bioaccumulation and health risk assessment. Environ. Int. 2014, 65, 135–146. [Google Scholar] [CrossRef]
- Dsikowitzky, L.; Nordhaus, I.; Andarwulan, N.; Irianto, H.E.; Lioe, H.N.; Ariyani, F.; Kleinertz, S.; Schwarzbauer, J. Accumulation patterns of lipophilic organic contaminants in surface sediments and in economic important mussel and fish species from Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2016, 110, 767–777. [Google Scholar] [CrossRef]
- Gewurtz, S.B.; Lazar, R.; Haffner, G.D. Comparison of polycyclic aromatic hydrocarbon and polychlorinated biphenyl dynamics in benthic invertebrates of Lake Erie, USA. Environ. Toxicol. Chem. Int. J. 2000, 19, 2943–2950. [Google Scholar] [CrossRef]
- Poliakova, O.V.; Lebedev, A.T.; Petrosyan, V.S.; Hanninen, O.; Renzoni, A.; Sawa, D.; Walker, C. Accumulation of persistent organic pollutants in the food chain of Lake Baikal. Toxicol. Environ. Chem. 2000, 75, 235–243. [Google Scholar] [CrossRef]
- Wan, Y.; Jin, X.; Hu, J.; Jin, F. Trophic dilution of polycyclic aromatic hydrocarbons (PAHs) in a marine food web from Bohai Bay, North China. Environ. Sci. Technol. 2007, 41, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Cheikyula, J.O.; Koyama, J.; Uno, S. Comparative study of bioconcentration and EROD activity induction in the Japanese flounder, red sea bream, and Java medaka exposed to polycyclic aromatic hydrocarbons. Environ. Toxicol. Int. J. 2008, 23, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Jafarabadi, A.R.; Bakhtiari, A.R.; Yaghoobi, Z.; Yap, C.K.; Maisano, M.; Cappello, T. Distributions and compositional patterns of polycyclic aromatic hydrocarbons (PAHs) and their derivatives in three edible fishes from Kharg coral Island, Persian Gulf, Iran. Chemosphere 2019, 215, 835–845. [Google Scholar] [CrossRef]
- Oliva, A.L.; La Colla, N.S.; Arias, A.H.; Blasina, G.E.; Cazorla, A.L.; Marcovecchio, J.E. Distribution and human health risk assessment of PAHs in four fish species from a SW Atlantic estuary. Environ. Sci. Pollut. Res. 2017, 24, 18979–18990. [Google Scholar] [CrossRef]
- Logan, D.T. Perspective on ecotoxicology of PAHs to fish. Hum. Ecol. Risk Assess. 2007, 13, 302–316. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, L.; Cai, Y.; Chen, Y. Distribution of polycyclic aromatic hydrocarbon (PAH) residues in several tissues of edible fishes from the largest freshwater lake in China, Poyang Lake, and associated human health risk assessment. Ecotoxicol. Environ. Saf. 2014, 104, 323–331. [Google Scholar] [CrossRef]
- Kaag, N.H.; Foekema, E.M.; Scholten, M.C.T.; van Straalen, N.M. Comparison of contaminant accumulation in three species of marine invertebrates with different feeding habits. Environ. Toxicol. Chem. Int. J. 1997, 16, 837–842. [Google Scholar] [CrossRef]
- Monikh, F.A.; Hosseini, M.; Rahmanpour, S. The effect of size and sex on PCB and PAH concentrations in crab Portunus pelagicus. Environ. Monit. Assess. 2014, 186, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Baumard, P.; Budzinski, H.; Garrigues, P. Polycyclic aromatic hydrocarbons in sediments and mussels of the western Mediterranean Sea. Environ. Toxicol. Chem. Int. J. 1998, 17, 765–776. [Google Scholar] [CrossRef]
- Cheung, K.C.; Leung, H.M.; Kong, K.Y.; Wong, M.H. Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment. Chemosphere 2007, 66, 460–468. [Google Scholar] [CrossRef]
- Grung, M.; Petersen, K.; Fjeld, E.; Allan, I.; Christensen, J.H.; Malmqvist, L.M.; Meland, S.; Ranneklev, S. PAH related effects on fish in sedimentation ponds for road runoff and potential transfer of PAHs from sediment to biota. Sci. Total Environ. 2016, 566, 1309–1317. [Google Scholar] [CrossRef]
- Huang, L.; Chernyak, S.M.; Batterman, S.A. PAHs, nitro-PAHs, hopanes, and steranes in lake trout from Lake Michigan. Environ. Toxicol. Chem. 2014, 33, 1792–1801. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.B.; An, Y.R.; Choi, S.G.; Choi, M.; Choi, H.G. Accumulation of PAHs and synthetic musk compound in minke whales (Balanoptera acutorostrata) and long-beaked common dolphins (Delphinus capensis) from Korean coastal waters. Environ. Toxicol. Chem. 2012, 31, 477–485. [Google Scholar] [CrossRef]
- Net, S.; Henry, F.; Rabodonirina, S.; Diop, M.; Merhaby, D.; Mahfouz, C.; Amara, R.; Ouddane, B. Accumulation of PAHs, Me-PAHs, PCBs and total mercury in sediments and marine species in coastal areas of Dakar, Senegal: Contamination level and impact. Int. J. Environ. Res. 2015, 9, 419–432. [Google Scholar] [CrossRef]
- Macek, K.J.; Rodgers, C.R.; Stalling, D.L.; Korn, S. The uptake, distribution and elimination of dietary 14C-DDT and 14C-dieldrin in rainbow trout. Trans. Am. Fish. Soc. 1970, 99, 689–695. [Google Scholar] [CrossRef]
- Zhang, G.; Pan, Z.; Wang, X.; Mo, X.; Li, X. Distribution and accumulation of polycyclic aromatic hydrocarbons (PAHs) in the food web of Nansi Lake, China. Environ. Monit. Assess. 2015, 187, 173. [Google Scholar] [CrossRef]
- Baumann, P.C.; Mac, M.J.; Smith, S.B.; Harshbarger, J.C. Tumor frequencies in walleye (Stizostedion vitreum) and brown bullhead (Ictalurus nebulosus) and sediment contaminants in tributaries of the Laurentian Great Lakes. Can. J. Fish. Aquat. Sci. 1991, 48, 1804–1810. [Google Scholar] [CrossRef]
- Eadie, B.J.; Landrum, P.F.; Faust, W. Polycyclic aromatic hydrocarbons in sediments, pore water and the amphipod Pontoporeia hoyi from Lake Michigan. Chemosphere 1982, 11, 847–858. [Google Scholar] [CrossRef]
- Levengood, J.M.; Schaeffer, D.J. Polycyclic aromatic hydrocarbons in fish and crayfish from the Calumet region of southwestern Lake Michigan. Ecotoxicology 2011, 20, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Metcalfe, T.L.; Riddle, G.; Haffner, G.D. Aromatic hydrocarbons in biota from the Detroit River and western Lake Erie. J. Great Lakes Res. 1997, 23, 160–168. [Google Scholar] [CrossRef]
- Ridgway, L.L.; Chapleau, F.; Comba, M.E.; Backus, S.M. Population characteristics and contaminant burdens of the white sucker (Catostomus commersoni) from the St. Lawrence River near Cornwall, Ontario and Massena, New York. J. Great Lakes Res. 1999, 25, 567–582. [Google Scholar] [CrossRef] [Green Version]
- Zabik, M.E.; Booren, A.; Zabik, M.J.; Welch, R.; Humphrey, H. Pesticide residues, PCBs and PAHs in baked, charbroiled, salt boiled and smoked Great Lakes lake trout. Food Chem. 1996, 55, 231–239. [Google Scholar] [CrossRef]
- Landrum, P.F.; Fisher, S.W. Influence of lipids on the bioaccumulation and trophic transfer of organic contaminants in aquatic organisms. In Lipids in Freshwater Ecosystems; Arts, M.T., Wainman, B.C., Eds.; Springer: New York, NY, USA, 1999; pp. 203–234. ISBN 978-1-4612-0547-0. [Google Scholar] [CrossRef]
- Liang, Y.; Tse, M.F.; Young, L.; Wong, M.H. Distribution patterns of polycyclic aromatic hydrocarbons (PAHs) in the sediments and fish at Mai Po Marshes Nature Reserve, Hong Kong. Water Res. 2007, 41, 1303–1311. [Google Scholar] [CrossRef]
- Mashroofeh, A.; Bakhtiari, A.R.; Pourkazemi, M. Distribution and composition pattern of polycyclic aromatic hydrocarbons in different tissues of sturgeons collected from Iranian coastline of the Caspian Sea. Chemosphere 2015, 120, 575–583. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, Q.; Gu, Y.; Du, F.; Wang, X.; Shi, F.; Ke, C.; Xiang, M.; Yu, Y. Bioaccumulation of polycyclic aromatic hydrocarbons (PAHs) in wild marine fish from the coastal waters of the northern South China Sea: Risk assessment for human health. Ecotoxicol. Environ. Saf. 2019, 180, 742–748. [Google Scholar] [CrossRef]
- Soltani, N.; Moore, F.; Keshavarzi, B.; Sorooshian, A.; Javid, R. Potentially toxic elements (PTEs) and polycyclic aromatic hydrocarbons (PAHs) in fish and prawn in the Persian Gulf, Iran. Ecotoxicol. Environ. Saf. 2019, 173, 251–265. [Google Scholar] [CrossRef]
- Beyer, J.; Jonsson, G.; Porte, C.; Krahn, M.M.; Ariese, F. Analytical methods for determining metabolites of polycyclic aromatic hydrocarbon (PAH) pollutants in fish bile: A review. Environ. Toxicol. Pharmacol. 2010, 30, 224–244. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, I.; Bayona, J.M.; Albaigés, J. Aliphatic and polycyclic aromatic hydrocarbons and sulfur/oxygen derivatives in northwestern Mediterranean sediments: Spatial and temporal variability, fluxes, and budgets. Environ. Sci. Technol. 1996, 30, 2495–2503. [Google Scholar] [CrossRef]
- Batista, D.; Tellini, K.; Nudi, A.H.; Massone, T.P.; Scofield, A.D.L.; de LR Wagener, A. Marine sponges as bioindicators of oil and combustion derived PAH in coastal waters. Mar. Environ. Res. 2013, 92, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Bourgeault, A.; Gourlay-Francé, C. Monitoring PAH contamination in water: Comparison of biological and physico-chemical tools. Sci. Total Environ. 2013, 454, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Ghaeni, M.; Pour, N.A.; Hosseini, M. Bioaccumulation of polychlorinated biphenyl (PCB), polycyclic aromatic hydrocarbon (PAH), mercury, methyl mercury, and arsenic in blue crab Portunus segnis from Persian Gulf. Environ. Monit. Assess. 2015, 187, 253. [Google Scholar] [CrossRef]
- Rust, A.J.; Burgess, R.M.; Brownawell, B.J.; McElroy, A.E. Relationship between metabolism and bioaccumulation of benzo [α] pyrene in benthic invertebrates. Environ. Toxicol. Chem. Int. J. 2004, 23, 2587–2593. [Google Scholar] [CrossRef]
- Chandurvelan, R.; Marsden, I.D.; Glover, C.N.; Gaw, S. Assessment of a mussel as a metal bioindicator of coastal contamination: Relationships between metal bioaccumulation and multiple biomarker responses. Sci. Total Environ. 2015, 511, 663–675. [Google Scholar] [CrossRef]
- Monirith, I.; Ueno, D.; Takahashi, S.; Nakata, H.; Sudaryanto, A.; Subramanian, A. Asia-Pacific mussel watch: Monitoring contamination of persistent organochlorine compounds in coastal waters of Asian countries. Mar. Pollut. Bull. 2003, 46, 281–300. [Google Scholar] [CrossRef]
- Bouzas, A.; Aguado, D.; Martí, N.; Pastor, J.M.; Herráez, R.; Campins, P.; Seco, A. Alkylphenols and polycyclic aromatic hydrocarbons in eastern Mediterranean Spanish coastal marine bivalves. Environ. Monit. Assess. 2011, 176, 169–181. [Google Scholar] [CrossRef]
- Eduok, S.I.; Ebong, G.A.; Udoinyang, E.P.; Njoku, J.N.; Eyen, E.A. Bacteriological and polycyclic aromatic hydrocarbon accumulation in mangrove oyster (Crassostrea tulipa) from Douglas Creek, Nigeria. Pak. J. Nutr. 2010, 9, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Uno, S.; Tanaka, H.; Miki, S.; Kokushi, E.; Yamamoto, M.; Koyama, J. Distribution of parent and alkylated PAHs in bivalves collected from Osaka Bay, Japan. Jpn. J. Environ. Toxicol. 2015, 18, 11–24. [Google Scholar] [CrossRef]
- Ramdine, G.; Fichet, D.; Louis, M.; Lemoine, S. Polycyclic aromatic hydrocarbons (PAHs) in surface sediment and oysters (Crassostrea rhizophorae) from mangrove of Guadeloupe: Levels, bioavailability, and effects. Ecotoxicol. Environ. Saf. 2012, 79, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Y.; Suarez, P.; Alonso, A.; Longo, E.; Villaverde, A.; San Juan, F. Environmental quality of mussel farms in the Vigo estuary: Pollution by PAHs, origin and effects on reproduction. Environ. Pollut. 2011, 159, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.; Tanaka, H.; Miki, S.; Kokushi, E.; Ito, K.; Yamamoto, M.; Koyama, J. Bioaccumulation of nitroarenes in bivalves at Osaka Bay, Japan. Mar. Pollut. Bull. 2011, 63, 477–481. [Google Scholar] [CrossRef]
- Tanaka, H.; Onduka, T. Background Levels of PAHs in the coastal waters of Japan based on residual concentrations of bivalves. J. Environ. Chem. 2010, 20, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Beyer, J.; Trannum, H.C.; Bakke, T.; Hodson, P.V.; Collier, T.K. Environmental effects of the Deepwater Horizon oil spill: A review. Mar. Pollut. Bull. 2016, 110, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Hickey, C.W.; Roper, D.S.; Holland, P.T.; Trower, T.M. Accumulation of organic contaminants in two sediment-dwelling shellfish with contrasting feeding modes: Deposit-(Macomona liliana) and filter-feeding (Austrovenus stutchburyi). Arch. Environ. Contam. Toxicol. 1995, 29, 221–231. [Google Scholar] [CrossRef]
- Meador, J.P.; Casillas, E.; Sloan, C.A.; Varanasi, U. Comparative bioaccumulation of polycyclic aromatic hydrocarbons from sediment by two infaunal invertebrates. Mar. Ecol. Prog. Ser. 1995, 123, 107–124. [Google Scholar] [CrossRef] [Green Version]
- Ikenaka, Y.; Ito, Y.; Eun, H.; Watanabe, E.; Miyabara, Y. Characteristics of accumulation patterns of polycyclic aromatic hydrocarbons in the organisms inhabited in Lake Suwa. J. Environ. Chem. 2008, 18, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Thomann, R.V.; Komlos, J. Model of biota-sediment accumulation factor for polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. Int. J. 1999, 18, 1060–1068. [Google Scholar] [CrossRef]
- Incardona, J.P.; Collier, T.K.; Scholz, N.L. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004, 196, 191–205. [Google Scholar] [CrossRef] [PubMed]
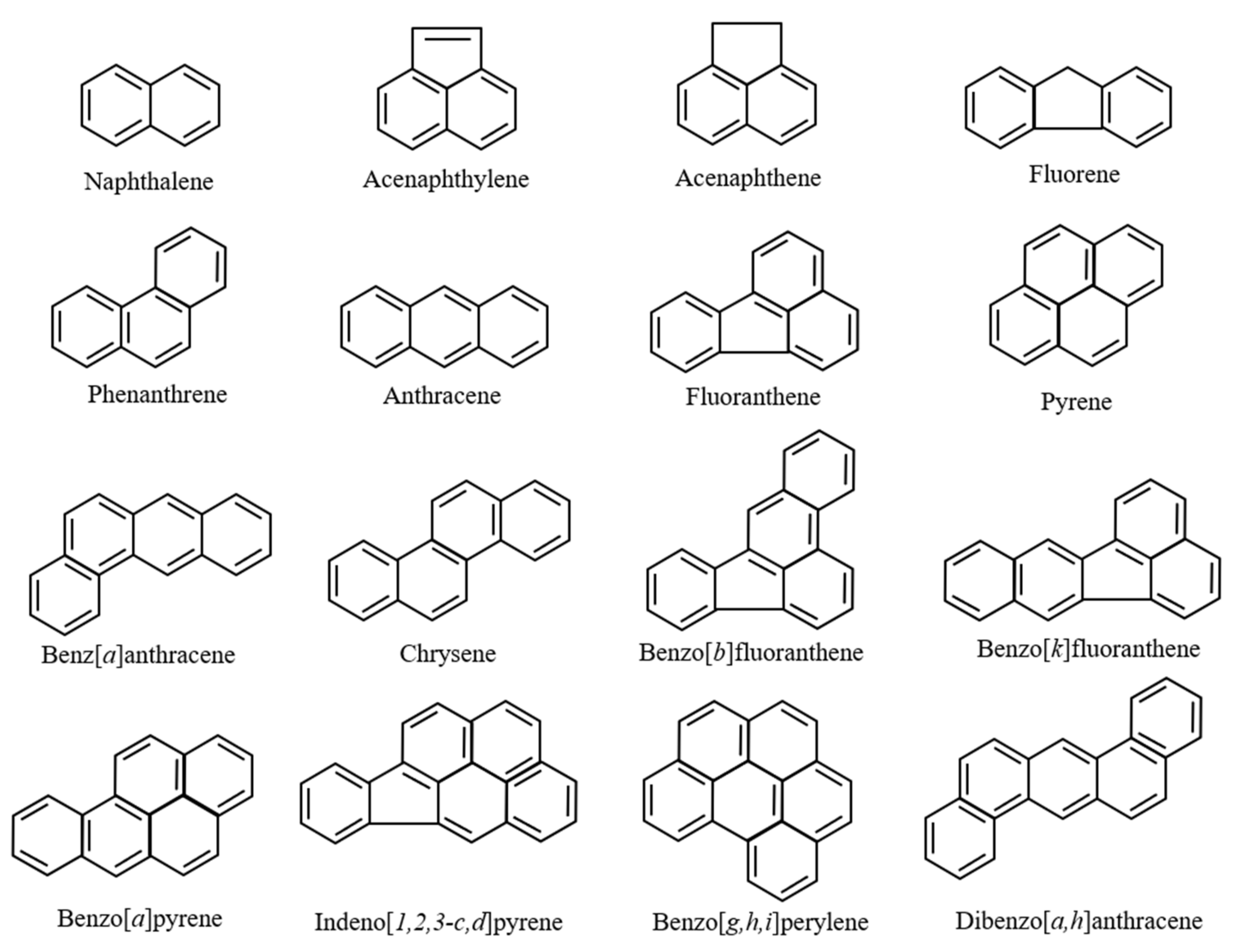

| Sampling Points | Attached PAHs Concentrations | Reference |
|---|---|---|
| Canary Islands (Spain) beach sediments | 52.1–17023.6 ng/g (in pellets) 35.1–8725.8 ng/g (in fragments) | [46] |
| South Atlantic coastline (Brazil) beach sediments | 1454 to 6002 ng/g (in pellets) | [47] |
| Beijiang River (China) surface water | 427.3 ng/g (in expanded polystyrene) 364.2 ng/g (in polyethylene) 282.4 ng/g (in polypropylene) | [114] |
| PAH Compounds | Caenorhabditis elegans | Daphnia magna | Artemia salina | Chironomus tentans | Eisenia fetida |
|---|---|---|---|---|---|
| Acenaphthene | 70573 (72 h) a | 41000 (48 h) e | - | - | - |
| Phenanthrene | 4771 (48 h) a 3758 (72 h) a | 843 (48 h) d | - | 490 (48 h)g | 114.02 (72 h) h |
| Anthracene | 2561 (48 h) a 1560 (72 h) a | 20 (1 h) c | 20 (1 h) c | - | * |
| Fluoranthene | 2719 (48 h) a 1955 (72 h) a | 4 (1 h) c | 40 (1 h) c | 250 (48 h) f | * |
| Pyrene | 2418 (48 h) a 1653 (72 h) a | 4 (1 h) c | 8 (1 h) c | - | * |
| Benzo[a]pyrene | 174 (48 h) a 80 (72 h) a | 250 (48 h) b | - | - | - |
| Group | Species | Feeding Habitat | Location | No. of PAHs Measured | Total PAH Concentrations (ng/g wet wt) | Reference |
|---|---|---|---|---|---|---|
| Fish | Lake trout | Carnivorous | Lake Michigan | 16 USEPA priority | Male: 0.56 ± 0.29 Female:0.53 ± 0.18 Eggs: 0.30 ± 0.11 | Huang et al. [148] |
| Lake trout | Carnivorous | Lake Michigan | 27 | Lean:1.52 ± 0.38 | Zabik et al. [158] | |
| Lake Superior | 27 | Fat/siscowet:6.34 ± 0.94 | Levengood et al. [155] | |||
| Minnows-fathead | Omnivorous | Calumet region of southwestern Lake Michigan | 15 (16 USEPA priority excluding NAP *) | 10–350 (range) | Levengood et al. [155] | |
| Green sunfish | Omnivorous | Calumet region of southwestern Lake Michigan | 15 (16 USEPA priority excluding NAP) | 10–80 (range) | Levengood et al. [155] | |
| Alewife | Omnivorous | Calumet region of southwestern Lake Michigan | 15 (16 USEPA priority excluding NAP) | 15–1064 (range) | Levengood et al. [155] | |
| Round goby | Carnivorous | Calumet region of southwestern Lake Michigan | 15 (16 USEPA priority excluding NAP) | 55 (mean) | Levengood et al. [155] | |
| Yellow perch | Carnivorous | Calumet region of southwestern Lake Michigan | 15 (16 USEPA priority excluding NAP) | 20 (mean) | Levengood et al. [155] | |
| Crayfish | Omnivorous | Calumet region of southwestern Lake Michigan | 15 (16 USEPA priority excluding NAP) | 10–130 (range) | Levengood et al. [155] | |
| White sucker | Bottom feeder | Upstream and downstream of the Moses-Saunders power dam | 33 (including 17 methyl PAHs) | Upstream: 166 Downstream: 116 | Ridgway et al. [157] | |
| Brown bullhead | Omnivorous | Lake Michigan tributaries | 5 | 20–24 (range) | Baumann et al. [153] | |
| St. Mary’s River tributary | 5 | 24 (mean) | ||||
| Lake Erie tributary | 5 | 220 (mean) | ||||
| Invertebrates | Amphipod: Pontoporeia hoyi | Lake Michigan | 7 | 4000–7000 (range) | Eadie et al. [154] | |
| Oligochaete worms | Lake Erie | 8 | 300–400 (range) | Eadie et al. [154] | ||
| Chironomid midges | Lake Erie | 8 | 400–800 (range) | Eadie et al. [154] | ||
| Bivalves: Zebra mussel | Detroit River and western Lake Erie | 16 USEPA priority | 12.6–8.7 (range) | Metcralfe et al. [156] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. https://doi.org/10.3390/ijerph17041363
Honda M, Suzuki N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. International Journal of Environmental Research and Public Health. 2020; 17(4):1363. https://doi.org/10.3390/ijerph17041363
Chicago/Turabian StyleHonda, Masato, and Nobuo Suzuki. 2020. "Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals" International Journal of Environmental Research and Public Health 17, no. 4: 1363. https://doi.org/10.3390/ijerph17041363
APA StyleHonda, M., & Suzuki, N. (2020). Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. International Journal of Environmental Research and Public Health, 17(4), 1363. https://doi.org/10.3390/ijerph17041363