Concentrations of Formic Acid, Acetic Acid, and Ammonia in Newly Constructed Houses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Houses
2.2. Sampling and Analysis of Target Compounds in Indoor and Outdoor Air
2.3. Statics Analysis
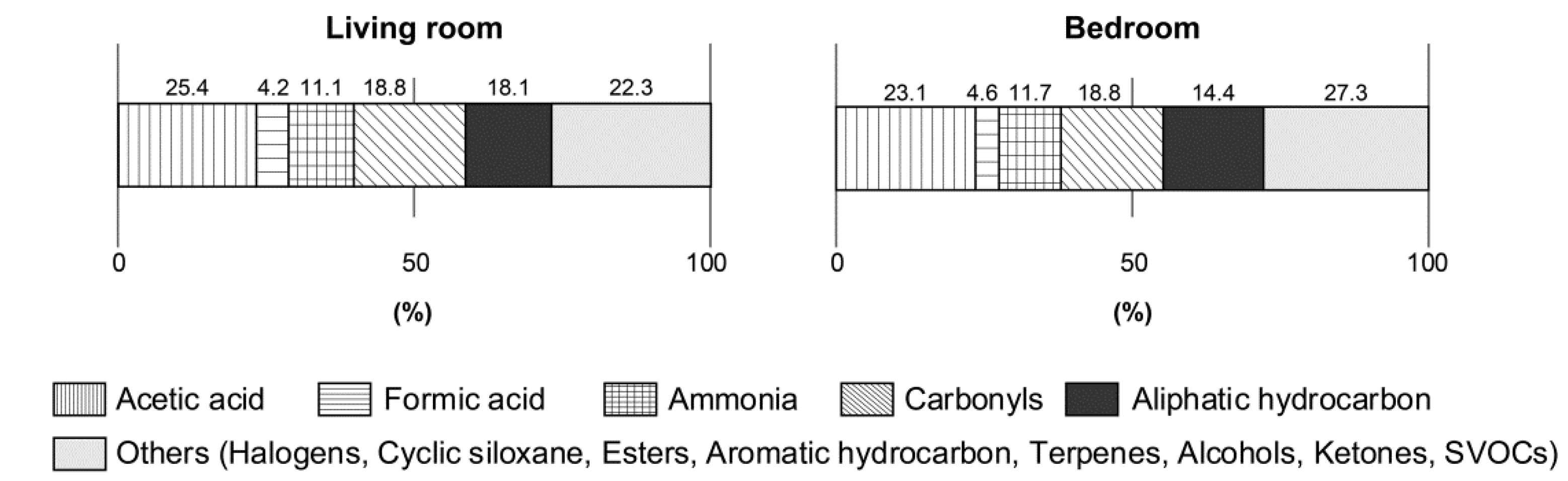
3. Results
4. Discussion
4.1. Formic Acid
4.2. Acetic Acid
4.3. Ammonia
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Bai, Z.; Yu, H.; Zhang, J.; Zhu, T. Regulatory standards related to building energy conservation andindoor-air-quality during rapid urbanization in China. Energy Build. 2004, 36, 1299–1308. [Google Scholar] [CrossRef]
- McGill, G.; Oyedele, L.O.; McAllister, K. An investigation of indoor air quality, thermal comfort and sick building syndrome symptoms in UK energy efficient homes. Smart Sustain. Built. Environ. 2015, 4, 329–348. [Google Scholar] [CrossRef]
- Engvall, K.; Wickman, P.; Norbäck, D. Sick building syndrome and perceived indoor environment in relation to energy saving by reduced ventilation flow during heating season: A 1 year intervention study in dwellings. Indoor Air 2005, 15, 120–126. [Google Scholar] [CrossRef] [PubMed]
- SeppȨnen, O. Ventilation strategies for good indoor air quality and energy efficiency. Int. J. Vent. 2008, 6, 297–306. [Google Scholar]
- Yu, C.W.F.; Jeong Tai Kim, J.T. Building pathology, investigation of sick buildings—VOC emissions. Indoor Built. Environ. 2010, 19, 30–39. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Alexis, N.; Bacchus, H.; Bernstein, I.L.; Fritz, P.; Horner, E.; Li, N.; Mason, S.; Nel, A.; Oullette, J.; et al. The health effects of nonindustrial indoor air pollution. J. Allergy Clin. Immunol. 2008, 121, 585–591. [Google Scholar] [CrossRef]
- Yan, M.; Zhai, Y.; Shi, P.; Hu, Y.; Yang, H.; Zhao, H. Emission of volatile organic compounds from new furniture products and its impact on human health. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1886–1906. [Google Scholar] [CrossRef]
- The Ministry of Health, Labor, and Welfare of Japan Guideline Values of Chemicals in Indoor Air. (In Japanese). Available online: https://www.mhlw.go.jp/web/t_doc?dataId=00tc3866&dataType=1&pageNo=1 (accessed on 30 January 2020).
- Saito, I.; Onuki, A.; Todaka, E.; Nakaoka, H.; Mori, C.; Hosaka, M.; Ogata, A. Recent trends in indoor air pollution: Health risks from unregulated chemicals. Jpn. J. Risk Anal. 2011, 21, 91–100. [Google Scholar]
- Steinemann, A. Human exposure, health hazards, and environmental regulations. Environ. Impact Assess. Rev. 2004, 24, 695–710. [Google Scholar] [CrossRef]
- Myers, I.; Maynard, R.L. Polluted air–outdoors and indoors. Occup. Med. 2005, 55, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Salonen, H.J.; Pasanen, A.L.; Lappalainen, S.K.; Riuttala, H.M.; Tuomi, T.M.; Pasanen, P.O.; Bäck, B.C.; Reijula, K.E. Airborne concentrations of volatile organic compounds, formaldehyde and ammonia in Finnish office buildings with suspected indoor air problems. J. Occup. Environ. Hyg. 2009, 6, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Uchiyama, I.; Uchiyama, S.; Kunugita, N. Assessment of inhalation exposure to indoor air pollutants: Screening for health risks of multiple pollutants in Japanese dwellings. Environ. Res. 2016, 145, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Tomizawa, T.; Tokoro, A.; Aoki, M.; Hishiki, M.; Yamada, T.; Tanaka, R.; Sakamoto, H.; Yoshida, T.; Bekki, K.; et al. Gaseous chemical compounds in indoor and outdoor air of 602 houses throughout Japan in winter and summer. Environ. Res. 2015, 137, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ernstgärd, L.; Iregren, A.; Sjögren, B.; Johanson, G. Acute effects of exposure to vapors of acetic acid in humans. Toxicol. Lett. 2006, 165, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Ihrig, A.; Triebig, G. Exposition Study on the Work Medicine—Significance of Ammonia-Associated Health Effects. Arbeitsmed Sozialmed Umweltmed 2004, 39, 390–401. [Google Scholar]
- European Commission, EU-LCI Values. Available online: https://ec.europa.eu/growth/sectors/construction/eu-lci/values_en (accessed on 30 January 2020).
- Finnish Society of Indoor Air Quality and Climate (FiSIAQ). Classification of Indoor Climate; Publication 5 E.; FiSIAQ: Espoo, Finland, 2000. [Google Scholar]
- Leduc, D.; Gris, P.; Lheureux, P.; Gevenois, P.A.; De Vuyst, P.; Yernault, J.C. Acute and long term respiratory damage following inhalation of ammonia. Thorax 1992, 47, 755–757. [Google Scholar] [CrossRef] [Green Version]
- Liesivuori, J.; Savolainen, H. Methanol and formic acid toxicity: Biochemical mechanisms. Pharmacol. Toxicol. 1991, 69, 157–163. [Google Scholar] [CrossRef]
- Shusterman, D.; Tarun, A.; Murphy, M.A.; Morris, J. Seasonal allergic rhinitic and normal subjects respond differentially to nasal provocation with acetic acid vapor. Inhal. Toxicol. 2005, 17, 147–152. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakaoka, H.; Nakayama, Y.; Takaya, K.; Tsumura, K.; Hanazato, M.; Tanaka, S.; Matsushita, K.; Iwayama, R.; Mori, C. Changes in the concentration of volatile organic compounds and aldehydes in newly constructed houses over time. Int. J. Environ. Sci. Technol. 2020, 17, 333–342. [Google Scholar] [CrossRef] [Green Version]
- JIS 1964 Indoor Air-Sampling Strategy for Volatile Organice Compounds (VOCs). Available online: https://kikakurui.com/a1/A1964-2015-01.html (accessed on 22 February 2020).
- ISO 16000-5:2007 Indoor Air-Part5: Sampling Strategy for Volatile Organic Compounds (VOCs). Available online: https://www.iso.org/standard/37388.html (accessed on 22 February 2020).
- Pharmaceutical Society of Japan. Standard Methods of Analysis for Hygienic Chemists Commentary; Yah Long Publishing: Taipei, Taiwan, 1996; pp. 1004–1008. [Google Scholar]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 30 January 2020).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Shusterman, D. Occupational irritant and allergic rhinitis. Curr. Allergy Asthma Rep. 2014, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Cometto-Muniz, J.E.; Jalowayski, A.A.; Dalton, P.; Kendall-Reed, M.; Hodgson, M. Assessment of upper respiratory tract and ocular irritative effects of volatile chemicals in humans. Crit. Rev. Toxicol. 2004, 34, 85–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffman, S.S.; Williams, C.M. Science of odor as a potential health issue. J. Environ. Qual. 2005, 34, 129–138. [Google Scholar] [PubMed]
- American Industrial Hygiene Association, Odor Thresholds for Chemicals with Established Health Standards, 2nd Edition. Available online: https://www.pdo.co.om/hseforcontractors/Health/Documents/HRAs/ODOR%20THRESHOLDS.pdf (accessed on 22 February 2020).
- International Chemical Safety Cards (ICSCs). Available online: https://www.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0485&p_version=2 (accessed on 30 January 2020).
- Sekine, Y.; Nishimura, A.; Suenaga, Y.; Komine, H. Field measurements and reduction experiments of formaldehyde and formic acid in indoor air. J. Archit. Plann. Environ. Eng. AIJ 2001, 548, 51–55. (In Japanese) [Google Scholar] [CrossRef]
- Zhang, J.; Wilson, W.E.; Lioy, P.J. Indoor air chemistry: Formation of organic acids and aldehydes. Environ. Sci. Technol. 1994, 28, 1975–1982. [Google Scholar] [CrossRef]
- Nagata, Y. Measurement of odor threshold by Triangle Odor Bag Method. Odor measurement review, Researches and developments on odor measurement. Jpn. Minist. Environ. Gov. FO Jpn. 1990, 118–127. Available online: http://www.env.go.jp/en/air/odor/measure/02_3_2.pdf (accessed on 30 January 2020).
- Leonardos, G.; Kendall, D.; Barnard, N. Odor threshold determinations of 53 odorant chemicals. J. Air Pollut. Contr. Assoc. 1969, 19, 91–95. [Google Scholar] [CrossRef] [Green Version]
- The Ministry of the Environment of Japan the Offensive Odor Control Low in Japan. Available online: https://www.env.go.jp/en/laws/air/offensive_odor/all.pdf (accessed on 30 January 2020).
- Feron, V.J.; Art, J.H.; Mojet, J. 2001 Approach to setting occupational exposure limits for sensory irritants in the Netherlands. AIHAJ 2001, 62, 733–735. [Google Scholar] [CrossRef]
- Rohr, A. The health significance of gas- and particle- phase terpene oxidation products: A review. Environ. Int. 2013, 60, 145–162. [Google Scholar] [CrossRef]
- Wolkoff, P. Indoor air pollutants in office environments: Assessment of comfort, health, and performance. Int. J. Hyg. Environ. Health 2013, 216, 371–394. [Google Scholar] [CrossRef]
- Saito, I.; Onuki, A.; Uehara, S.; Seto, H.; Kurita, M.; Ogata, A. Research on emission source of volatile organic compounds and aldehydes in a newly built wooden house. Indoor Environ. 2001, 13, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]


| Living Room | Bedroom | Outdoor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD (±) | Max | Min | Mean | SD (±) | Max | Min | Mean | SD (±) | Max | Min | ||
| Temperature | °C | 23.8 | 5.7 | 31.3 | 7.3 | 25.1 | 4.3 | 33.2 | 8.0 | 26.5 | 7.0 | 37.7 | 4.4 |
| Relative humidity | % | 62.3 | 14.1 | 82.2 | 24.6 | 58.0 | 10.5 | 77.5 | 24.4 | 54.3 | 17.4 | 94.2 | 23.4 |
| Ventilation rates | Per hour | 1.2 | 0.5 | 2.1 | 0.6 | ― | ― | ― | ― | ― | ― | ― | ― |
| Our study (2015–2016) | S. Uchiyama et al. (2015) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Living Room, n = 47 | Bedroom, n = 47 | Outdoor, n = 47 | n = 602 | |||||||||||||||||
| LOQ (a) | Mean | SD (±) | Median | Max | Min | Frequency | Mean | SD (±) | Median | Max | Min | Frequency | Mean | SD (±) | Median | Max | Min | Frequency | Median (Summer) | |
| (μg m−3) | (μg m−3) | (μg m−3) | (μg m−3) | (μg m−3) | (%) | (μg m−3) | (μg m−3) | (μg m−3) | (μg m−3) | (μg m−3) | (%) | (μg m−3) | (μg m−3) | (μg m−3) | (μg m−3) | (μg m−3) | (%) | (μg m−3) | ||
| Acetic acid | 3.0 | 169 | 99 | 160 | 410 | 20 | 100 | 148 | 89 | 130 | 400 | 0.8 | 100 | 49 | 37 | 39 | 160 | ND(b) | 100 | 130 |
| Formic acid | 3.0 | 28 | 11 | 28 | 62 | 7.0 | 100 | 30 | 15 | 28 | 91 | 1.6 | 100 | 20 | 7.1 | 18 | 38 | 5.6 | 100 | 28 |
| Ammonia | 3.0 | 73 | 30 | 68 | 160 | 13 | 100 | 77 | 32 | 75 | 160 | 10 | 100 | 21 | 13 | 17 | 59 | 5.9 | 100 | 37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, N.; Nakaoka, H.; Eguchi, A.; Hanazato, M.; Nakayama, Y.; Tsumura, K.; Takaguchi, K.; Takaya, K.; Todaka, E.; Mori, C. Concentrations of Formic Acid, Acetic Acid, and Ammonia in Newly Constructed Houses. Int. J. Environ. Res. Public Health 2020, 17, 1940. https://doi.org/10.3390/ijerph17061940
Suzuki N, Nakaoka H, Eguchi A, Hanazato M, Nakayama Y, Tsumura K, Takaguchi K, Takaya K, Todaka E, Mori C. Concentrations of Formic Acid, Acetic Acid, and Ammonia in Newly Constructed Houses. International Journal of Environmental Research and Public Health. 2020; 17(6):1940. https://doi.org/10.3390/ijerph17061940
Chicago/Turabian StyleSuzuki, Norimichi, Hiroko Nakaoka, Akifumi Eguchi, Masamichi Hanazato, Yoshitake Nakayama, Kayo Tsumura, Kohki Takaguchi, Kazunari Takaya, Emiko Todaka, and Chisato Mori. 2020. "Concentrations of Formic Acid, Acetic Acid, and Ammonia in Newly Constructed Houses" International Journal of Environmental Research and Public Health 17, no. 6: 1940. https://doi.org/10.3390/ijerph17061940
APA StyleSuzuki, N., Nakaoka, H., Eguchi, A., Hanazato, M., Nakayama, Y., Tsumura, K., Takaguchi, K., Takaya, K., Todaka, E., & Mori, C. (2020). Concentrations of Formic Acid, Acetic Acid, and Ammonia in Newly Constructed Houses. International Journal of Environmental Research and Public Health, 17(6), 1940. https://doi.org/10.3390/ijerph17061940







