Concentrations of Particulate Matter and PM-Bound Polycyclic Aromatic Hydrocarbons Released during Combustion of Various Types of Materials and Possible Toxicological Potential of the Emissions: The Results of Preliminary Studies
Abstract
1. Introduction
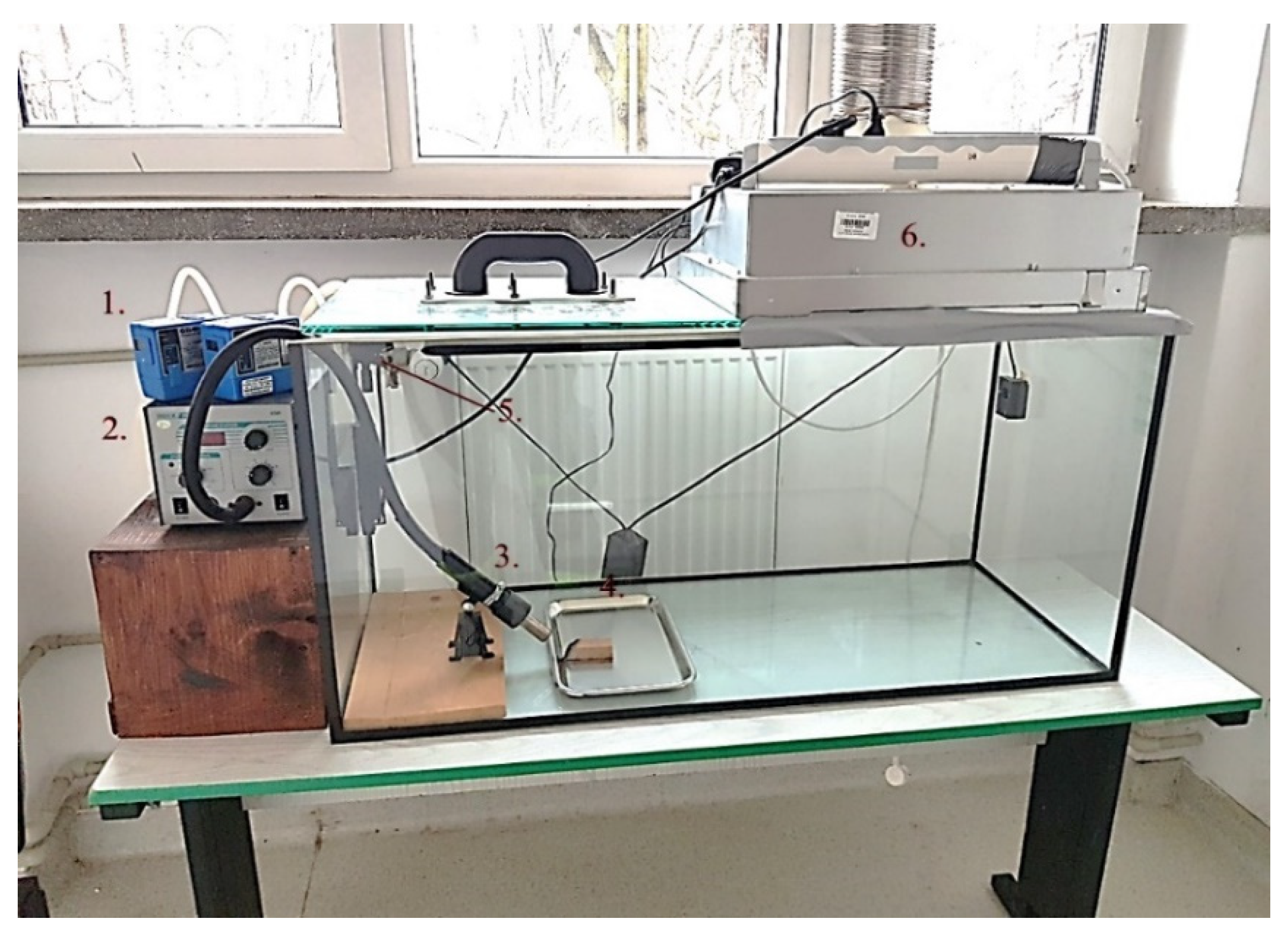
2. Materials and Methods
2.1. PM and PAH Analysis
2.2. Assessment of Carcinogenic, Mutagenic, and Toxic Potential of PM-Bound PAH Compounds Released during Combustion
+ [BghiP]) + 0.1 × ([BaA] + [BbF] + [BkF] + [IcdP]) + 1 × [BaP] + 1 × [DahA]
1 ×[BaP] + 0.31 × [IcdP] + 0.29 × [DahA] + 0.19 × [BghiP]
0.00203 × [DahA] + 0.00253 × [BbF] + 0.00487 × [BkF]
3. Results
3.1. PM Concentrations
3.2. PAH Concentrations
3.3. Toxicological Potential of PM-Bound PAH-Compounds
4. Conclusions
- The amount and type of hazardous substances present in fire smoke depend on the material that has been burned. Fires often involve objects/places where a combination of combustible materials occurs. The conducted tests are only the preliminary stage. They indicate the direction of further research and can be the basis and premise for research in more extensive forms, e.g., repeating an experiment with the same materials with longer sampling time and more repetitions, combining materials or using other materials, changing fire conditions (larger combustion chamber, use of other measuring equipment), measurement of the concentration of other combustion products, measurement of the concentration of other PM fractions (e.g., PM1, PM2.5, PM10).
- Although the measurements and calculations include only the concentrations of PM and PM-bound PAHs, the toxicological potential of emissions are high compared to other studies and may indicate high probability of adverse health effects during the combustion of all analyzed types of materials. Toxicological potential of emissions would be even higher if other PM-bound substances (e.g., metals, alkylated- and oxygenated-PAH compounds, and PAH-compounds containing heteroatoms) or gaseous pollutants released during fires were examined.
- Despite the fact that each fire is unique in terms of the type and amount of burning material and combustion conditions, additional studies are needed to better characterize the effects of fuel type and fire stage on exposure levels. Although smoke is a complex mixture and it was not possible to measure all of its components or potential health effects, we were able to demonstrate excessive or unhealthy exposures on the PM and PM-bound PAHs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Brand-Rauf, P.W.; Fallon, L.F.; Tarantin, T.; Idema, C.; Andrews, L. Health hazards of fire fighters: Exposure assessment. Br. J. Ind. Med. 1988, 45, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Stec, A.; Hull, T. Fire Toxicity; Oxford CRC Press: Cambridge, UK, 2010. [Google Scholar]
- Urbanski, S.P.; Hao, W.M.; Baker, S. Chemical composition of wildland fire emissions. In Developments in Environmental Science; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- George, I.; Black, R.; Geron, C.; Aurell, J.; Hays, M.; Preston, W.; Gullett, B. Volatile and semivolatile organic compounds in laboratory peat fire. Atmos. Environ. 2016, 132, 163–170. [Google Scholar] [CrossRef]
- Fent, K.W.; Alexander, B.; Roberts, J.; Robertson, S.; Toennis, C.; Sammons, D.; Bertke, S.; Kerber, S.; Smith, D.; Horn, D. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Environ. Hyg. 2017, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, G.; Aklilua, Y.; Landis, M.; Hsu, Y.M. Impacts of a large boreal wildfire on ground level atmospheric concentrations of PAHs, VOCs and ozone. Atmos. Environ. 2018, 178, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Stec, A.; Dickens, K.; Salden, M.; Hewitt, F.; Watts, D.; Houldsworth, P.; Martin, F. Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Sci. Rep. 2018, 8, 2476. [Google Scholar] [CrossRef] [PubMed]
- Stec, A.; Dickens, K.; Barnes, J.; Bedford, C. Environmental contamination following the Grenfell Tower fire. Chemosphere 2019, 226, 576–586. [Google Scholar] [CrossRef]
- Robertson, K.; Hsieh, Y.; Bugna, G. Fire environment effects on particulate matter emission factors in southeastern U.S. pine-grasslands. Atmos. Environ. 2014, 99, 104–111. [Google Scholar] [CrossRef]
- Goo, J. Study on the real-time size distribution of smoke particles for each fire stage by using a steady-state tube furnace method. Fire Saf. J. 2015, 78, 96–101. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Jiang, J.; Duan, L.; Ge, S.; Zhang, Q.; Deng, J.; Wang, S.; Hao, H. Semi-coke briquettes: Towards reducing emissions of primary PM2.5, particulate carbon and carbon monoxide from household coal combustion in China. Sci. Rep. 2016, 6, 19306. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, D.; Luo, G.; Yao, H. Temperature effect on central-mode particulate matter formation in combustion of coals with different mineral compositions. Energy Fuel 2015, 29, 5245–5252. [Google Scholar] [CrossRef]
- Szramowiat-Sala, K.; Korzeniewska, A.; Sornek, K.; Marczak, M.; Wierońska, F.; Berent, K.; Gołaś, J.; Filipowicz, M. The properties of particulate matter generated during wood combustion in in-use stoves. Fuel 2019, 253, 792–801. [Google Scholar] [CrossRef]
- European Committee for Standardization (CEN). Workplace Atmospheres-Size Fraction Definitions for Measurement of Airborne Particles (Report No. EN 481:1993); CEN: London, UK, 1993. [Google Scholar]
- American Conference of Governmental Industrial Hygienist. TLVs and BEIs: Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices; ACGIH: Cincinnati, OH, USA, 2005. [Google Scholar]
- Brown, J.; Gordon, T.; Price, O.; Asgharian, B. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol. 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Navarro, K.; Kleinman, M.; Mackay, C.; Reinhardt, T.; Balmes, J.; Broyles, G.; Ottmar, R.; Naher, L.; Domitrovich, J. Wildland firefighter smoke exposure and risk of lung cancer and cardiovascular disease mortality. Environ. Res. 2017, 173, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Chemical agents and related occupations. In Monographs on the Evaluation of the Carcinogenic Risks to Humans; IARC: Lyon, France, 2012. [Google Scholar]
- Armstrong, B.; Hutchinson, E.; Unwin, J.; Fletcher, T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: A review and meta-analysis. Environ. Health Perspect. 2004, 112, 970–978. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010. [Google Scholar]
- Dalgao-Saborit, J.M.; Stark, C.; Harrison, R.M. Carcinogenic potential, levels and sources of polycyclic aromatic hydrocarbon mixtures in indoor and outdoor environments and their implications for air quality standards. Environ. Int. 2011, 37, 383–392. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Kozielska, B.; Klejnowski, K. Concentration, origin and health hazard from fine partcile-bound PAH at three characteristic sites in Southern Poland. Bull. Environ. Contam. Toxicol. 2013, 91, 349–355. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Farmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Durant, J.L.; Busby, W.F.J.r.; Lafleur, A.L.; Penman, B.W.; Crespi, C.L. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat. Res. Gen. Toxicol. 1996, 371, 123–157. [Google Scholar] [CrossRef]
- Baxter, C.S.; Hoffman, J.D.; Knipp, M.J.; Reponen, T.; Haynes, E.N. Exposure of firefighters to particulates and polycyclic aromatic hydrocarbons. J. Occup. Environ. Hyg. 2014, 11, D85–D91. [Google Scholar] [CrossRef]
- Miranda, A.; Martins, V.; Cascão, P.; Amorim, J.; Tavares, R.; Borrego, C.; Tchepel, O.; Ferreira, A.; Cordeiro, C.; Viegas, D.; et al. Monitoring of firefighters exposure to smoke during fire experiments in Portugal. Environ. Int. 2010, 36, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Slezakova, K.; Alves, M.J.; Fernandes, A.; Teixeira, J.P.; Delerue-Matos, C.; Pereira, M.; Morais, S. Polycyclic aromatic hydrocarbons at fire stations: Firefighters’ exposure monitoring and biomonitoring, and assessment of the contribution to total internal dose. J. Hazard. Mater. 2017, 323, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.; Chappell, P.; Entwistle, J.; Kelly, F.; Deary, M. A study of particulate emissions during 23 major industrial fires: Implications for human health. Environ. Int. 2018, 112, 310–323. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Standardization. Ambient Air—Standard Gravimetric Measurement Method for the Determination of the PM10 or PM2.5 Mass Concentration of Suspended Particulate Matter; CEN: Dublin, Ireland, 2014. [Google Scholar]
- International Organization for Standardization. Workplace Atmospheres—Pumps for Personal Sampling of Chemical and Biological Agents—Requirements and Test Methods; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- International Organization for Standardization. Stationary Source Emissions—Determination of Gas and Particle-Phase Polycyclic Aromatic Hydrocarbons—Part 2: Sample Preparation, Clean-Up and Determination; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Hinds, W.C. Aerosol Technology. Properties, Behavior, and Measurement of Airborne Particles, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Rogula-Kozłowska, W. Size-segregated urban particulate matter: Mass closure, chemical composition, and primary and secondary matter content. Air Qual. Atmos. Health 2016, 9, 533–550. [Google Scholar] [CrossRef]
- Łukaszczuk, P. The application of nanotechnology in fire protection. Saf. Fire Technol. 2016, 42, 95–102. [Google Scholar] [CrossRef]
- Drysdale, D. An Introduction to fire Dynamics, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Sayadi, A.; Tapia, J.; Neitzert, T.; Clifton, G. Effects of expanded polystyrene (EPS) particles on fire resistance, thermal conductivity and compressive strength of foamed concrete. Constr. Build. Mater. 2016, 112, 716–724. [Google Scholar] [CrossRef]
- Delegou, E.T.; Apostolopoulou, M.; Ntoutsi, I.; Thoma, M.; Keramidas, V.; Papatrechas, C.; Economou, G.; Moropoulou, A. The effect of fire on building materials: The case-study of the Varnakova Monastery Cells in central Greece. Heritage 2019, 2, 80. [Google Scholar] [CrossRef]
- Samsonov, Y.N.; Ivanov, V.A.; McRae, D.J.; Baker, S.P. Chemical and dispersal characteristics of particulate emissions from forest fires in Siberia. Int. J. Wildland Fire 2012, 21, 818–827. [Google Scholar] [CrossRef]
- Huang, R.; Hu, Y.; Russell, A.G.; Mulholland, J.A.; Odman, M.T. The impacts of prescribed fire on PM2.5 air quality and human health: Application to asthma-related emergency room visits in Georgia, USA. Int. J. Environ. Res. Public Health 2019, 16, 2312. [Google Scholar] [CrossRef]
- Macintyre, H.L.; Heaviside, C.; Neal, L.S.; Agnew, P.; Thornes, J.; Vardoulakis, S. Mortality and emergency hospitalizations associated with atmospheric particulate matter episodes across the UK in spring 2014. Environ. Int. 2016, 97, 108–116. [Google Scholar] [CrossRef]
- Prado, G.; Zanetta, D.; Arbex, M.; Braga, A.; Pereira, L.; Rodrigues de Marchi, M.; de Melo Loureiro, A.; Marcourakis, T.; Sugauara, L.; Gattás, G.; et al. Burnt sugarcane harvesting: Particulate matter exposure and the effects on lung function, oxidative stress, and urinary 1-hydroxypyrene. Sci. Total Environ. 2012, 437, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.M.; Jones, A.D.; Turn, S.Q.; Williams, R.B. Emission Factors for Polycyclic Aromatic Hydrocarbons from Biomass Burning. Environ. Sci. Technol. 1996, 30, 2462–2469. [Google Scholar] [CrossRef]
- Mayer, A.C.; Fent, K.W.; Bartke, S.; Horn, G.P.; Smith, D.L.; Kerber, S.; Guardia, M.J. Firefighter hood contamination: Efficiency of laundering to remove PAHs and FRs. J. Occup. Environ. Hyg. 2019, 16, 129–140. [Google Scholar] [CrossRef]
- Blomqvist, P.; McNamee, M.; Andersson, P.; Lönnermark, A. Polycyclic aromatic hydrocarbons (PAHs) quantified in large-scale fire experiments. Fire Technol. 2012, 48, 513–528. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Majder, M.; Jureczko, I.; Ciuka-Witrylak, M.; Łukaszek-Chmielewska, A. Polycyclic aromatic hydrocarbons in the firefighter workplace: The results from the first in Poland short-term measuring campaign. E3S Web Conf. 2018, 45, 00075. [Google Scholar] [CrossRef]





| Risk Indicator | Beech | Oak | Pine | Paper | Cotton | Polypropylene | Polyurethane | OSB | |
|---|---|---|---|---|---|---|---|---|---|
| CEQ [µg/m3] | PM4 | 3.3 | 1.9 | 53.3 | 6.5 | 0.6 | 1.0 | 11.4 | 85.8 |
| TSP | 3.7 | 15.0 | 53.7 | 28.8 | 4.3 | 1.5 | 18.1 | 76.4 | |
| MEQ [µg/m3] | PM4 | 3.8 | 2.0 | 40.6 | 6.5 | 0.5 | 0.9 | 11.0 | 68.2 |
| TSP | 4.2 | 12.4 | 42.3 | 33.8 | 4.2 | 1.4 | 17.6 | 59.2 | |
| TEQ [ng/m3] | PM4 | 3.7 | 0.3 | 0.5 | 0.8 | 0.3 | 0.4 | 2.5 | 26.4 |
| TSP | 4.6 | 3.8 | 0.4 | 15.7 | 1.4 | 0.3 | 2.8 | 21.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bralewska, K.; Rakowska, J. Concentrations of Particulate Matter and PM-Bound Polycyclic Aromatic Hydrocarbons Released during Combustion of Various Types of Materials and Possible Toxicological Potential of the Emissions: The Results of Preliminary Studies. Int. J. Environ. Res. Public Health 2020, 17, 3202. https://doi.org/10.3390/ijerph17093202
Bralewska K, Rakowska J. Concentrations of Particulate Matter and PM-Bound Polycyclic Aromatic Hydrocarbons Released during Combustion of Various Types of Materials and Possible Toxicological Potential of the Emissions: The Results of Preliminary Studies. International Journal of Environmental Research and Public Health. 2020; 17(9):3202. https://doi.org/10.3390/ijerph17093202
Chicago/Turabian StyleBralewska, Karolina, and Joanna Rakowska. 2020. "Concentrations of Particulate Matter and PM-Bound Polycyclic Aromatic Hydrocarbons Released during Combustion of Various Types of Materials and Possible Toxicological Potential of the Emissions: The Results of Preliminary Studies" International Journal of Environmental Research and Public Health 17, no. 9: 3202. https://doi.org/10.3390/ijerph17093202
APA StyleBralewska, K., & Rakowska, J. (2020). Concentrations of Particulate Matter and PM-Bound Polycyclic Aromatic Hydrocarbons Released during Combustion of Various Types of Materials and Possible Toxicological Potential of the Emissions: The Results of Preliminary Studies. International Journal of Environmental Research and Public Health, 17(9), 3202. https://doi.org/10.3390/ijerph17093202





