Abstract
Aim: Diabetes and periodontal disease are both chronic pathological conditions linked by several underlying biological mechanisms, in which the inflammatory response plays a critical role, and their association has been largely recognized. Recently, attention has been given to diabetes as an important mediator of vascular endothelial growth factor (VEGF) overexpression in periodontal tissues, by virtue of its ability to affect microvasculature. This review aims to summarize the findings from studies that explored VEGF expression in diabetic patients with periodontitis, compared to periodontally healthy subjects. Materials and Methods: A systematic literature review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A PubMed search of select medical subject heading (MeSH) terms was carried out to identify all studies reporting findings about VEGF expression in periodontal tissues of diabetic patients up to May 2018. The inclusion criteria were studies on VEGF expression in periodontally diseased tissues of diabetic patients compared with nondiabetic subjects, with any method of analysis, and published in the English language. Results: Eight articles met the inclusion criteria. Immunohistochemistry was used in six of the studies, reverse transcriptase polymerase chain reaction (real-time RT-PCR) aiming to quantify mRNA VEGF expression was used in one study, and ELISA analysis was used for one study. Compared with nondiabetic patients, a higher VEGF expression in gingival tissue and gingival crevicular fluid (GCF) samples in diabetic patients with periodontitis was reported. Conclusions: Overall, novel evidence for the VEGF expression within the periodontal tissue of diabetic patients paves the way for further studies on the role of this protein in neovascularization physiology and pathophysiology in microvasculature of the periodontium.
1. Introduction
Diabetes and periodontal disease are both chronic pathological conditions linked by several underlying biological mechanisms, in which the inflammatory response plays a critical role, and their association has been largely recognized [1,2]. Periodontitis is an inflammatory disease affecting supporting tissues of teeth [3,4]. Its chronicization is a key determinant of clinical manifestations and underlying microvasculature alterations leading to irreversible damage [5]. It has been demonstrated that diabetic patients are more prone to developing periodontitis, and, conversely [6,7], uncontrolled diabetes is associated with higher risk for periodontitis onset and progression. Recently, in addition to the established relationship between periodontitis and diabetes, attention has been given to diabetes as an important mediator of vascular endothelial growth factor (VEGF) overexpression in periodontal tissues, by virtue of its ability to induce vasoreactivity in many organs [8,9,10,11]. Vascular endothelial growth factor (VEGF), a highly conserved heparin-binding homodimeric [12,13], disulfide-bound glycoprotein of 45kDa, is a key mediator of angiogenesis, a complex process which leads to the new growth of blood vessels from preexisting differentiated endothelial cells in the vascular network [14]. VEGF is a multifunctional growth factor implicated in embryonic development, and it is a powerful angiogenic agent in physiological and pathological neovascularization [13,14,15,16]. The vascular endothelial growth factor (VEGF) belongs to the endothelial growth factor family (VEGFs), currently consisting of seven known members with a regulatory role in different vascular systems: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PIGF [17]. VEGF exerts its biological effects through interaction with specific (VEGF-R) receptors, which belong to the receptor tyrosine kinase (RTK) subfamily [3,5]; the fms-like tyrosine kinase Flt-1 (VEGFR-1/Flt-1), the fetal liver kinase (VEGFR-2/KDR/Flk-1), and Flk-4 (VEGFR-3), causing proliferation of blood vessels (VEGF-A, VEGF-B, VEGF-C and VEGF-E) and lymphangiogenesis (VEGF-C and VEGF-D) [14,15,16]. The vascular endothelial growth factor receptors are one of the most important signaling pathways that regulate angiogenesis [17,18,19,20]. VEGFR-1 and VEGFR-2 are primarily implicated in this process, and there is a growing body of evidence suggesting that VEGFR-1 is involved in both physiological and pathological neovascularization [21,22,23,24,25]. The first evidence for the role of VEGFR-1 during angiogenesis came from the finding that abnormal blood vessel growth caused early embryogenic lethality when VEGFR-1 was targeted and deleted [2]. The negative role of VEGFR-1 in angiogenesis has been demonstrated by animal studies, observing an increase of endothelial cell progenitors and resulting in vascular disorganization as a consequence of loss of VEGFR-1/flt-1 [26,27]. However, more recent in vitro evidence suggests that selective activation of VEGFR-1 ligand is not linked to the proliferation of endothelial cells [28]. While the precise role of VEGFR-1 in angiogenesis remains to be determined, the role of VEGFR-2 in neovascularization is well recognized [28,29,30]. Upon binding, VEGFR-2 homo- or heterodimerizes with monomer receptors, triggering autophosphorylation of its tyrosine residues with receptors that activate broad signaling cascades, leading to different biological responses involving the activation of receptor tyrosine kinase (RTKs) [30,31,32]. The binding of VEGF to its receptor promotes the activation of relay proteins that transmit a signal into the nucleus of the endothelial cell. Subsequently, the nuclear signal induces a group of genes to release molecules needed for new endothelial cell growth [33,34]. Among the biological actions of VEGF, a role for this molecule in the direct control function of periodontal damage in diabetic patients has been recently suggested [35,36,37]. Several lines of evidence confirm that VEGF is a positive regulator of angiogenesis in physiological and pathological conditions [35,38], stimulating extracellular matrix degradation, proliferation and migration of endothelial cells, and regulating vascular permeability [39,40,41,42,43]. Several factors have been demonstrated as inductors of mRNA VEGF transcription, including PDGF, EGF, TNF-α, TGF-β, and IL-1. Importantly, it has been found that VEGF levels are also regulated via the hypoxia exposure; the tissue tension oxygen induces the expression of VEGF irreversibly, through increased transcription and mRNA stabilization [44]. Pathological angiogenesis is correlated with diabetic microvasculopathy in many organs, playing a critical role in diabetic retinopathy [45,46], nephropathy [31,35], neuropathy [47], impaired collateral vessel formation, and other systemic conditions. Several factors related to diabetes lead to angiogenic stimulation, and, primarily, the vascular endothelial growth factor (VEGF) signaling pathway is involved [48,49]. Specifically, it has been demonstrated that diabetes causes defective VEGF signaling leading to impairment of tyrosine kinase receptors Flk-1 activation, the receptor implicated in different angiogenesis processes and in transmitting VEGF signaling [35]. This reduced activity results in increased serum VEGF levels, causing pathologic angiogenesis [15]. Sasso et al. [50] have shown that the reduction of Flt-1 and Flk-1 receptors influenced the VEGF expression in the myocardium of diabetic patients, leading to a greater expression when compared to healthy subjects. Waltenberger et al. [35] demonstrated that Flk-1 activation was abnormal in diabetic conditions. Furthermore, there other several factors involved in abnormal angiogenesis in diabetes, including: (a) a chronic inflammatory status with consequent secretions of pro-inflammatory molecules, characterizing diabetes mellitus, which increases VEGF transcription hypoxia-inducible factor-1α (HIF-1α) [51,52,53,54,55,56,57]; (b) the hypoxic condition, resulting in the upregulation of hypoxia inducible factors, which triggers cells to upregulate VEGF and other pro-angiogenic agents [58]; (c) the presence of oxidative stress, which has been shown to characterize diabetes, and is responsible for the secretion of proinflammatory cytokines such as TNF-α, transforming growth factors alpha (TGF-α) and beta (TGF-β), and interleukins 6 (IL-6) and 8 (IL-8); and (d) hyperglycemia and advanced glycation end products (AGEs), which contribute to impaired angiogenic potential [59,60,61,62,63,64] in vitro and other excess tissue factors.
2. Materials and Methods
A systematic literature review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A PubMed search of select medical subject heading (MeSH) terms to identify all studies that reported VEGF-expression in diabetic patients with periodontitis findings to May 2019 was performed: “vascular endothelial growth factors “ OR “vascular” AND “endothelial” AND “growth” AND “factors” OR “vascular endothelial growth factors” OR “vascular” AND “endothelial” AND “growth” AND “factors” OR “vascular endothelial growth factors” AND “diabetes mellitus” OR “diabetes” AND “mellitus” OR “diabetes mellitus” OR “diabetes” OR “diabetes insipidus” OR “diabetes” AND “insipidus” OR “diabetes insipidus” AND “periodontitis” OR “periodontitis”. To be eligible, every study had to include the assessment of the expression of vascular endothelial growth factor (VEGF) in diabetic patients with periodontal disease and provide a healthy comparison, including non-diabetic patients with or without periodontal disease. The following outcomes were extracted for each study, where available: type of analysis, number of patients, setting, and analysis method, as shown in Table 1 and Table 2.

Table 1.
Data extraction table.

Table 2.
Data extraction table.
3. Results
The electronic search yielded a total of 26 articles. After examining the full text of articles, 18 of them were excluded from the review, as showed in Figure 1.
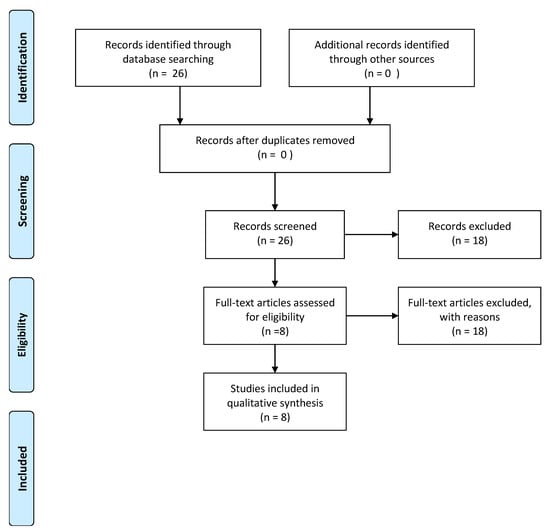
Figure 1.
Flow diagram of information through the different phases of a systematic review.
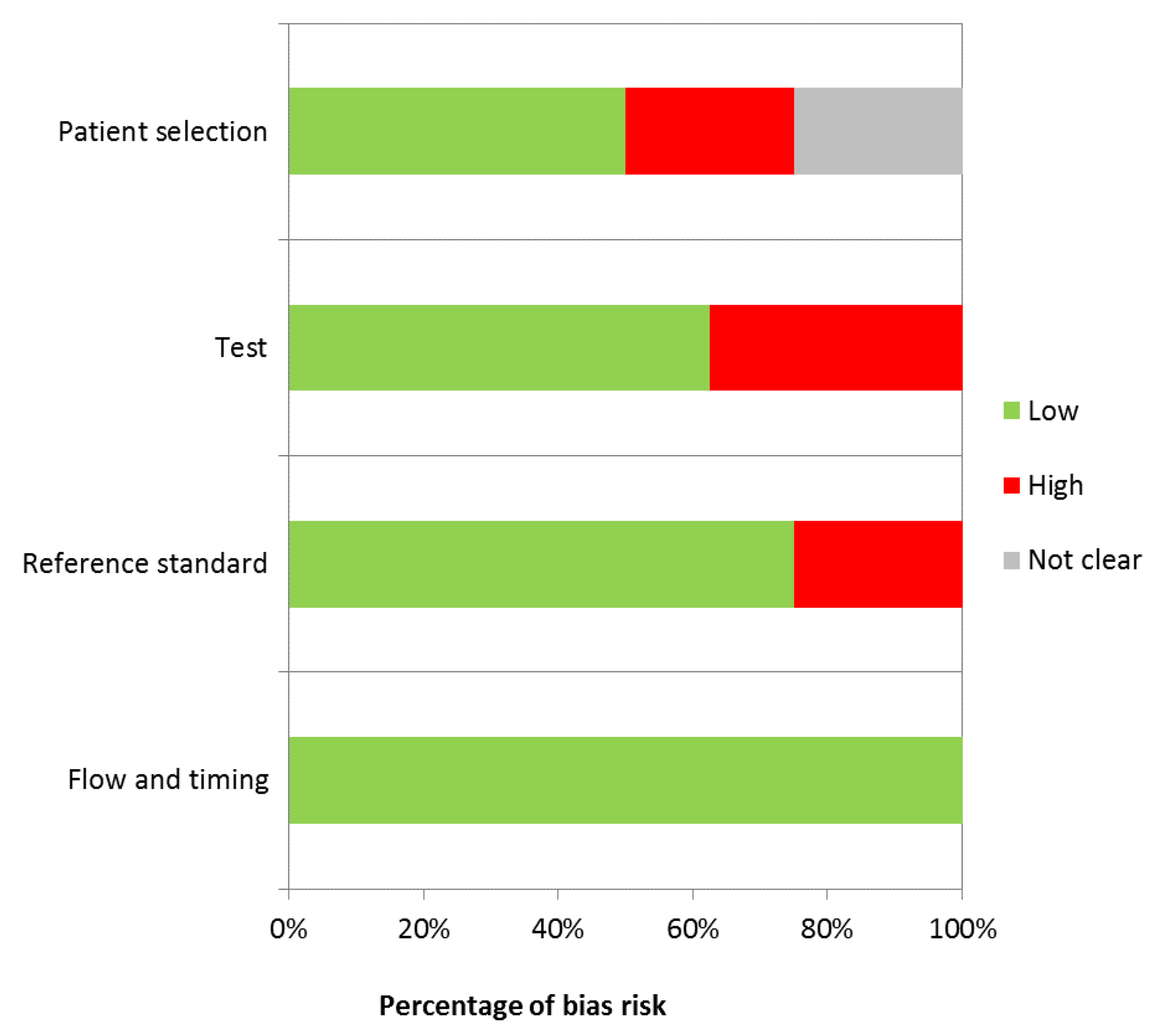
Of the eight remaining articles, six reported the results of immunochemistry quantification, one of ELISA analysis, and one real-time RT-PCR which aimed to quantify mRNA VEGF expression. A total of 330 patients were enrolled in the studies, and 424 gingival samples were analyzed. Furthermore, two studies also analyzed the VEGF expression into gingival crevicular fluid. The risk of bias for each study has been reported (Table 3).

Table 3.
Evaluation of bias risk.
In the study conducted by Lucarini et al. [65], 16 gingival samples corresponding to non-diabetic patients with periodontitis and 32 gingival samples from diabetic patients with periodontitis were selected. As a control for specificity of detection, 16 gingival samples from healthy subjects without periodontitis were included in the study. The detection of expression of VEGF was conducted in epithelial cells and connective tissue cells and in endothelial cells of sub-epithelial connective tissue vessels by immunohistochemical labeling using specific monoclonal antibodies. Their analysis demonstrated that epithelial and endothelial cells of the diabetic patients’ group and periodontal group without diabetes showed strong VEGF immunostaining. Otherwise, endothelial cells and cells derived from the gingival epithelium of healthy subjects showed moderate immunostaining. The study of Aspriello et al. [66] described VEGF cytoplasmic expression by immunohistochemical staining (IHC) in endothelial cells of gingival tissue samples of 66 patients with periodontitis, including 22 patients with type II diabetes mellitus (T2DM), 22 patients with type I diabetes mellitus (T1DM), and 22 systemically healthy subjects. Cryostat sections of gingival tissue were immunostained with the monoclonal antibodies anti-VEGF. For the immunohistochemical study, the semiquantitative was used. The immunohistochemical data revealed a significant difference in the periodontal expression of VEGF among the study patients. There was significantly more immunoreactivity to VEGF in the epithelium of T1DM and T2DM than in the control group (p <0.05). Endothelial cells of patients with T1DM showed a greater mean (±SD) degree of immunostaining than those of patients with T2DM and patients without diabetes (p <0.05). Kranti et al. [67] conducted a similar analysis, assessing the expression of cytoplasmic VEGF in macrophages and fibroblasts. Fifty subjects were analyzed for VEGF expression by immunohistochemistry. Expression of both line cells VEGF was more moderate in healthy subjects with periodontitis and patients with controlled type II diabetes mellitus and periodontitis than in the periodontally healthy group (p <0.01). The discrepancies with the previous study are likely due to the fact that analysis of VEGF was taken after periodontal treatment, leading to a decrease in inflammatory response. In contrast, the one study that aimed to analyze the VEGF mRNA expression in gingival samples, using real-time RT-PCR, failed to detect this overexpression in diseased periodontal tissues of diabetic patients (Keles et al.) [68]. Specifically, a higher significantly positive amplification of the mRNA encoding VEGF was observed in healthy subjects than in periodontal groups with or without diabetes, while the amount of VEGF mRNA did not differ between the diseased groups, and no statistically significant difference was observed between the groups (p >0.05). This different response may be attributable to physiological angiogenesis in the periodontium vasculature. Ramya and colleagues [69] published the results of their research, showing that diseased periodontal sites in the diabetic patients were correlated with significantly higher levels of VEGF expression when compared to healthy sites, in accordance with the previous studies (p >0.001). The authors concluded that their findings may be due to insulin resistance, which appears to stimulate the induction of VEGF expression. On the other hand, Ünlü et al. [70] detected VEGF subcellular localization using immunochemistry methods. They reported that positive staining was significantly localized to cytoplasm of monocytes and macrophages in healthy tissue samples and periodontal sites of diabetic patients, compared to healthy subjects. Similarly, an increased VEGF expression in diabetic patients was observed by Sakallioglu et al. [71]. In their study, gingival tissues and GCF samples were drawn from analyzed sixteen type II diabetes mellitus patients under a good metabolic control condition (HbA1c value less than seven), and 15 patients under systemically healthy conditions, all with periodontitis. VEGF protein expression was analyzed by enzyme-linked immunosorbent assay (ELISA). Of interest, compared with the control group, a slightly higher VEGF concentration of GCF was observed in diabetic patients with periodontitis, and the difference was not statistically significant (p >0.05). The latter findings indicated an even stronger gingival tissue response to the diabetic condition when compared to the nondiabetic patients, showing a higher VEGF concentration, and showing that the intensity of this pro-angiogenic agent expression in periodontal tissues may be correlated with diabetes. These results were not in agreement with the previous study conducted by Güneri et al. [72] suggesting that VEGF expression in periodontal tissues is primarily dependent on the periodontal status and that diabetes might have an additive effect. The differences among the studies may be due to the characteristics of gingival tissue samples derived from different degrees of severity of periodontitis in the diabetic group, conditioning the VEGF expression. A comparison analysis between the studies was not possible because, although achieved staining by the immunohistochemistry technique was analogous to the results acquired with the ELISA test (enzyme-linked immunosorbent assay), the interpretation of immunohistochemistry expression is defined in a subjective manner, and the majority of authors have revealed the VEGF expression with subjective scoring, while the application of the ELISA test is established as an objective quantitative method.
4. Discussion
Angiogenesis is a multi-sequential process characterized by the interaction of a wide variety of regulatory mediators, in which vascular endothelial growth factor (VEGF) is a protein primarily involved. The processes underlying the pathogenesis of the microvascular and macrovascular complications of diabetes have been primarily ascribed to angiogenic factors. Vascular endothelial growth factors expression is induced by hyperglycemia, advanced glycation end products, and oxidative stress [72]. This response may result in pathologic neovascularization and altered vascular permeability, leading to detrimental effects in microvascular tissues, such as the retina. In addition, VEGF is involved in the pathogenesis of diabetic renal disease, resulting in increased expression in the epithelial cells of the glomerulus [73], podocyte [74], and collecting ducts. In addition, the clinical observations relating to diabetic cardiovascular disease have demonstrated a significant pathologic role of VEGF, showing a much greater expression in human arteriole smooth muscle cells and infiltrating macrophages after myocardial infarction (MI). Periodontal vasculature, during development of disease, is subjected to the microvasculature alterations which lead to supporting tissue destruction. There is increasing evidence that microvasculature of the periodontium is severely affected in diabetic patients with periodontitis, showing a higher vascular endothelial growth factor (VEGF) expression when compared to healthy subjects, with or without periodontitis. More recently, evidence has accumulated supporting the concept that microangiopathy induced by diabetes substantially contributes to periodontal vasculature alteration in periodontal tissues, inducing the VEGF expression through its capability to induce microvasculopathy in many organs. However, these results are in contrast with previous studies reporting that VEGF levels expression was increased in periodontal sites of systemically healthy patients, compared to patients without periodontitis [55]. Our review showed limited information. Limitations that may be encountered with this review include discrepancies associated with different VEGF expression quantification methods (Table 3). The interpretation of immunohistochemistry expression is generally made in a qualitative and subjective manner, whereas quantification is considered of little or no importance. A majority of studies used the qualitative (positive or negative) or semiquantitative (0, 1, 2, 3) interpretation of immunostaining, a key limitation of HIC staining. The analysis using staining scales is subject to interobserver variability and is often based on cellular presence or absence of a specific molecule. In addition, the evaluation of immunostaining may be influenced by heterogeneity of biologic VEGF expression between tissue samples and gingival crevicular fluid, which is susceptible to the saliva concentration fluctuation. In the majority of studies, a positive result referred essentially to the presence of brown staining (peroxidase), leading to confounding factors [41,42,43]. Similarly, two authors assessed VEGF expression analysis in which the results of the immunohistochemical investigation revealed marked cytoplasmic staining, but this analysis is considered to be non-specific. Additionally, difficulty in the interpretation and comparison of immunohistochemical studies included variability in patient selection, different immunohistochemical criteria used for determining the staining degree, and bias associated with the statistical method of analysis data. For instance, studies applying immunochemistry analysis revealed a globally increased VEGF expression in periodontal sites in diabetic patients compared to healthy subjects. Interestingly, although the reduced VEGF expression level in patients after periodontal treatment was obvious, this result might be of relevance, because improvement of inflammatory status has been shown to reduce the angiogenesis [45,54]. However, although from these results we can assume that the periodontal vasculature is affected by diabetes, and the VEGF expression may influence periodontal disease progression, further investigation establishing the degree of disorder at different disease stages of diabetic patients with periodontal disease are needed [63], because the duration and degree of hyperglycemia in diabetes may also be critical, as well as the periodontal status. Nonetheless, as suggested by this, we cannot exclude the involvement of preexisting periodontal inflammation, as the author hypothesized that preexisting periodontal disease in patients with diabetes might partly explain increased VEGF expression and impact on outcome.
5. Conclusions
The current paradigm of linkage between type 2 diabetes and periodontitis must be integrated. We recognize an additional factor impacting the risk of periodontitis in patients with diabetes that may inform this discussion of new paradigms. This review has highlighted the detrimental effect of diabetes on periodontal tissues, resulting also from the induction of overexpression of VEGF, responsible for pathological angiogenesis. To date, an extremely limited number of studies have addressed the overexpression of VEGF in the periodontium of diabetic patients, and partially conflicting results have been reported. Expression of VEGF in diabetic patients with periodontitis has been demonstrated [21], but no conclusive data on inductive effect of periodontal damage have been published. However, the pivotal role of diabetes, and its signaling system in periodontal tissues, remains largely unexplored. Although expression of VEGF in the periodontium has been preliminarily reported, further investigations are needed for an appropriate conclusion in this linkage. In addition, clinical trials exploring new treatment strategies that target both diseases are required.
Author Contributions
Conceptualization: B.R.; investigation: B.R., E.F., and G.M.N.; resources: I.C., F.C., and E.F.; data curation: B.R. and E.F.; validation: G.M.N., F.C., B.R., S.S., R.G., and A.G.; writing—original draft preparation: B.R., E.F., and G.M.N.; writing—review and editing: B.R., E.F., F.R.G., A.G., and G.M.N.; visualization: A.G. and S.S.; supervision: E.F., G.M.N., and B.R.; project administration: B.R. and G.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Witmer, A.N.; Vrensen, G.F.; Van Noorden, C.J.; Schlingemann, R.O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003, 22, 1–29. [Google Scholar] [CrossRef]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.K.; Quinn, A.M. Protein kinase catalytic domain sequence database: Identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991, 200, 38–62. [Google Scholar]
- Corsalini, M.; Rapone, B.; Grassi, F.R.; Di Venere, D. A study on oral rehabilitation in stroke patients: Analysis of a group of 33 patients. Gerodontology 2010, 27, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signalling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef]
- Houck, K.A.; Ferrara, N.; Winer, J.; Cachianes, G.; Li, B.; Leung, D.W. The vascular endothelial growth factor family: Identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 1991, 5, 1806–1814. [Google Scholar] [CrossRef]
- Di Comite, M.; Crincoli, V.; Fatone, L.; Ballini, A.; Mori, G.; Rapone, B.; Boccaccio, A.; Pappalettere, C.; Grassi, F.R.; Favia, A. Quantitative analysis of defects at the dentin-post space in endodontically treated teeth. Materials 2015, 8, 3268–3283. [Google Scholar] [CrossRef]
- Kalemaj, Z.; Scarano, A.; Valbonetti, L.; Rapone, B.; Grassi, F.R. Bone response to four dental implants with different surface topography: A histologic and histometric study in minipigs. Int. J. Periodontics Restor. Dent. 2016, 36, 745–754. [Google Scholar] [CrossRef]
- Rapone, B.; Nardi, G.M.; Di Venere, D.; Pettini, F.; Grassi, F.R.; Corsalini, M. Oral hygiene in patients with oral cancer undergoing chemotherapy and/or radiotherapy after prosthesis rehabilitation: Protocol proposal. Oral Implantol. 2016, 9, 90–97. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Rapone, B.; Stefanachi, G.; Laforgia, A.; Pettini, F. Evidence of signs and symptoms of Craniomandibular Disorders in Fibromyalgia patients. Open Dent. J. 2017, 11, 91–98. [Google Scholar] [CrossRef]
- Grassi, F.R.; Pappalettere, C.; Di Comite, M.; Corsalini, M.; Mori, G.; Ballini, A.; Crincoli, V.; Pettini, F.; Rapone, B.; Boccaccio, A. Effect of different irrigating solutions and endodontic sealers on bond strength of the dentin-post interface with and without defects. Int. J. Med. Sci. 2012, 9, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Di Venere, D.; Pettini, F.; Nardi, G.M.; Laforgia, A.; Stefanachi, G.; Notaro, V.; Rapone, B.; Grassi, F.R.; Corsalini, M. Correlation between parodontal indexes and orthodontic retainers: Prospective study in a group of 16 patients. Oral Implantol. 2017, 10, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.R.; Grassi, R.; Rapone, B.; Gianfranco, A.; Balena, A.; Kalemaj, Z. Dimensional changes of buccal bone plate in immediate implants inserted through open flap, open flap and bone grafting, and flapless technique. A CBCT randomized controlled clinical trial. Clin. Oral Implants Res. 2019, 30, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Di Venere, D.; Nardi, G.M.; Lacarbonara, V.; Laforgia, A.; Stefanachi, G.; Corsalini, M.; Grassi, F.R.; Rapone, B.; Pettini, F. Early mandibular canine-lateral incisor transposition: Case Report. Oral Implantol. 2017, 10, 181–189. [Google Scholar] [CrossRef]
- Di Venere, D.; Corsalini, M.; Nardi, G.M.; Laforgia, A.; Grassi, F.R.; Rapone, B.; Pettini, F. Obstructive site localization in patients with Obstructive Sleep Apnea Syndrome: A comparison between otolaryngologic data and cephalometric values. Oral Implantol. 2017, 10, 295–310. [Google Scholar] [CrossRef]
- Grassi, F.R.; Rapone, B.; Scarano Catanzaro, F.; Corsalini, M.; Kalemaj, Z. Effectiveness of computer-assisted anesthetic delivery system (STA™) in dental implant surgery: A prospective study. Oral Implantol. 2017, 10, 381–389. [Google Scholar] [CrossRef]
- Rapone, B.; Converti, I.; Santacroce, L.; Cesarano, F.; Vecchiet, F.; Cacchio, L.; Scacco, S.; Grassi, R.; Grassi, F.R.; Gnoni, A.; et al. Impact of Periodontal Inflammation on Nutrition and Inflammation Markers in Hemodialysis Patients. Antibiotics 2019, 8, 209. [Google Scholar] [CrossRef]
- Quaglia, E.; Moscufo, L.; Corsalini, M.; Coscia, D.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Rapone, B.; Carossa, M.; Carossa, S. Polyamide vs silk sutures in the healing of postextraction sockets: A split mouth study. Oral Implantol. 2018, 11, 115–120. [Google Scholar]
- Nardi, G.M.; Grassi, R.; Grassi, F.R.; Aragona, S.E.; Rapone, B.; Della Vella, F.; Sabatini, S. Use of photobiomodulation induced by polarized polychromatic non-coherent light in the management of adult chronic periodontitis. J. Biol. Regul. Homeost. Agents 2019, 33, 293–297. [Google Scholar]
- Corsalini, M.; Di Venere, D.; Carossa, M.; Ripa, M.; Sportelli, P.; Cantatore, F.; Cagnetta, C.; De Rinaldis, C.; Montemurro, N.; De Giacomo, A.; et al. Comparative clinical study between zirconium-ceramic and metal-ceramic fixed rehabilitations. Oral Implantol. 2018, 11, 150–160. [Google Scholar]
- Corsalini, M.; Di Venere, D.; Sportelli, P.; Magazzino, D.; Ripa, M.; Cantatore, F.; Cagnetta, C.; De Rinaldis, C.; Montemurro, N.; De Giacomo, A.; et al. Evaluation of prosthetic quality and masticatory efficiency in patients with total removable prosthesis: Study of 12 cases. Oral Implantol. 2018, 11, 230–240. [Google Scholar]
- Corsalini, M.; Rapone, B.; Di Venere, D.; Petruzzi, M. Removable prosthetic treatment in oral pemphigus vulgaris: Report of three cases. J. Int. Soc. Prevent Communit. Dent. 2019, 9, 423–426. [Google Scholar]
- Taurino, F.; Stanca, E.; Vonghia, L.; Siculella, L.; Sardanelli, A.M.; Papa, S.; Zanotti, F.; Gnoni, A. Short-term type-1 diabetes differentially modulates 14-3-3 proteins in rat brain and liver. Eur. J. Clin. Investig. 2014, 44, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Giudetti, A.M. Dietary long-chain unsaturated fatty acids acutely and differently reduce the activities of lipogenic enzymes and of citrate carrier in rat liver. J. Physiol. Biochem. 2016, 72, 485–494. [Google Scholar] [CrossRef]
- Priore, P.; Cavallo, A.; Gnoni, A.; Damiano, F.; Gnoni, G.V.; Siculella, L. Modulation of hepatic lipid metabolism by olive oil and its phenols in nonalcoholic fatty liver disease. UBMB Life 2015, 67, 9–17. [Google Scholar] [CrossRef]
- Fong, G.H.; Rossant, J.; Gertsenstein, M.; Breitman, M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Corsalini, M.; Rapone, B.; Cagnetta, G.; Carossa, M.; Sportelli, P.; De Giacomo, A.; Laforgia, A.; Di Venere, D. Orofacial functions and chewing effiency in elderly patients with Parkinsons’s disease rehabilitated with removable prostheses. Open Dent. 2020, 14, 13–18. [Google Scholar]
- Fong, G.H.; Zhang, L.; Bryce, D.M.; Peng, J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development 1999, 126, 3015–3025. [Google Scholar]
- Hiratsuka, S.; Minowa, O.; Kuno, J.; Noda, T.; Shibuya, M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl. Acad. Sci. USA 1998, 95, 9349–9354. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.D.; Singh, A.; Majnoun, F.; Latz, C.; Lashkari, K.; Rahimi, N. Substitution of C-terminus of VEGFR-2 with VEGFR-1 promotes VEGFR-1 activation and endothelial cell proliferation. Oncogene 2004, 23, 5523–5531. [Google Scholar] [CrossRef]
- Grassi, F.R.; Ciccolella, F.; D'Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; De Matteo, M.; et al. Effect of low-level laser irradiation on osteoblast proliferation and bone formation. J. Biol. Regul. Homeost. Agents 2011, 25, 603–614. [Google Scholar] [PubMed]
- Meyer, R.D.; Singh, A.J.; Rahimi, N.J. The carboxyl terminus controls ligand-dependent activation of VEGFR-2 and its signaling. Biol. Chem. 2004, 279, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Tetè, S.; Scattarella, A.; Cantore, S.; Mastrangelo, F.; Papa, F.; Nardi, G.M.; Perillo, L.; Crincoli, V.; Gherlone, E.; et al. The role of anti-cyclic citrullinated peptide antibody in periodontal disease. Int. J. Immunopathol. Pharmacol. 2010, 23, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Waltenberger, J.; Claesson-Welsh, L.; Siegbahn, A.; Shibuya, M.; Heldin, C.H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 1994, 269, 26988–26995. [Google Scholar]
- Wilkinson-Berka, J.L. Vasoactive factors and diabetic retinopathy: Vascular endothelial growth factor, cycoloxygenase-2 and nitric oxide. Curr. Pharm. Des. 2004, 10, 3331–3348. [Google Scholar] [CrossRef]
- Osterby, R.; Nyberg, G. New vessel formation in the renal corpuscles in advanced diabetic glomerulopathy. J. Diabet Complicat. 1987, 1, 122–127. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Cesarano, F.; Arazzi, M.; Di Liberato, L.; Scacco, S.; Grassi, R.; Grassi, F.R.; Gnoni, A.; et al. Periodontal microbiological status influences the occurrence of cyclosporine-A and Tacrolimus-induced gingival overgrowth. Antibiotics 2019, 8, 124. [Google Scholar] [CrossRef]
- Ballini, A.; Scattarella, A.; Crincoli, V.; Carlaio, R.G.; Papa, F.; Perillo, L.; Romanazzo, T.; Bux, M.V.; Nardi, G.M.; Dituri, A.; et al. Surgical treatment of gingival overgrowth with 10 years of follow-up. Head Face Med. 2010, 6, 19. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A.; Archibald, V.; Dines, K.C.; Maxfield, E.K. Anti-oxidant and pro-oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non-diabetic and streptozotocin-diabetic rats. Diabetologia 1994, 37, 449–459. [Google Scholar] [CrossRef]
- Waltenberger, J. Impaired collateral vessel development in diabetes: Potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001, 49, 554–560. [Google Scholar] [CrossRef]
- Ziyadeh, F.N.; Hoffman, B.B.; Han, D.C.; Iglesias-De La Cruz, M.C.; Hong, S.W.; Isono, M.; Chen, S.; McGowan, T.A.; Sharma, K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 8015–8020. [Google Scholar] [CrossRef] [PubMed]
- Scattarella, A.; Ballini, A.; Grassi, F.R.; Carbonara, A.; Ciccolella, F.; Dituri, A.; Nardi, G.M.; Cantore, S.; Pettini, F. Treatment of oroantral fistula with autologous bone graft and application of a non-reabsorbable membrane. Int. J. Med. Sci. 2010, 7, 267–271. [Google Scholar]
- Bottaro, D.P.; Liotta, L.A. Cancer: Out of air is not out of action. Nature 2003, 423, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef]
- Freedman, S.B.; Isner, J.M. Therapeutic angiogenesis for coronary artery disease. Ann. Intern. Med. 2002, 136, 54–71. [Google Scholar] [CrossRef]
- Williams, B. A potential role for angiotensin II-induced vascular endothelial growth factor expression in the pathogenesis of diabetic nephropathy? Min. Electrolyte Metab. 1998, 24, 400–405. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Fatone, L.; Montenegro, V.; De Vito, D.; Pettini, F.; Crincoli, V.; Antelmi, A.; Romita, P.; Rapone, B.; et al. Transmission of nonviral sexually transmitted infections and oral sex. J. Sex Med. 2012, 9, 372–384. [Google Scholar] [CrossRef]
- Sasso, F.C.; Torella, D.; Carbonara, O.; Ellison, G.M.; Torella, M.; Scardone, M.; Marra, C.; Nasti, R.; Marfella, R.; Cozzolino, D.; et al. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J. Am. Coll. Cardiol. 2005, 46, 827–834. [Google Scholar] [CrossRef]
- Teixeira, A.S.; Andrade, S.P. Glucose-induced inhibition of angiogenesis in the rat sponge granuloma is prevented by aminoguanidine. Life Sci. 1999, 64, 655–662. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling-in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Nanda, T.; Singh, B.; Sharma, P.; Arora, K.S. Cyclosporine A and amlodipine induced gingival overgrowth in a kidney transplant recipient: Case presentation with literature review. BMJ Case Rep. CP 2019, 12, e229587. [Google Scholar] [CrossRef] [PubMed]
- Yaziji, H.; Barry, T. Diagnostic Immunohistochemistry: What can go wrong? Adv. Anat. Pathol. 2006, 13, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamaguchi, S.; Chida, K.; Shibuya, M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001, 20, 2768–2778. [Google Scholar] [CrossRef]
- Seidal, T.; Balaton, A.J.; Battifora, H. Interpretation and quantification of immunostains. Am. J. Surg. Pathol. 2001, 25, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.A. Quantification of immunohistochemistry-issues concerning methods, utility and semiquantitative assessment I. Histopathology 2006, 49, 406–410. [Google Scholar] [CrossRef]
- Taylor, C.R.; Levenson, R.M. Quantification of immunohistochemistry-issues concerning methods, utility and semiquantitative assessment II. Histopathology 2006, 49, 411–424. [Google Scholar] [CrossRef]
- Booth, V.; Young, S.; Cruchley, A.; Taichman, N.S.; Paleolog, E. Vascular endothelial growth factor in human periodontal disease. J. Periodontal Res. 1998, 33, 491–499. [Google Scholar] [CrossRef]
- Beral, A.; Veli, Ö.Ö.; Çiğdem, P.; Emir, B.; Timur, K.; Gülnur, E. Gingival crevicular fluid and salivary HIF-1α, VEGF, and TNF-α levels in periodontal health and disease. J. Periodontol. 2019, 90, 788–797. [Google Scholar]
- Ben-Av, P.; Crofford, L.J.; Wilder, R.L.; Hla, T. Induction of vascular endothelial growth factor in synovial fibroblasts by prostaglandin E and interleukin-1: A potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995, 372, 83–87. [Google Scholar] [CrossRef]
- Kuroki, M.; Voest, E.E.; Amano, S. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J. Clin. Investig. 1996, 7, 1667–1675. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nature Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Notaro, V.; Rapone, B.; Cagnetta, G.; Sportelli, P.; Nardi, G.M.; Corsalini, M. Resonance frequency evaluation on immediate loading implants with angled abutments: Case series. Annali di Stomatologia 2018, 9, 91–96. [Google Scholar]
- Lucarini, G.; Zizzi, A.; Aspriello, S.D.; Ferrante, L.; Tosco, E.; Lo Muzio, L.; Foglini, P.; Mattioli-Belmonte, M.; Di Primio, R.; Piemontese, M. Involvement of vascular endothelial growth factor, CD44 and CD133 in periodontal disease and diabetes: An immunohistochemical study. J. Clin. Periodontol. 2009, 36, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Aspriello, S.D.; Zizzi, A.; Lucarini, G.; Rubini, C.; Faloia, E.; Boscaro, M.; Tirabassi, G.; Piemontese, M. Vascular Endothelial Growth Factor and Microvessel Density in Periodontitis Patients With and Without Diabetes. J. Periodontol. 2009, 80, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Kranti, K.; Mani, R.; Elizabeth, A. Immunoexpression of Vascular Endothelial Growth Factor and Ki-67 in Human Gingival Samples: An Observational Study. Indian J. Dent. 2015, 6, 69–74. [Google Scholar] [CrossRef]
- Keles, G.C.; Cetinkaya, B.O.; Eroglu, C.; Simsek, S.B.; Kahraman, H. Vascular Endothelial Growth Factor Expression Levels of Gingiva in Gingivitis and Periodontitis Patients With/Without Diabetes Mellitus. Inflamm. Res. 2010, 59, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.K. Expression of VEGF in Periodontal Tissues of Type II Diabetes Mellitus Patients with Chronic Periodontitis—An Immunohistochemical Study. J. Clin. Diagn. Res. 2014, 8, ZC01–ZC03. [Google Scholar]
- Unlü, F.; Güneri, P.G.; Hekimgil, M.; Yeşilbek, B.; Boyacioğlu, H. Expression of Vascular Endothelial Growth Factor in Human Periodontal Tissues: Comparison of Healthy and Diabetic Patients. J. Periodontol. 2003, 74, 181–187. [Google Scholar] [CrossRef]
- Sakallioğlu, E.E.; Aliyev, E.; Lütfioğlu, M.; Yavuz, U.; Açikgöz, G. Vascular Endothelial Growth Factor (VEGF) Levels of Gingiva and Gingival Crevicular Fluid in Diabetic and Systemically Healthy Periodontitis Patients. Clin. Oral Investig. 2007, 11, 115–120. [Google Scholar] [CrossRef]
- Güneri, P.; Unlü, F.; Yeşilbek, B.; Bayraktar, F.; Kokuludağ, A.; Hekimgil, M.; Boyacioğlu, H.J. Vascular Endothelial Growth Factor in Gingival Tissues and Crevicular Fluids of Diabetic and Healthy Periodontal Patients. Periodontol. 2004, 75, 91–97. [Google Scholar] [CrossRef]
- Williams, B. A potential role for angiotensin II-induced vascular endothelial growth factor expression in the pathogenesis of diabetic nephropathy? Min. Electrolyte Metab. 1999, 24, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Grone, H.J.; Johren, O.; Kullmer, J.; Plate, K.H.; Risau, W.; Fuchs, E. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am. J. Physiol. 1995, 268, F240–F250. [Google Scholar] [CrossRef] [PubMed]
- Pupilli, C.; Lasagni, L.; Romagnani, P.; Bellini, F.; Mannelli, M.; Misciglia, N.; Mavilia, C.; Vellei, U.; Villari, D.; Serio, M. Angiotensin II stimulates the synthesis and secretion of vascular permeability factor/vascular endothelial growth factor in human mesangial cells. J. Am. Soc. Nephrol. 1999, 10, 245–255. [Google Scholar]
- Gruden, G.; Thomas, S.; Burt, D.; Zhou, W.; Chusney, G.; Gnudi, L.; Viberti, G. Interaction of angiotensin II and mechanical stretch on vascular endothelial growth factor production by human mesangial cells. J. Am. Soc. Nephrol. 1999, 10, 730–737. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).