Characteristic Human Individual Puffing Profiles Can Generate More TNCO than ISO and Health Canada Regimes on Smoking Machine When the Same Brand Is Smoked
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Recruitment of Participants
2.3. Study Protocol
2.4. Cigarette Brand
2.5. CReSSmicro™ Analysis
2.6. Machine Smoking and Chemical Analysis
2.7. CReSSmicro™ Device Evaluation Experiment
3. Results
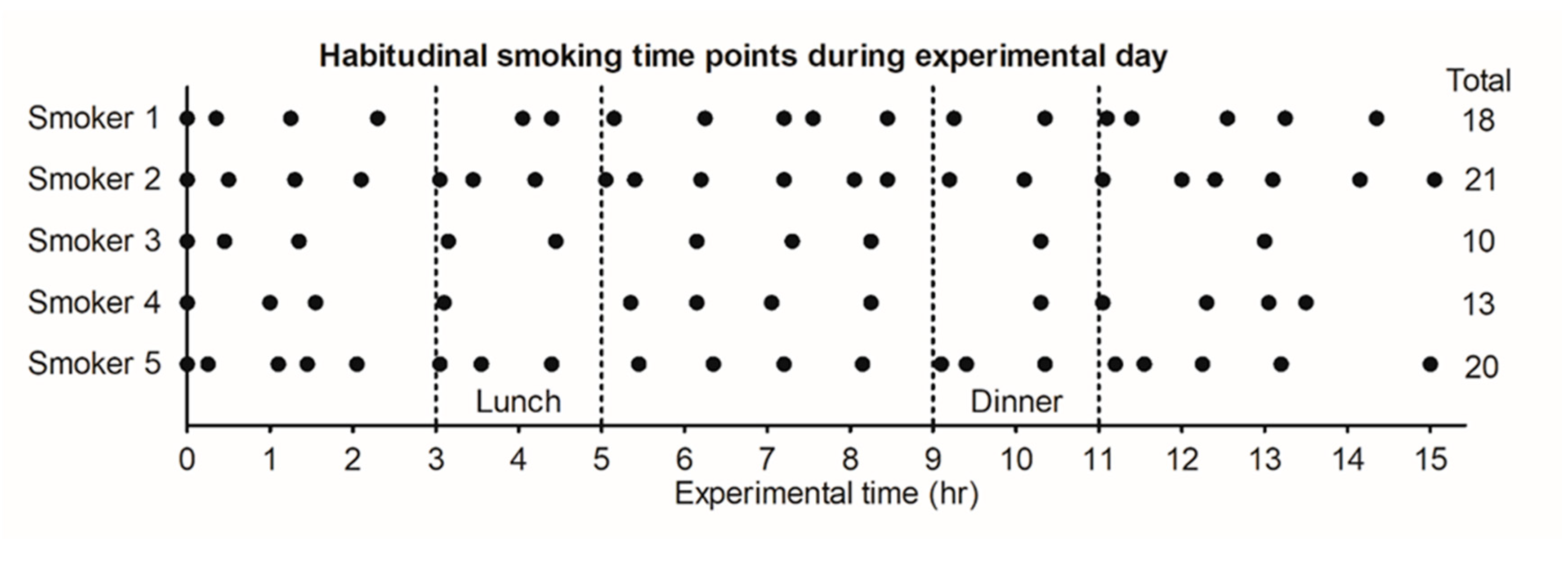
3.1. Smoking Timepoints
3.2. Personal Puffing Profile
3.3. Puff Profile over the Course of the Cigarette
3.4. Machine Smoking Puffing Profile
3.5. Evaluation of CReSSmicro™ Device
4. Discussion
4.1. Characterizing Human Smoking Behavior over the Course of the Day
4.2. Puffing Topography in Literature and the Use of CReSSmicro™ Device
4.3. TNCO Yields Produced by Machine Smoking with Personal Human Puffing Regximes, ISO Regime and HCI Regime
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collaborators, G.B.D.T. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- Hammond, D.; Wiebel, F.; Kozlowski, L.T.; Borland, R.; Cummings, K.M.; O’Connor, R.J.; McNeill, A.; Connolly, G.N.; Arnott, D.; Fong, G.T. Revising the machine smoking regime for cigarette emissions: Implications for tobacco control policy. Tob. Control 2007, 16, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Marian, C.; O’Connor, R.J.; Djordjevic, M.V.; Rees, V.W.; Hatsukami, D.K.; Shields, P.G. Reconciling human smoking behavior and machine smoking patterns: Implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3305–3320. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Goel, R.; Bitzer, Z.; Elias, R.J.; Foulds, J.; Muscat, J.; Richie, J.P., Jr. Effects of Topography-Related Puff Parameters on Carbonyl Delivery in Mainstream Cigarette Smoke. Chem. Res. Toxicol. 2017, 30, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Talhout, R.; Richter, P.A.; Stepanov, I.; Watson, C.V.; Watson, C.H. Cigarette Design Features: Effects on Emission Levels, User Perception, and Behavior. Tob. Regul. Sci. 2018, 4, 592–604. [Google Scholar] [CrossRef] [PubMed]
- WHO Study Group on Tobacco Product Regulation. Report on the Scientific Basis of Tobacco Product Regulation: Fifth Report of a WHO Study Group (989); WHO Study Group on Tobacco Product Regulation: Geneva, Switserland, 2015. [Google Scholar]
- Melikian, A.A.; Djordjevic, M.V.; Hosey, J.; Zhang, J.; Chen, S.; Zang, E.; Muscat, J.; Stellman, S.D. Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob. Res. 2007, 9, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Song, M.A.; Benowitz, N.L.; Berman, M.; Brasky, T.M.; Cummings, K.M.; Hatsukami, D.K.; Marian, C.; O’Connor, R.; Rees, V.W.; Woroszylo, C.; et al. Cigarette Filter Ventilation and its Relationship to Increasing Rates of Lung Adenocarcinoma. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Scherer, G. Smoking behaviour and compensation: A review of the literature. Psychopharmacology 1999, 145, 1–20. [Google Scholar] [CrossRef]
- Saidi, M.S.; Mhaisekar, A.; Hajaligol, M.R.; Subbiah, M. Mathematical Modeling of a Lit-End Cigarette: Puffing Cycle and Effects of Puff Counts. Beitr. Tab. Int. Contrib. Tob. Res. 2008, 23, 46–62. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.C.; Wang, L.; Liu, C.; McAdam, K.G.; Wang, B. Gas-phase pressure and flow velocity fields inside a burning cigarette during a puff. Thermochim. Acta 2016, 623, 22–28. [Google Scholar] [CrossRef]
- Baker, R.R.; Bishop, L.J. The pyrolysis of tobacco ingredients. J. Anal. Appl. Pyrolysis 2004, 71, 223–311. [Google Scholar] [CrossRef]
- Liang, Q.; Roethig, H.J.; Lipowicz, P.J.; Jin, Y.; Mendes, P.E. The effect of cigarette burn time on exposure to nicotine and carbon monoxide in adult smokers. Regul. Toxicol. Pharmacol. 2008, 50, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Purkis, S.W.; Troude, V.; Hill, C.A. Effect of puffing intensity on cigarette smoke yields. Regul. Toxicol. Pharmacol. 2013, 66, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Pazo, D.Y.; Moliere, F.; Sampson, M.M.; Reese, C.M.; Agnew-Heard, K.A.; Walters, M.J.; Holman, M.R.; Blount, B.C.; Watson, C.H.; Chambers, D.M. Mainstream Smoke Levels of Volatile Organic Compounds in 50 U.S. Domestic Cigarette Brands Smoked With the ISO and Canadian Intense Protocols. Nicotine Tob. Res. 2016, 18, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.T.; Taylor, K.M.; Holman, M.R.; Ding, Y.S.; Hearn, B.; Watson, C.H. Polycyclic Aromatic Hydrocarbons in the Mainstream Smoke of Popular U.S. Cigarettes. Chem. Res. Toxicol. 2015, 28, 1616–1626. [Google Scholar] [CrossRef]
- Pauwels, C.; Klerx, W.N.M.; Pennings, J.L.A.; Boots, A.W.; van Schooten, F.J.; Opperhuizen, A.; Talhout, R. Cigarette Filter Ventilation and Smoking Protocol Influence Aldehyde Smoke Yields. Chem. Res. Toxicol. 2018, 31, 462–471. [Google Scholar] [CrossRef]
- WHO Tobacco Laboratory Network. WHO SOP 01 Standard Operating Procedure for Intense Smoking of Cigarettes; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 3308:2012. Routine Analytical Cigarette-Smoking Machine -Definitions and Standard Conditions; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Hammond, D.; Fong, G.T.; Cummings, K.M.; O’Connor, R.J.; Giovino, G.A.; McNeill, A. Cigarette yields and human exposure: A comparison of alternative testing regimens. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1495–1501. [Google Scholar] [CrossRef]
- ISO 10315:2014. Cigarettes—Determination of Nicotine in Smoke Condensates—Gas-Chromatographic Method; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- Chen, A.; Krebs, N.M.; Zhu, J.; Muscat, J.E. Nicotine metabolite ratio predicts smoking topography: The Pennsylvania Adult Smoking Study. Drug Alcohol Depend. 2018, 190, 89–93. [Google Scholar] [CrossRef]
- Ross, K.C.; Dempsey, D.A.; St Helen, G.; Delucchi, K.; Benowitz, N.L. The Influence of Puff Characteristics, Nicotine Dependence, and Rate of Nicotine Metabolism on Daily Nicotine Exposure in African American Smokers. Cancer Epidemiol. Biomark. Prev. 2016, 25, 936–943. [Google Scholar] [CrossRef]
- Farris, S.G.; Aston, E.R.; Abrantes, A.M.; Zvolensky, M.J. Tobacco demand, delay discounting, and smoking topography among smokers with and without psychopathology. Drug Alcohol Depend. 2017, 179, 247–253. [Google Scholar] [CrossRef]
- Chung, S.; Kim, S.S.; Kini, N.; Fang, H.J.; Kalman, D.; Ziedonis, D.M. Smoking topography in Korean American and white men: Preliminary findings. J. Immigr. Minor. Health 2015, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Appleton, S.; Liu, J.; Lipowicz, P.J.; Sarkar, M. Effect of cigarette design on biomarkers of exposure, puffing topography and respiratory parameters. Inhal. Toxicol. 2015, 27, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.V.; Richter, P.; de Castro, B.R.; Sosnoff, C.; Potts, J.; Clark, P.; McCraw, J.; Yan, X.; Chambers, D.; Watson, C. Smoking Behavior and Exposure: Results of a Menthol Cigarette Cross-over Study. Am. J. Health Behav. 2017, 41, 309–319. [Google Scholar] [CrossRef] [PubMed]
- June, K.M.; Norton, K.J.; Rees, V.W.; O’Connor, R.J. Influence of measurement setting and home smoking policy on smoking topography. Addict. Behav. 2012, 37, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.G.; Frandsen, M.; Dunbar, M.S.; Shiffman, S. Gender and stimulus control of smoking behavior. Nicotine Tob. Res. 2015, 17, 431–437. [Google Scholar] [CrossRef]
- Piasecki, T.M.; Hedeker, D.; Dierker, L.C.; Mermelstein, R.J. Progression of nicotine dependence, mood level, and mood variability in adolescent smokers. Psychol. Addict. Behav. 2016, 30, 484–493. [Google Scholar] [CrossRef]
- Grainge, M.J.; Shahab, L.; Hammond, D.; O’Connor, R.J.; McNeill, A. First cigarette on waking and time of day as predictors of puffing behaviour in UK adult smokers. Drug Alcohol Depend. 2009, 101, 191–195. [Google Scholar] [CrossRef]
- Chapman, S.; Haddad, S.; Sindhusake, D. Do work-place smoking bans cause smokers to smoke “harder”? Results from a naturalistic observational study. Addiction 1997, 92, 607–610. [Google Scholar]
- Lee, E.M.; Malson, J.L.; Waters, A.J.; Moolchan, E.T.; Pickworth, W.B. Smoking topography: Reliability and validity in dependent smokers. Nicotine Tob. Res. 2003, 5, 673–679. [Google Scholar] [CrossRef]
- Blank, M.D.; Disharoon, S.; Eissenberg, T. Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation. Nicotine Tob. Res. 2009, 11, 896–903. [Google Scholar] [CrossRef]
- Perkins, K.A.; Karelitz, J.L.; Giedgowd, G.E.; Conklin, C.A. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob. Res. 2012, 14, 490–494. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, S.; Hsin, A.; Faulkner, G.; Prapavessis, H. A systematic review and analysis of data reduction techniques for the CReSS smoking topography device. J. Smok. Cessat. 2013, 10, 12–28. [Google Scholar] [CrossRef]
- Shiffman, S.; Scholl, S. Increases in Cigarette Consumption and Decreases in Smoking Intensity When Nondaily Smokers Are Provided With Free Cigarettes. Nicotine Tob. Res. 2018, 20, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.M.; Leyro, T.M.; Rosen, R.L.; Zvolensky, M.J.; Farris, S.G. Negative urgency and ad-libitum smoking topography. Drug Alcohol Depend. 2019, 201, 220–226. [Google Scholar] [CrossRef]
- Mikheev, V.B.; Buehler, S.S.; Brinkman, M.C.; Granville, C.A.; Lane, T.E.; Ivanov, A.; Cross, K.M.; Clark, P.I. The application of commercially available mobile cigarette topography devices for e-cigarette vaping behavior measurements. Nicotine Tob. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/brand_preference/index.htm (accessed on 9 April 2020).
- Statista Research. Available online: https://www.statista.com/statistics/822213/market-share-marlboro-in-the-netherlands/ (accessed on 9 April 2020).
- Oldham, M.J.; Plunkett, S.E.; Fisher, M.T.; Shafer, K.H.; Morton, M.J. Laboratory Evaluation of the CReSSmicro™ Portable Topography Device: Implications for Clinical Research. Contrib. Tob. Res. 2014, 26. [Google Scholar] [CrossRef]
- ISO 4387:2000/A1:2008. Cigarettes—Determination of Total and Nicotine-Free Dry Particulate Matter Using a Routine Analytical Smoking Machine; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- ISO 8454:2007/A1:2009. Cigarettes—Determination of Carbon Monoxide in the Vapour Phase of Cigarette Smoke—NDIR Method; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Hammond, D.; Fong, G.T.; Cummings, K.M.; Hyland, A. Smoking topography, brand switching, and nicotine delivery: Results from an in vivo study. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1370–1375. [Google Scholar] [CrossRef]
- Brinkman, M.C.; Kim, H.; Chuang, J.C.; Kroeger, R.R.; Deojay, D.; Clark, P.I.; Gordon, S.M. Comparison of True and Smoothed Puff Profile Replication on Smoking Behavior and Mainstream Smoke Emissions. Chem. Res. Toxicol. 2015, 28, 182–190. [Google Scholar] [CrossRef]
- Guyatt, A.R.; Kirkham, A.J.; Baldry, A.G.; Dixon, M.; Cumming, G. How does puffing behavior alter during the smoking of a single cigarette? Pharmacol. Biochem. Behav. 1989, 33, 189–195. [Google Scholar] [CrossRef]
- Kolonen, S.; Tuomisto, J.; Puustinen, P.; Airaksinen, M.M. Puffing behavior during the smoking of a single cigarette in a naturalistic environment. Pharmacol. Biochem. Behav. 1992, 41, 701–706. [Google Scholar] [CrossRef]
- Collins, C.C.; Epstein, D.H.; Parzynski, C.S.; Zimmerman, D.; Moolchan, E.T.; Heishman, S.J. Puffing behavior during the smoking of a single cigarette in tobacco-dependent adolescents. Nicotine Tob. Res. 2010, 12, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, J.C.; Kassel, J.D.; Heinz, A.J.; Braun, A.; Wardle, M.C.; Greenstein, J.; Evatt, D.P.; Conrad, M. Predictors and sequelae of smoking topography over the course of a single cigarette in adolescent light smokers. J. Adolesc. Health 2011, 48, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.A.; Higby, R.; Stutt, K. Puff-by-Puff Analysis of Selected Mainstream Smoke Constituents in The Kentucky Reference 2R4F Cigarette. Beitr. Tab. Int. Contrib. Tob. Res. 2005, 21, 273–279. [Google Scholar] [CrossRef]
- Norton, K.J.; June, K.M.; O’Connor, R.J. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob. Induc. Dis. 2014, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.A.; Ashare, R.L.; Kozlowski, L.T.; Pickworth, W.B. The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol. Biochem. Behav. 2005, 82, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, M.C.; Chuang, J.C.; Gordon, S.M.; Kim, H.; Kroeger, R.R.; Polzin, G.M.; Richter, P.A. Exposure to and deposition of fine and ultrafine particles in smokers of menthol and nonmenthol cigarettes. Inhal. Toxicol. 2012, 24, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Tidey, J.W.; Cassidy, R.N.; Miller, M.E. Smoking Topography Characteristics of Very Low Nicotine Content Cigarettes, With and Without Nicotine Replacement, in Smokers With Schizophrenia and Controls. Nicotine Tob. Res. 2016, 18, 1807–1812. [Google Scholar] [CrossRef]
- Djordjevic, M.V.; Stellman, S.D.; Zang, E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J. Natl. Cancer Inst. 2000, 92, 106–111. [Google Scholar] [CrossRef]
- Dickens, C.; McGrath, C.; Warren, N.; Biggs, P.; McAughey, J. Puffing and inhalation behaviour in cigarette smoking: Implications for particle diameter and dose. J. Phys. 2009, 151, 012019. [Google Scholar] [CrossRef]
- Rostron, B.L.; Corey, C.G.; Chang, J.T.; van Bemmel, D.M.; Miller, M.E.; Chang, C.M. Associations of Cigarettes Smoked Per Day with Biomarkers of Exposure among U.S. Adult Cigarette Smokers in the Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Cancer Epidemiol. Biomark. Prev. 2019, 28, 1443–1453. [Google Scholar] [CrossRef]
- Corley, R.A.; Kabilan, S.; Kuprat, A.P.; Carson, J.P.; Jacob, R.E.; Minard, K.R.; Teeguarden, J.G.; Timchalk, C.; Pipavath, S.; Glenny, R.; et al. Comparative Risks of Aldehyde Constituents in Cigarette Smoke Using Transient Computational Fluid Dynamics/Physiologically Based Pharmacokinetic Models of the Rat and Human Respiratory Tracts. Toxicol. Sci. 2015, 146, 65–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yu, S. Smoking Topography among Korean Smokers: Intensive Smoking Behavior with Larger Puff Volume and Shorter Interpuff Interval. Int. J. Environ. Res. Public Health 2018, 15, 1024. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Inaba, Y.; Yamaguchi, I.; Endo, O.; Hammond, D.; Uchiyama, S.; Suzuki, G. Smoking topography and biomarkers of exposure among Japanese smokers: Associations with cigarette emissions obtained using machine smoking protocols. Environ. Health Prev. Med. 2013, 18, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Breland, A.B.; Kleykamp, B.A.; Eissenberg, T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine Tob. Res. 2006, 8, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.; Kochhar, N.; Prasad, K.; Shepperd, J.; Warburton, D.M. The influence of changing nicotine to tar ratios on human puffing behaviour and perceived sensory response. Psychopharmacology 2003, 170, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, D.; Morgan, S.F.; Pickens, R.W.; Hughes, J.R. Smoking topography in a nonlaboratory environment. Int. J. Addict. 1987, 22, 719–725. [Google Scholar] [CrossRef]


| Participant or Smoking Regime | Cigarette Count | Puffs per Cigarette | Puff Volume (mL) | Puff Flow (mL/sec) | Puff Duration (sec) | Inter-Puff Interval (sec) | Total Puffing Volume per Cigarette (mL) | Total Cigarette Volume per Day (L) & |
|---|---|---|---|---|---|---|---|---|
| # | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Smoker 1 | 18 | 26 (8.9) | 80 (11.4) | 43 (7.7) | 1.9 (0.2) | 11 (2.4) | 2127 (904) | 38.3 |
| Smoker 2 | 21 | 13 (1.3) | 60 (5.7) | 48 (3.0) | 1.4 (0.1) | 21 (5.8) | 778 (123) | 16.3 |
| Smoker 3 | 10 | 17 (2.3) | 93 (13.9) | 57 (7.3) | 1.6 (0.1) | 10 (4.6) | 1582 (296) | 15.8 |
| Smoker 4 | 13 | 11 (2.3) | 44 (3.8) | 51 (4.0) | 0.9 (0.1) | 26 (8.1) | 495 (125) | 6.4 |
| Smoker 5 | 20 | 11 (1.0) | 67 (4.1) | 27 (2.3) | 2.6 (0.2) | 16 (3.1) | 740 (79) | 14.8 |
| ISO * | 8 | 35 | 17.5 | 2 | 60 | 280$ | ||
| HCI * | 9 | 55 | 27.5 | 2 | 30 | 495$ |
| Participant or Smoking Regime | Puffs Smoking Machine (SD) | Total Puffing Volume mL/cig (SD) | Tar mg/cig (SD) | Nicotine mg/cig (SD) | CO mg/cig (SD) |
|---|---|---|---|---|---|
| Smoker 1 | 12 (1.4) | 1005 (116) | 58 (9.6) | 3.4 (0.4) | 49 (0.8) |
| Smoker 2 | 12 (0.9) | 710 (45) | 32 (1.1) | 2.1 (0.1) | 18 (0.2) |
| Smoker 3 | 14 (1.0) | 1200 (108) | 59 (8.1) | 3.3 (0.3) | 27 (3.3) |
| Smoker 4 | 12 (0) | 530 (0) | 21 (1.4) | 1.5 (0.1) | 16 (&) |
| Smoker 5 | 11 (0.7) | 754 (38) | 38 (1.5) | 2.4 (0.1) | 20 (0.1) |
| ISO * | 8 | 280 $ | 10.37 | 0.8 | 8.51 |
| HCI * | 9 | 495 $ | 34.04 | 1.97 | 26.27 |
| Puff Parameter | Smoking Machine or CReSSmicro™ Device | Way of Cigarette Insertion in CReSSmicro™ Device | |||||
|---|---|---|---|---|---|---|---|
| Normal | Loose | Tight | |||||
| Regime 1 | Regime 2 | Regime 1 | Regime 2 | Regime 1 | Regime 2 | ||
| Puff count | Smoking machine | 11 | 11 | 10 | 10 | 11 | 12 |
| CReSSmicro™ device | 18 | 20 | 18 | 26 | 11 | 13 | |
| Puff volume (mL) | Smoking machine | 55 | 65 | 55 | 65 | 55 | 65 |
| CReSSmicro™ device | 59 | 73 | 60 | 74 | 65 | 76 | |
| Puff duration (sec) | Smoking machine | 2 | 2 | 2 | 2 | 2 | 2 |
| CReSSmicro™ device | 2 | 2 | 2 | 2 | 2 | 2 | |
| Inter-puff interval (sec) | Smoking machine | 30 | 18 | 30 | 18 | 30 | 18 |
| CReSSmicro™ device | 27 | 16 | 29 | 16 | 27 | 16 | |
| Total puffing volume (mL) | Smoking machine | 990 | 1300 | 1008 | 1690 | 587 | 845 |
| CReSSmicro™ device | 1053 | 1453 | 1106 | 1932 | 692 | 983 | |
| Flow-dropouts | CReSSmicro™ device | 5 | 6 | 8 | 6 | 0 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauwels, C.G.G.M.; Boots, A.W.; Visser, W.F.; Pennings, J.L.A.; Talhout, R.; Van Schooten, F.-J.; Opperhuizen, A. Characteristic Human Individual Puffing Profiles Can Generate More TNCO than ISO and Health Canada Regimes on Smoking Machine When the Same Brand Is Smoked. Int. J. Environ. Res. Public Health 2020, 17, 3225. https://doi.org/10.3390/ijerph17093225
Pauwels CGGM, Boots AW, Visser WF, Pennings JLA, Talhout R, Van Schooten F-J, Opperhuizen A. Characteristic Human Individual Puffing Profiles Can Generate More TNCO than ISO and Health Canada Regimes on Smoking Machine When the Same Brand Is Smoked. International Journal of Environmental Research and Public Health. 2020; 17(9):3225. https://doi.org/10.3390/ijerph17093225
Chicago/Turabian StylePauwels, Charlotte G.G.M., Agnes W. Boots, Wouter F. Visser, Jeroen L.A. Pennings, Reinskje Talhout, Frederik-Jan Van Schooten, and Antoon Opperhuizen. 2020. "Characteristic Human Individual Puffing Profiles Can Generate More TNCO than ISO and Health Canada Regimes on Smoking Machine When the Same Brand Is Smoked" International Journal of Environmental Research and Public Health 17, no. 9: 3225. https://doi.org/10.3390/ijerph17093225
APA StylePauwels, C. G. G. M., Boots, A. W., Visser, W. F., Pennings, J. L. A., Talhout, R., Van Schooten, F.-J., & Opperhuizen, A. (2020). Characteristic Human Individual Puffing Profiles Can Generate More TNCO than ISO and Health Canada Regimes on Smoking Machine When the Same Brand Is Smoked. International Journal of Environmental Research and Public Health, 17(9), 3225. https://doi.org/10.3390/ijerph17093225





