Influence of Benz[a]anthracene on Bone Metabolism and on Liver Metabolism in Nibbler Fish, Girella punctata
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Effects of BaA on Plasma Ca and Pi Levels in Nibbler Fish
2.3. Osteoblastic and Osteoclastic Marker mRNA Expression in BaA-Treated Nibbler Fish Scales
2.4. Measurement of Plasma Components in BaA-Injected and Untreated Nibbler Fish
2.5. Statistical Analysis
3. Results
3.1. Effects of BaA on Plasma Ca and Pi Levels in BaA-Treated or Untreated Nibbler Fish
3.2. Effects of BaA on Osteoclastic and Osteoblastic Marker mRNA Expression in The Scales of BaA-Treated or Untreated Nibbler Fish
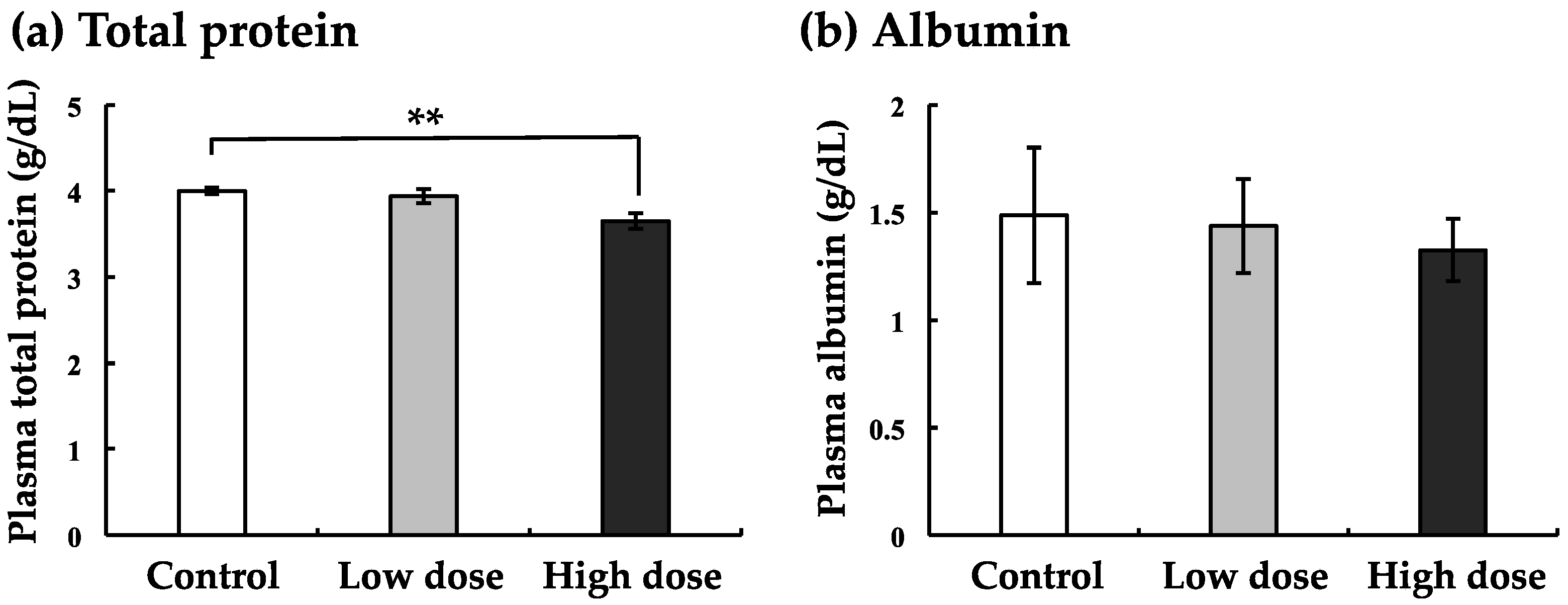
3.3. Effects of BaA on Total Protein and Albumin in The Plasma of BaA-Treated or Untreated Nibbler Fish
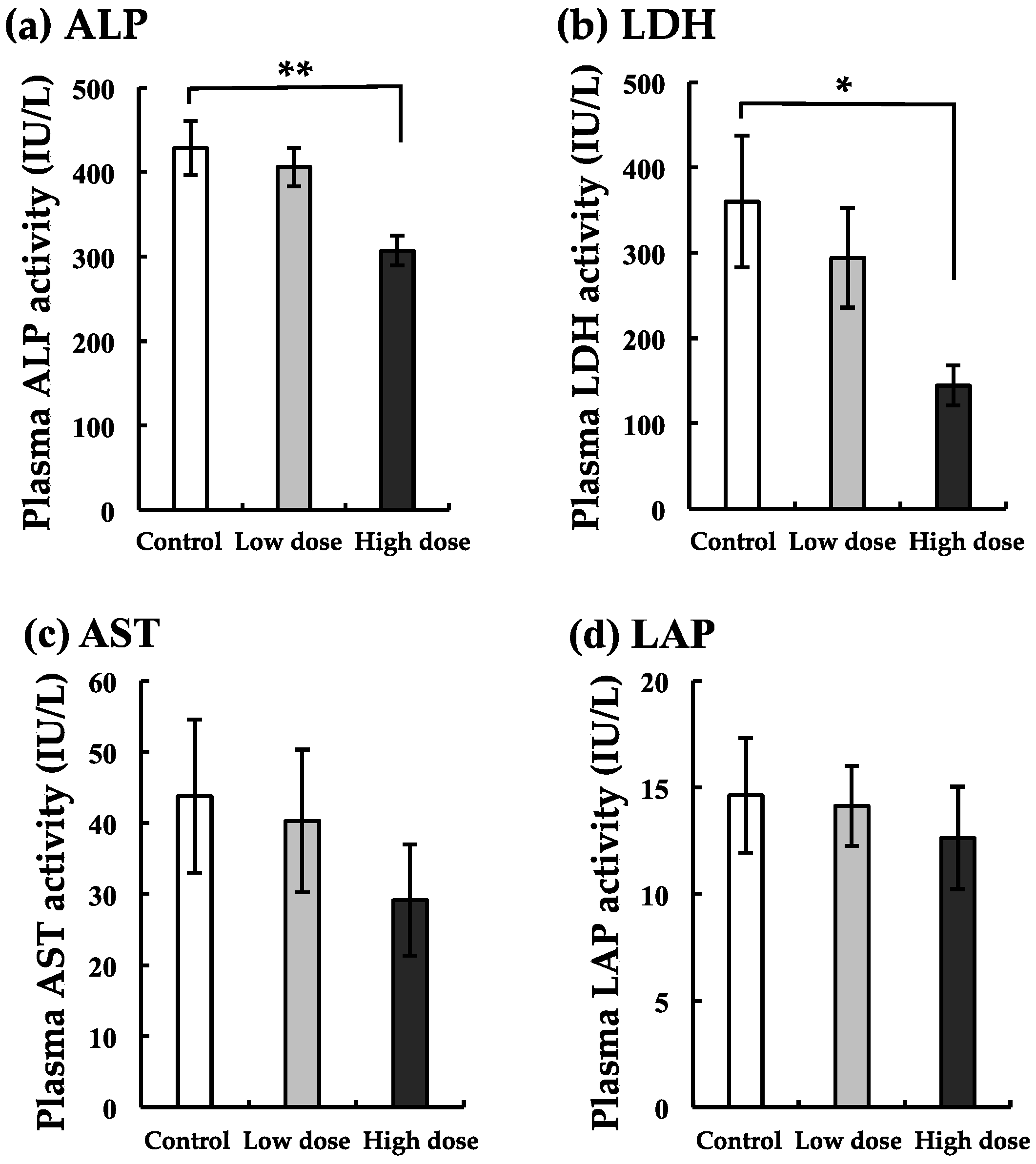
3.4. Changes in The Enzyme Markers of The Liver with BaA Treatment
3.5. Changes in Markers of Lipid Metabolism with BaA Treatment
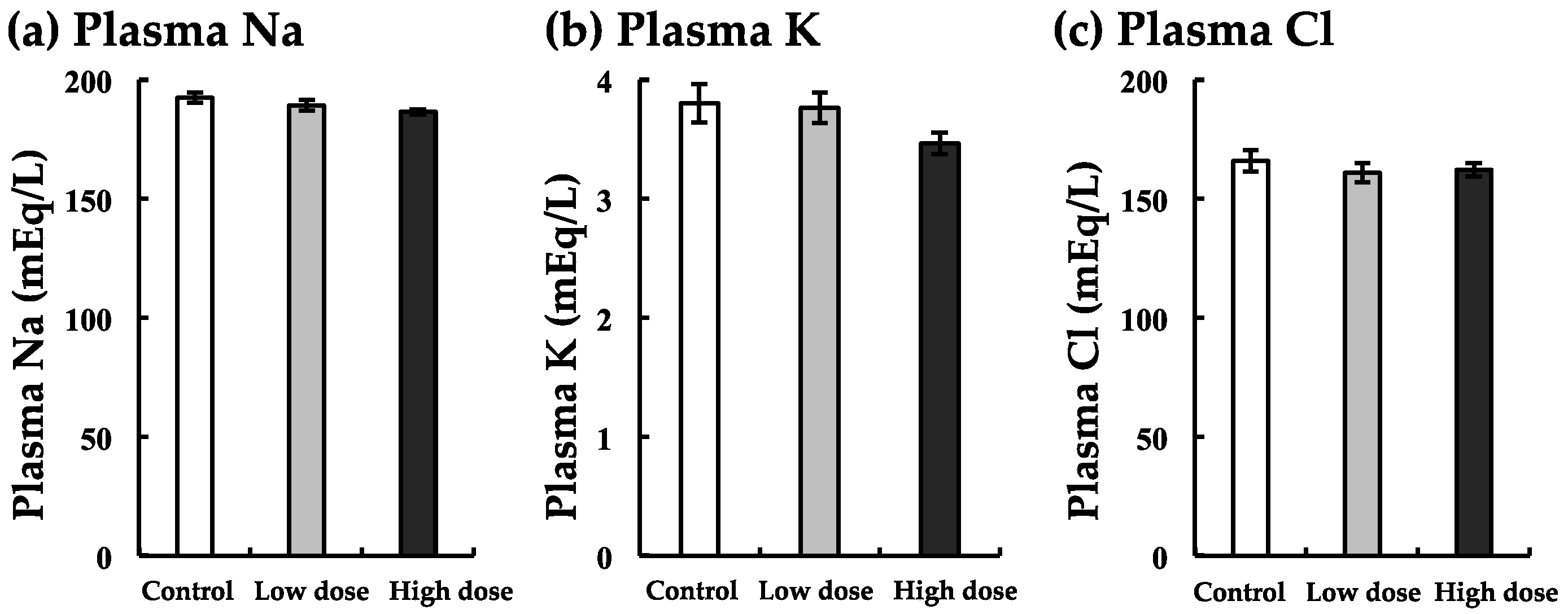
3.6. Changes in Na, K, and Cl Levels with BaA Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lima, A.L.C.; Eglinton, T.I.; Reddy, C.M. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ. Sci. Technol. 2003, 37, 53–61. [Google Scholar] [CrossRef]
- Lee, L.L.; Lee, J.S.C.; Waldman, S.D.; Casper, R.F.; Grynpas, M.D. Polycyclic aromatic hydrocarbons present in cigarette smoke cause bone loss in an ovariectomized rat model. Bone 2002, 30, 917–923. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Y.; Bian, S.; Zhao, C.; Jin, Y.; Yu, D.; Wu, X.; Zhang, D.; Cao, W.; Jing, F.; et al. Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in U.S. adults, NHANES 2005-2010. Environ. Pollut. 2018, 240, 209–218. [Google Scholar] [CrossRef]
- Li, D.; Daler, D. Ocean pollution from land-based sources: East China Sea, China. Ambio 2004, 33, 107–113. [Google Scholar] [CrossRef]
- Barron, M.G.; Carls, M.G.; Heintz, R.; Rice, S.D. Evaluation of fish early life-stage toxicity models of chronic embryonic exposures to complex polycyclic aromatic hydrocarbon mixtures. Toxicol. Sci. 2004, 78, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Billiard, S.M.; Timme-Laragy, A.R.; Wassenberg, D.M.; Cockman, C.; Di Giulio, R.T. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006, 92, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Bereiter-Hahn, J.; Zylberberg, L. Regeneration of teleost fish scale. Comp. Biochem. Physiol. Part A 1993, 105, 625–641. [Google Scholar] [CrossRef]
- Suzuki, N.; Suzuki, T.; Kurokawa, T. Suppression of osteoclastic activities by calcitonin in the scales of goldfish (freshwater teleost) and nibbler fish (seawater teleost). Peptides 2000, 21, 115–124. [Google Scholar] [CrossRef]
- Azuma, K.; Kobayashi, M.; Nakamura, M.; Suzuki, N.; Yashima, S.; Iwamuro, S.; Ikegame, M.; Yamamoto, T.; Hattori, A. Two osteoclastic markers expressed in multinucleate osteoclasts of goldfish scales. Biochem. Biophys. Res. Commun. 2007, 362, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Kitamura, K.; Nemoto, T.; Shimizu, N.; Wada, S.; Kondo, T.; Tabata, M.J.; Sodeyama, F.; Ijiri, K.; Hattori, A. Effect of vibration on osteoblastic and osteoclastic activities: Analysis of bone metabolism using goldfish scale as a model for bone. Adv. Space Res. 2007, 40, 1711–1721. [Google Scholar] [CrossRef]
- Suzuki, N.; Somei, M.; Seki, A.; Reiter, R.J.; Hattori, A. Novel bromomelatonin derivatives as potentially effective drugs to treat bone diseases. J. Pineal Res. 2008, 45, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Mugiya, Y.; Watabe, N. Studies on fish scale formation and resorption II: Effect of estradiol on calcium homeostasis and skeletal tissue resorption in the goldfish, Carassius auratus, and the killifish, Fundulus heteroclitus. Comp. Biochem. Physiol. Part A 1977, 57, 197–202. [Google Scholar] [CrossRef]
- Lake, J.L.; Ryba, S.A.; Serbst, J.R.; Libby, A.D. Mercury in fish scales as an assessment method for predicting muscle tissue mercury concentrations in largemouth bass. Arch. Environ. Contam. Toxicol. 2006, 50, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Camusso, M.; Vigano, L.; Balestrini, R. Bioconcentration of trace metals in rainbow trout: A field study. Ecotoxicol. Environ. Saf. 1995, 31, 133–141. [Google Scholar] [CrossRef]
- Cobelo-García, A.; Morán, P.; Almécija, C.; Caballero, P. Historical record of trace elements (1983–2007) in scales from Atlantic salmon (Salmo salar): Study of past metal contamination from a copper mine (Ulla River, NW Iberian Peninsula). Chemosphere 2017, 188, 18–24. [Google Scholar] [CrossRef]
- Hayakawa, K.; Onoda, Y.; Tachikawa, C.; Hosoi, S.; Yoshita, M.; Chung, S.W.; Kizu, R.; Toriba, A.; Kameda, T.; Tang, N. Estrogenic/antiestrogenic activities of polycyclic aromatic hydrocarbons and their monohydroxylated derivatives by yeast two-hybrid assay. J. Health Sci. 2007, 53, 562–570. [Google Scholar] [CrossRef]
- Suzuki, N.; Hayakawa, K.; Kameda, T.; Toriba, A.; Tang, N.; Tabata, M.J.; Takada, K.; Wada, S.; Omori, K.; Srivastav, A.K.; et al. Monohydroxylated polycyclic aromatic hydrocarbons inhibit both osteoclastic and osteoblastic activities in teleost scales. Life Sci. 2009, 84, 482–488. [Google Scholar] [CrossRef]
- Suzuki, N.; Sato, M.; Nassar, F.H.; Abdel-Gawad, F.K.; Bassem, S.M.; Yachiguchi, K.; Tabuchi, Y.; Endo, M.; Sekiguchi, T.; Urata, M.; et al. Seawater polluted with highly concentrated polycyclic aromatic hydrocarbons suppresses osteoblastic activity in the scales of goldfish, Carassius auratus. Zool. Sci. 2016, 33, 407–413. [Google Scholar] [CrossRef]
- Matsunaka, T.; Nagao, S.; Inoue, M.; Mundo, R.; Tang, N.; Suzuki, N.; Ogiso, S.; Hayakawa, K. Temporal variations of polycyclic aromatic hydrocarbons in the seawater at Tsukumo Bay, Noto Peninsula, Japan, during 2014–2018. Int. J. Environ. Res. Public Health 2020, 17, 873. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakano, J.; Kawabe, K.; Toriba, A.; Hayakawa, K.; Tang, N.; Sekiguchi, T.; Tabuchi, Y.; Ikegame, M.; Shimizu, N.; et al. Benz[a]anthracene decreases plasma calcium levels resulting from influence of scale osteoclastic and osteoblastic activities in goldfish. Int. J. Zool. Inv. 2017, 3, 72–81. [Google Scholar]
- Sato, M.; Yachiguchi, K.; Motohashi, K.; Yaguchi, Y.; Tabuchi, Y.; Kitani, Y.; Ikaria, T.; Ogiso, S.; Sekiguchi, T.; Hai, T.N.; et al. Sodium fluoride influences calcium metabolism resulting from the suppression of osteoclasts in the scales of nibbler fish Girella Punctata. Fish. Sci. 2017, 83, 543–550. [Google Scholar] [CrossRef]
- Yachiguchi, K.; Sekiguchi, T.; Nakano, M.; Hattori, A.; Yamamoto, M.; Kitamura, K.; Maeda, M.; Tabuchi, Y.; Kondo, T.; Kamauchi, H.; et al. Effect of inorganic mercury and methylmercury on osteoclasts and osteoblasts in the scales of the marine teleost as a model system of bone. Zool. Sci. 2014, 31, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Danks, J.A.; Maruyama, Y.; Ikegame, M.; Sasayama, Y.; Hattori, A.; Nakamura, M.; Tabata, M.J.; Yamamoto, T.; Furuya, R.; et al. Parathyroid hormone 1 (1–34) acts on the scales and involves calcium metabolism in goldfish. Bone 2011, 48, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Ikegame, M.; Hattori, A.; Tabata, M.J.; Kitamura, K.; Tabuchi, Y.; Furusawa, Y.; Maruyama, Y.; Yamamoto, T.; Sekiguchi, T.; Matsuoka, R.; et al. Melatonin is a potential drug for the prevention of bone loss during space flight. J. Pineal Res. 2019, 67, e12594. [Google Scholar] [CrossRef] [PubMed]
- Danion, M.; Deschamps, M.H.; Thomas-Guyon, H.; Bado-Nilles, A.; Le Floch, S.; Quentel, C.; Sire, J.Y. Effect of an experimental oil spill on vertebral bone tissue quality in European sea bass (Dicentrarchus labrax L.). Ecotoxicol. Environ. Saf. 2011, 74, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Voronov, I.; Heersche, J.N.M.; Casper, R.F.; Tenenbaum, H.C.; Manolson, M.F. Inhibition of osteoclast differentiation by polycyclic aryl hydrocarbons is dependent on cell density and RANKL concentration. Biochem. Pharm. 2005, 70, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Cheng, Y.-W.; Chen, Y.-W.; Lee, H. Correlation between the amounts of polycyclic aromatic hydrocarbons and mutagenicity of airborne particulate samples from Taichung City, Taiwan. Environ. Res. 1998, 78, 43–49. [Google Scholar] [CrossRef]
- Wang, X.L.; Tao, S.; Dawson, R.W.; Xu, F.L. Characterizing and comparing risks of polycyclic aromatic hydrocarbons in a Tianjin wastewater-irrigated area. Environ. Res. 2002, 90, 201–206. [Google Scholar] [CrossRef]
- Souza, T.; Jennen, D.; van Delft, J.; van Herwijnen, M.; Kyrtoupolos, S.; Kleinjans, J. New insights into BaP-induced toxicity: Role of major metabolites in transcriptomics and contribution to hepatocarcinogenesis. Arch. Toxicol. 2016, 90, 1449–1458. [Google Scholar] [CrossRef]
- Dimai, H.P.; Linkhart, T.A.; Linkhart, S.G.; Donahue, L.R.; Beamer, W.G.; Rosen, C.J.; Farley, J.R.; Baylink, D.J. Alkaline phosphatase levels and osteoprogenitor cell numbers suggest bone formation may contribute to peak bone density differences between two inbred strains of mice. Bone 1998, 22, 211–216. [Google Scholar] [CrossRef]
- Bower, G.; Toma, T.; Harling, L.; Jiao, L.R.; Efthimiou, E.; Darzi, A.; Athanasiou, T.; Ashrafian, H. Bariatric surgery and non-alcoholic fatty liver disease: A systematic review of liver biochemistry and histology. Obes. Surg. 2015, 25, 2280–2289. [Google Scholar] [CrossRef]
- Shahid, S.; Masood, K. Assessing liver proteins and enzymes of medical workers exposed to ionizing radiation (IR). Clin. Exp. Med. 2018, 18, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Kitamura, K.; Hattori, A. Fish scale is a suitable model for analyzing determinants of skeletal fragility in type 2 diabetes. Endocrine 2016, 54, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Yoshikubo, H.; Suzuki, N.; Takemura, K.; Hoso, M.; Yashima, S.; Iwamuro, S.; Takagi, Y.; Tabata, M.J.; Hattori, A. Osteoblastic activity and estrogenic response in the regenerating scale of goldfish, a good model of osteogenesis. Life Sci. 2005, 76, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hattori, A. Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J. Pineal Res. 2002, 33, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hattori, A. Bisphenol A suppresses osteoclastic and osteoblastic activities in the cultured scales of goldfish. Life Sci. 2003, 73, 2237–2247. [Google Scholar] [CrossRef]
- Suzuki, N.; Tabata, M.J.; Kambegawa, A.; Srivastav, A.K.; Shimada, A.; Takeda, H.; Kobayashi, M.; Wada, S.; Katsumata, T.; Hattori, A. Tributyltin inhibits osteoblastic activity and disrupts calcium metabolism through an increase in plasma calcium and calcitonin levels in teleosts. Life Sci. 2006, 78, 2533–2541. [Google Scholar] [CrossRef]
- Yachiguchi, K.; Matsumoto, N.; Haga, Y.; Suzuki, M.; Matsumura, C.; Tsurukawa, M.; Okuno, T.; Nakano, T.; Kawabe, K.; Kitamura, K.; et al. Polychlorinated biphenyl (118) activates osteoclasts and induces bone resorption in goldfish. Environ. Sci. Poll. Res. 2014, 21, 6365–6372. [Google Scholar] [CrossRef]
- Suzuki, N.; Yamamoto, M.; Watanabe, K.; Kambegawa, A.; Hattori, A. Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: The scale is a good model for the evaluation of heavy metals in bone metabolism. J. Bone Miner. Metab. 2004, 22, 439–446. [Google Scholar] [CrossRef]
- Suzuki, N.; Yachiguchi, K.; Hayakawa, K.; Omori, K.; Takada, K.; Tabata, J.M.; Kitamura, K.; Endo, M.; Wada, S.; Srivastav, A.K.; et al. Effects of inorganic mercury on osteoclasts and osteoblasts of the goldfish scales in vitro. J. Fac. Agr. Kyushu Univ. 2011, 56, 47–51. [Google Scholar]
- Suzuki, N.; Watanabe, K.; Sekimoto, A.; Urata, M.; Zanaty, M.I.; Sekiguchi, T.; Kitani, Y.; Matsubara, H.; Srivastav, A.K.; Hattori, A. Gadolinium at low concentration suppresses both osteoclastic and osteoblastic activities in the scales of goldfish. Am. J. Environ. Sci. 2019, 15, 137–144. [Google Scholar] [CrossRef][Green Version]



| Name | Forward Primer | Reverse Primer | Accession Number |
|---|---|---|---|
| MMP-9 | TGTGGTGCTCAACCACCTACAACT | ATCCCTGCCTTGAGTGGTGCAT | LC198841 |
| COL1A1 | GTGAGGTCGCCAAGAAGAAC | ATGAGACGCAGGAAGGTCAG | AB874603 |
| EF-1α | GTATGGTCGTCACCTTTGCTC | GTGGGTCGTTCTTGCTGTC | AB874605 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanaty, M.I.; Sawada, N.; Kitani, Y.; Nassar, H.F.; Mahmoud, H.M.; Hayakawa, K.; Sekiguchi, T.; Ogiso, S.; Tabuchi, Y.; Urata, M.; et al. Influence of Benz[a]anthracene on Bone Metabolism and on Liver Metabolism in Nibbler Fish, Girella punctata. Int. J. Environ. Res. Public Health 2020, 17, 1391. https://doi.org/10.3390/ijerph17041391
Zanaty MI, Sawada N, Kitani Y, Nassar HF, Mahmoud HM, Hayakawa K, Sekiguchi T, Ogiso S, Tabuchi Y, Urata M, et al. Influence of Benz[a]anthracene on Bone Metabolism and on Liver Metabolism in Nibbler Fish, Girella punctata. International Journal of Environmental Research and Public Health. 2020; 17(4):1391. https://doi.org/10.3390/ijerph17041391
Chicago/Turabian StyleZanaty, Mohamed I., Niina Sawada, Yoichiro Kitani, Hossam F. Nassar, Hamada M. Mahmoud, Kazuichi Hayakawa, Toshio Sekiguchi, Shouzo Ogiso, Yoshiaki Tabuchi, Makoto Urata, and et al. 2020. "Influence of Benz[a]anthracene on Bone Metabolism and on Liver Metabolism in Nibbler Fish, Girella punctata" International Journal of Environmental Research and Public Health 17, no. 4: 1391. https://doi.org/10.3390/ijerph17041391
APA StyleZanaty, M. I., Sawada, N., Kitani, Y., Nassar, H. F., Mahmoud, H. M., Hayakawa, K., Sekiguchi, T., Ogiso, S., Tabuchi, Y., Urata, M., Matsubara, H., Takeuchi, Y., Hattori, A., Srivastav, A. K., Amornsakun, T., & Suzuki, N. (2020). Influence of Benz[a]anthracene on Bone Metabolism and on Liver Metabolism in Nibbler Fish, Girella punctata. International Journal of Environmental Research and Public Health, 17(4), 1391. https://doi.org/10.3390/ijerph17041391





