Promising Scaffold-Free Approaches in Translational Dentistry
Abstract
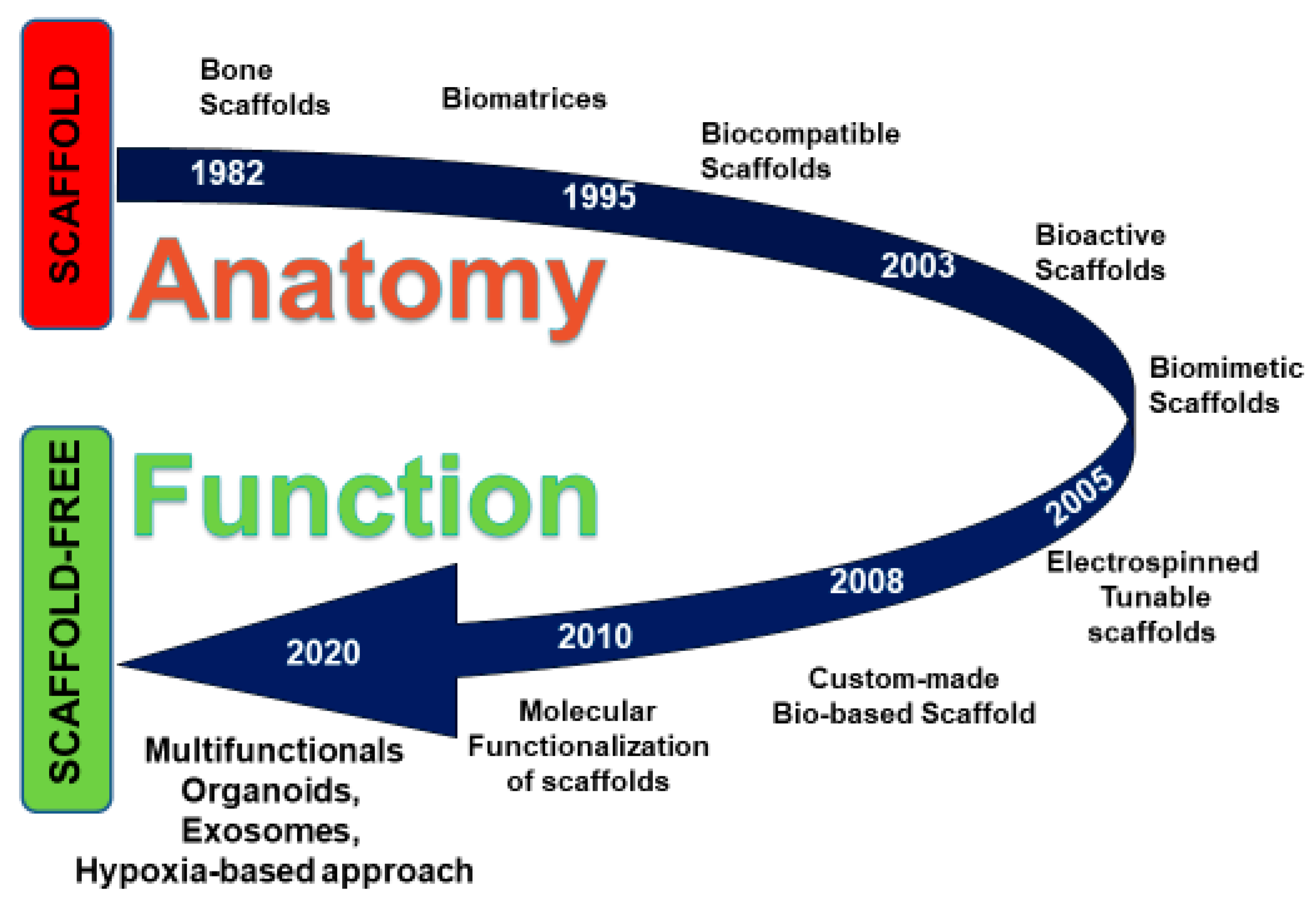
:1. Introduction
2. Searching Strategy
3. Scaffold-Free Approaches
3.1. Exosomes in Regenerative Dentistry
3.2. The Hypoxia-Based Approach in Regenerative Dentistry
3.3. Heat Shock Proteins (HSPs) in Regenerative Dentistry
4. Conclusions and Future Insights
Author Contributions
Funding
Conflicts of Interest
References
- Moller, J.F.; Petersen, J.K. Efficacy of a fibrin sealant on healing of extraction wounds. Int. J. Oral Maxillofac. Surg. 1988, 17, 142–144. [Google Scholar] [CrossRef]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Flace, P.; Girolamo, F.; Tarullo, A.; Laino, L.; et al. Regenerative surgery performed with platelet-rich plasma used in sinus lift elevation before dental implant surgery: An useful aid in healing and regeneration of bone tissue. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1222–1226. [Google Scholar] [PubMed]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Shah, R.; M.G.T.; Thomas, R.; Mehta, D.S. An Update on the Protocols and Biologic Actions of Platelet Rich Fibrin in Dentistry. Eur. J. Prosthodont. Restor. Dent. 2017, 25, 64–72. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e51–e55. [Google Scholar] [CrossRef]
- Marrelli, M.; Tatullo, M. Influence of PRF in the healing of bone and gingival tissues. Clinical and histological evaluations. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1958–1962. [Google Scholar]
- Barry, M.; Pearce, H.; Cross, L.; Tatullo, M.; Gaharwar, A.K. Advances in Nanotechnology for the Treatment of Osteoporosis. Curr. Osteoporos. Rep. 2016, 14, 87–94. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Odontogenic Differentiation of Human Dental Pulp Stem Cells on Hydrogel Scaffolds Derived from Decellularized Bone Extracellular Matrix and Collagen Type, I. PLoS ONE 2016, 11, e0148225. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Maletta, C.; Inchingolo, F.; Alfano, M.; Tatullo, M. Three-point bending tests of zirconia core/veneer ceramics for dental restorations. Int J Dent 2013, 2013, 831976. [Google Scholar] [CrossRef] [Green Version]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential use of human periapical cyst-mesenchymal stem cells (hpcy-mscs) as a novel stem cell source for regenerative medicine applications. Front. Cell Dev. Biol. 2017, 5, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschouris, K.; Firoozi, N.; Kang, Y. The application of cell sheet engineering in the vascularization of tissue regeneration. Regen. Med. 2016, 11, 559–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnke, P.H.; Wiltfang, J.; Springer, I.; Acil, Y.; Bolte, H.; Kosmahl, M.; Russo, P.A.J.; Sherry, E.; Lutzen, U.; Wolfart, S.; et al. Man as living bioreactor: Fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials 2006, 27, 3163–3167. [Google Scholar] [CrossRef] [PubMed]
- Mesimaki, K.; Lindroos, B.; Tornwall, J.; Mauno, J.; Lindqvist, C.; Kontio, R.; Miettinen, S.; Suuronen, R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 2009, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Tatullo, M.; Khariton, M.; Joshi, P.; Perniconi, B.; Gaharwar, A.K. Nanoengineered Osteoinductive and Elastomeric Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 27, 95–104. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1205. [Google Scholar] [CrossRef] [Green Version]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Saltotellez, M.; Timmers, L.; Lee, C.N.; el Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.S.; Suh, J.H.; Lee, H.J.; Bang, E.S.; Lee, J.M. Current Knowledge and Future Perspectives on Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Agent. Int. J. Mol. Sci. 2020, 21, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethő, A.; Chen, Y.; George, A. Exosomes in Extracellular Matrix Bone Biology. Curr. Osteoporos. Rep. 2018, 16, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Chun-Chieh, H.; Raghuvaran, N.; Satish, A.; Sriram, R. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue re generation. Biomaterials 2016, 111, 103–115. [Google Scholar]
- Chew, J.R.J.; Chuah, S.J.; Teo, K.Y.W.; Zhang, S.; Lai, R.C.; Fu., J.H.; Lim, L.P.; Lim, S.K.; Toh, W.S. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019, 89, 252–264. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliari-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.C.; Liu, X.B.; Huang, S.; Bi, X.Y.; Wang, H.X.; Xie, L.X.; Wang, Y.; Cao, X.; Lv, J.; Xiao, F. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012, 21, 3289–3297. [Google Scholar] [CrossRef]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef]
- Han, Y.D.; Bai, Y.; Yan, X.L.; Ren, J.; Zeng, Q.; Li, X.D.; Pei, X.T.; Han, Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem. Biophys. Res. Commun. 2018, 497, 305–312. [Google Scholar] [CrossRef]
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.J. Hypoxic Conditioned Medium from Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling. Stem Cells Transl. Med. 2016, 5, 816–825. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, B.; Huang, G.; Zeng, Q.; Wang, C. Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: The role of PI3K/AKT and HIF-1alpha. Hum. Cell 2019, 32, 64–74. [Google Scholar] [CrossRef]
- Showalter, M.R.; Wancewicz, B.; Fiehn, O.; Archard, J.A.; Clayton, S.; Wagner, J.; Deng, P.; Halmai, J.; Fink, K.D.; Bauer, G.; et al. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem. Biophys. Res. Commun. 2019, 512, 729–735. [Google Scholar] [CrossRef]
- Riccitiello, F.; De Luise, A.; Conte, R.; D’Aniello, S.; Vittoria, V.; Di Salle, A.; Calarco, A.; Peluso, G. Effect of resveratrol release kinetic from electrospun nanofibers on osteoblast and osteoclast differentiation. Eur. Polym. J. 2018, 99, 289–297. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Sorenson, C.M.; Sheibani, N. Role of angiogenesis in endodontics: Contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J. Endod. 2015, 41, 797–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimta, A.A.; Baru, O.; Badea, M.; Buduru, S.D.; Berindan-Neagoe, I. The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry. Int. J. Mol. Sci. 2019, 20, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Fan, G.C. Hypoxic exosomes promote angiogenesis. Blood 2014, 124, 3669–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eke, P.I.; Dye, B.A.; Wei, L.; Thorntonevans, G.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000 2004, 34, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Picciariello, V.; Inchingolo, A.D.; Dipalma, G.; Vermesan, D.; Cagiano, R. Clinical trial with bromelain in third molar exodontia. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 771–774. [Google Scholar]
- Henderson, B.; Pockley, A.G. Proteotoxic stress and circulating cell stress proteins in the cardiovascular diseases. Cell Stress Chaperones 2012, 17, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Tatullo, M.; Marrelli, M.; Scacco, S.; Lorusso, M.; Doria, S.; Sabatini, R.; Auteri, P.; Cagiano, R.; Inchingolo, F. Relationship between oxidative stress and “burning mouth syndrome” in female patients: A scientific hypothesis. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1218–1221. [Google Scholar]
- De, A.K.; Kodys, K.M.; Yeh, B.S.; Miller-Graziano, C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J. Immunol. 2000, 165, 3951–3958. [Google Scholar] [CrossRef] [Green Version]
- Laudanski, K.; De, A.; Miller-Graziano, C. Exogenous heat shock protein 27 uniquely blocks differentiation of monocytes to dendritic cells. Eur. J. Immunol. 2007, 37, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, F.; Donos, N.; Henderson, B.; Alagarswamy, R.; Pelekos, G.; Boniface, D.; Nibali, L. Association between circulating levels of heat-shock protein 27 and aggressive periodontitis. Cell Stress Chaperones 2018, 23, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballini, A.; Cantore, S.; Scacco, S.; Coletti, D.; Tatullo, M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine 2018. Stem Cells Int. 2018, 2018, 69274019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrazzo, P.; Paduano, F.; Palmieri, F.; Marrelli, M.; Tatullo, M. Highly Efficient In Vitro Reparative Behaviour of Dental Pulp Stem Cells Cultured With Standardised Platelet Lysate Supplementation. Stem Cells Int. 2016, 2016, 7230987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study Model | Type of Disease | Therapeutic Approaches | Main Results | References |
|---|---|---|---|---|
| Fibrin sealant | Extraction wounds | Human plasma derivatives (Cell/scaffold-free) | Fibrin sealants enhance the healing process | Moller et al. 1988 [1] |
| Platelet-rich plasma (PRP) | Postoperative injury sites | Human plasma derivatives (Cell/scaffold-free) | The PRP delivers growth factors at the treated site | Whitman et al. 1997 [2] |
| Platelet-rich plasma (PRP) | Maxillary sinus-lift | Human plasma derivatives (Cell/scaffold-free) | Improvement in implant-prosthetic rehabilitation | Inchingolo et al. 2012 [3] |
| Platelet-rich fibrin (PRF) | Posto-operative surgical sites | Human plasma derivatives (Cell/scaffold-free) | PRF appears to accelerate the physiologic healing process | Dohan et al. 2006 [6] |
| Platelet-rich fibrin (PRF) | Post-extractive dental implants | Human plasma derivatives (Cell/scaffold-free) | Improving the bone quality and the healing of soft tissues | Marrelli et al. 2013 [7] |
| DPSCs + hydrogel scaffolds (bECM) + GFs | In vitro experiments | Cell/scaffold/GFs | Up-regulation of osteogenic genes | Paduano et al. 2016 [9] |
| Cell sheets engineering (CSE) | In vitro experiments | Scaffold-free cell sheet engineering (CSE) | Improving of cell growth to improve the viability of the cell grafts | Moschouris et al. 2016 [12] |
| The microvascular custom-made ectopic bone flap | Hemi-maxillectomy | Cell/scaffold/GFs | Reconstruction of large defects | Mesimaki et al. 2009 [14] |
| DPSCs + Exosomes | In vitro experiments | Cell/biomolecules | Culture and growth of dental pulp-like tissue in a tooth-root model | Chun-Chieh et al. 2016 [23] |
| MSC-derived exosome-loaded collagen sponge | Periodontal defects | Cell/scaffold/biomolecules | Periodontal tissue regeneration through increased cellular mobilization and proliferation | Chew et al. 2019 [24] |
| Exosomes from hypoxia-cultured MSCs | In vitro experiments | Cell/biomolecules | Anti-inflammatory activity | Showalter et al. 2019 [31] |
| Exosomes-derived miRNA | In vitro experiments | Cell/biomolecules | Pro-angiogenic activity | Zimta et al. 2019 [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatullo, M.; Marrelli, B.; Palmieri, F.; Amantea, M.; Nuzzolese, M.; Valletta, R.; Zavan, B.; De Vito, D. Promising Scaffold-Free Approaches in Translational Dentistry. Int. J. Environ. Res. Public Health 2020, 17, 3001. https://doi.org/10.3390/ijerph17093001
Tatullo M, Marrelli B, Palmieri F, Amantea M, Nuzzolese M, Valletta R, Zavan B, De Vito D. Promising Scaffold-Free Approaches in Translational Dentistry. International Journal of Environmental Research and Public Health. 2020; 17(9):3001. https://doi.org/10.3390/ijerph17093001
Chicago/Turabian StyleTatullo, Marco, Benedetta Marrelli, Francesca Palmieri, Massimiliano Amantea, Manuel Nuzzolese, Rosa Valletta, Barbara Zavan, and Danila De Vito. 2020. "Promising Scaffold-Free Approaches in Translational Dentistry" International Journal of Environmental Research and Public Health 17, no. 9: 3001. https://doi.org/10.3390/ijerph17093001
APA StyleTatullo, M., Marrelli, B., Palmieri, F., Amantea, M., Nuzzolese, M., Valletta, R., Zavan, B., & De Vito, D. (2020). Promising Scaffold-Free Approaches in Translational Dentistry. International Journal of Environmental Research and Public Health, 17(9), 3001. https://doi.org/10.3390/ijerph17093001









