Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
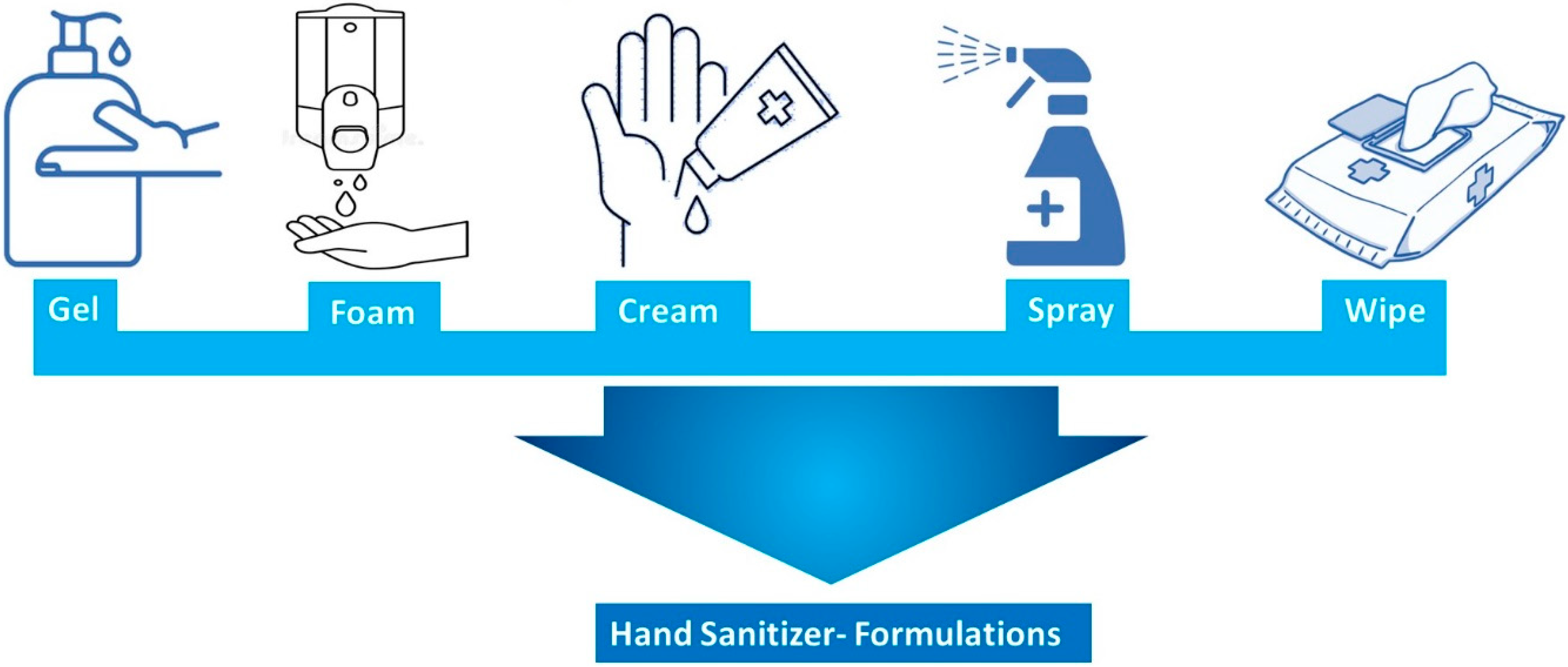
3.1. Types of Hand Sanitizer
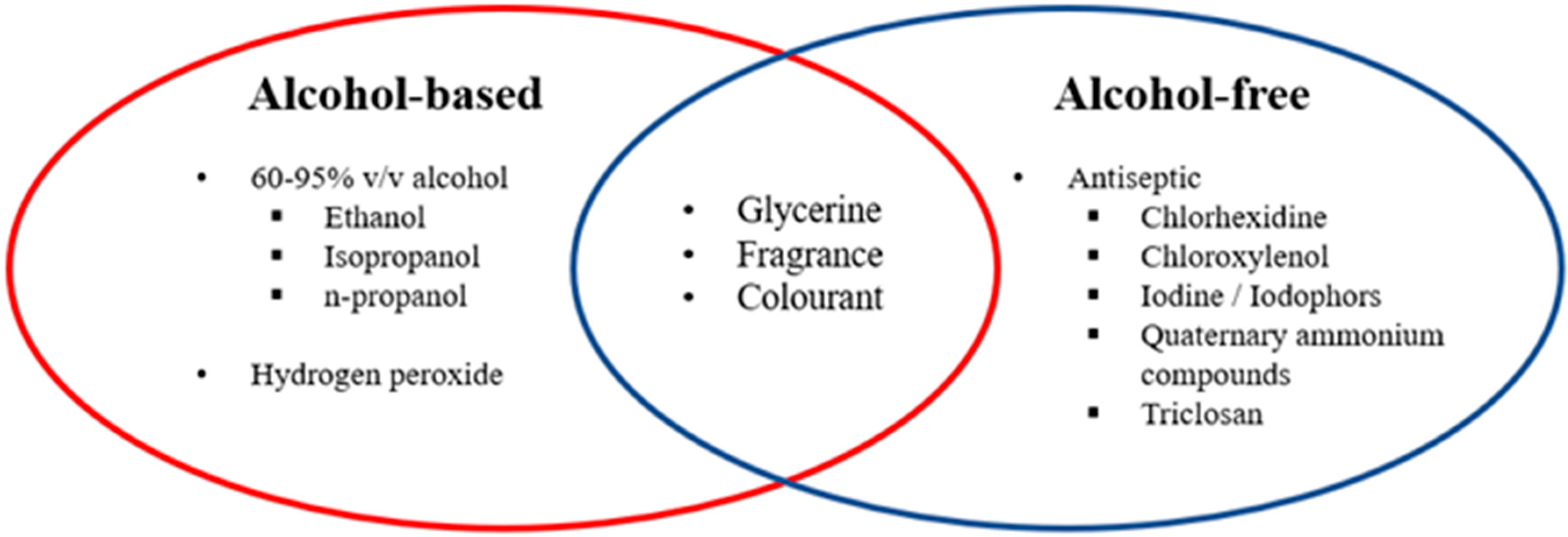
3.2. Alcohol and Soaps
3.3. Pharmaceutical Ingredients and Their Function
3.3.1. Chlorhexidine
3.3.2. Chloroxylenol
3.3.3. Iodine/Iodophors
3.3.4. Quaternary Ammonium Compounds
3.3.5. Triclosan
3.4. Physiology of Hand Skin
3.5. Efficacy of Alcohol-Based Hand Sanitizer against the Coronavirus
3.6. The Adverse Effects of Alcohol-Based Sanitizer or Handwashing Soaps
3.7. Recommendations to Minimize the Cutaneous Adverse Effects
3.8. Hand Hygiene Recommendations from CDC (USA), WHO and Malaysia Regulations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- COVID-19 Coronavirus 2019-nCov Statistics Update (Live): 4,122,912 Cases and 280,337 Deaths. Available online: https://virusncov.com/ (accessed on 8 May 2020).
- Situation Update Worldwide, as of 7 May 2020. Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 7 May 2020).
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.F.W.; Yuan, S.; Kok, K.H.; To, K.K.W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.Y.; Poon, R.W.S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Y.; Boquete-Suter, P.; Koch, D.; Pittet, D.; Kaiser, L. Survival of influenza virus on human fingers. Clin. Microbiol. Infect. 2014, 20, O58–O64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seto, W.H.; Tsang, D.; Yung, R.W.H.; Ching, T.Y.; Ng, T.K.; Ho, M.; Ho, L.M.; Peiris, J.S.M. Advisors of Expert SARS group of Hospital Authority Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003, 361, 1519–1520. [Google Scholar] [CrossRef] [Green Version]
- Kampf, G.; Kramer, A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin. Microbiol. Rev. 2004, 17, 863–893. [Google Scholar] [CrossRef] [Green Version]
- Hare, R.-M. Preferences of Possible People. In Preferences; Fehige, C., Ed.; W. de Gruyter: Berlin, Germany, 1998; Volume 29, pp. 399–405. [Google Scholar]
- Hulkower, R.L.; Casanova, L.M.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Inactivation of surrogate coronaviruses on hard surfaces by health care germicides. Am. J. Infect. Control 2011, 39, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Yu, I.T.; Xie, Z.H.; Tsoi, K.K.; Chiu, Y.L.; Lok, S.W.; Tang, X.P.; Hui, D.S.; Lee, N.; Li, Y.M.; Huang, Z.T.; et al. Why Did Outbreaks of Severe Acute Respiratory Syndrome Occur in Some Hospital Wards but Not in Others? Clin. Infect. Dis. 2007, 44, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Prevention of Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html (accessed on 8 May 2020).
- Manocha, S.; Walley, K.R.; Russell, J.A. Severe acute respiratory distress syndrome (SARS): A critical care perspective. Crit. Care Med. 2003, 31, 2684–2692. [Google Scholar] [CrossRef]
- Fendler, E.; Groziak, P. Efficacy of Alcohol-Based Hand Sanitizers Against Fungi and Viruses. Infect. Control Hosp. Epidemiol. 2002, 23, 61–62. [Google Scholar] [CrossRef]
- Gerberding, J.L.; Fleming, M.W.; Snider, D.E., Jr.; Thacker, S.B.; Ward, J.W.; Hewitt, S.M.; Wilson, R.J.; Heilman, M.A.; Doan, Q.M. Morbidity and Mortality Weekly Report Guideline for Hand Hygiene in Health-Care Settings; Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force; Centers for Disease Control: Atlanta, GA, USA, 2002; Volume 51.
- Ionidis, G.; Hübscher, J.; Jack, T.; Becker, B.; Bischoff, B.; Todt, D.; Hodasa, V.; Brill, F.H.H.; Steinmann, E.; Steinmann, J. Development and virucidal activity of a novel alcohol-based hand disinfectant supplemented with urea and citric acid. BMC Infect. Dis. 2016, 16, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, S.A.; Springthorpe, V.S.; Sattar, S.A.; Rivard, S.; Rahman, M. Potential role of hands in the spread of respiratory viral infections: Studies with human parainfluenza virus 3 and rhinovirus 14. J. Clin. Microbiol. 1991, 29, 2115–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattar, S.A. Microbicides and the environmental control of nosocomial viral infections. J. Hosp. Infect. 2004, 56, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Pandey, P.; Mahajan, R.; Dhasmana, D.C. Alcohol based hand sanitizers: Assurance and apprehensions revisited. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 558–563. [Google Scholar]
- Kramer, A.; Galabov, A.S.; Sattar, S.A.; Döhner, L.; Pivert, A.; Payan, C.; Wolff, M.H.; Yilmaz, A.; Steinmann, J. Virucidal activity of a new hand disinfectant with reduced ethanol content: Comparison with other alcohol-based formulations. J. Hosp. Infect. 2006, 62, 98–106. [Google Scholar] [CrossRef]
- Erasmus, V.; Daha, T.J.; Brug, H.; Richardus, J.H.; Behrendt, M.D.; Vos, M.C.; van Beeck, E.F. Systematic Review of Studies on Compliance with Hand Hygiene Guidelines in Hospital Care. Infect. Control Hosp. Epidemiol. 2010, 31, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Gold, N.A.; Avva, U. Alcohol Sanitizer; StatPearls Publishing: St. Petersburg, FL, USA, 2018. [Google Scholar]
- Mcdonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [Green Version]
- Van Asselt, A.J.; Te Giffel, M.C. Pathogen resistance and adaptation to disinfectants and sanitisers. In Understanding Pathogen Behaviour; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 484–506. [Google Scholar]
- Bloomfield, S.F.; Arthur, M. Mechanisms of inactivation and resistance of spores to chemical biocides. J. Appl. Bacteriol. 1994, 76, 91S–104S. [Google Scholar] [CrossRef]
- Visscher, M.; Davis, J.; Wickett, R. Effect of topical treatments on irritant hand dermatitis in health care workers. Am. J. Infect. Control 2009, 37, e1–e842. [Google Scholar] [CrossRef]
- Pittet, D. Compliance with hand disinfection and its impact on hospital-acquired infections. J. Hosp. Infect. 2001, 48, S40–S46. [Google Scholar] [CrossRef]
- Winnefeld, M.; Richard, M.A.; Drancourt, M.; Grob, J.J. Skin tolerance and effectiveness of two hand decontamination procedures in everyday hospital use. Br. J. Dermatol. 2000, 143, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, R.E.; Ormandy, K.; Fellows, C.; Hollowood, T. Impact of hand sanitizer format (gel/foam/liquid) and dose amount on its sensory properties and acceptability for improving hand hygiene compliance. J. Hosp. Infect. 2018, 100, 195–201. [Google Scholar] [CrossRef]
- Food and Drug Administration; HHS. Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Final Rule. Available online: https://www.fda.gov/media/109956/download (accessed on 8 May 2020).
- Compounding Expert Committee. Compounding Alcohol-Based Hand Sanitizer during COVID-19 Pandemic. Available online: https://www.usp.org/sites/default/files/usp/document/about/public-policy/usp-covid19-handrub.pdf (accessed on 8 May 2020).
- Song, X.; Vossebein, L.; Zille, A. Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: A review. Antimicrob. Resist. Infect. Control 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Hand Hygiene in Healthcare Settings; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Blaney, D.D.; Daly, E.R.; Kirkland, K.B.; Tongren, J.E.; Kelso, P.T.; Talbot, E.A. Use of alcohol-based hand sanitizers as a risk factor for norovirus outbreaks in long-term care facilities in northern New England: December 2006 to March 2007. Am. J. Infect. Control 2011, 39, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Oughton, M.T.; Loo, V.G.; Dendukuri, N.; Fenn, S.; Libman, M.D. Hand Hygiene with Soap and Water Is Superior to Alcohol Rub and Antiseptic Wipes for Removal of Clostridium difficile. Infect. Control Hosp. Epidemiol. 2009, 30, 939–944. [Google Scholar] [CrossRef]
- Kampf, G.; Marschall, S.; Eggerstedt, S.; Ostermeyer, C. Efficacy of ethanol-based hand foams using clinically relevant amounts: A cross-over controlled study among healthy volunteers. BMC Infect. Dis. 2010, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Stebbins, S.; Cummings, D.A.T.; Stark, J.H.; Vukotich, C.; Mitruka, K.; Thompson, W.; Rinaldo, C.; Roth, L.; Wagner, M.; Wisniewski, S.R.; et al. Reduction in the incidence of influenza A but not influenza B associated with use of hand sanitizer and cough hygiene in schools: A randomized controlled trial. Pediatr. Infect. Dis. J. 2011, 30, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Pinhas, A.R. A kinetic study using evaporation of different types of hand-rub sanitizers. J. Chem. Educ. 2010, 87, 950–951. [Google Scholar] [CrossRef]
- Coronado, G.D.; Holte, S.E.; Vigoren, E.M.; Griffith, W.C.; Barr, D.B.; Faustman, E.M.; Thompson, B. Do workplace and home protective practices protect farm workers? findings from the “For Healthy Kids” study. J. Occup. Environ. Med. 2012, 54, 1163–1169. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge: Clean Care Is Safer Care; World Health Organisation: Geneva, Switzerland, 2009. [Google Scholar]
- Centers for Disease Control and Prevention. Chemical Disinfectants—Guideline for Disinfection and Sterilization in Healthcare Facilities; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016.
- World Health Organization. Guide To Local Production: Who-Recommended Handrub Formulations; World Health Organisation: Geneva, Switzerland, 2015. [Google Scholar]
- Larson, E.L. APIC guidelines for handwashing and hand antisepsis in health care settings. AJIC Am. J. Infect. Control 1995, 23, 251–269. [Google Scholar] [CrossRef]
- Ison, S.; Beattie, M. Disinfection, sterilization and preservation (5th ed). Aust. Infect. Control 2002, 7, 74. [Google Scholar] [CrossRef]
- Walsh, B.; Blakemore, P.H.; Drabu, Y.J. The effect of handcream on the antibacterial activity of chlorhexidine gluconate. J. Hosp. Infect. 1987, 9, 30–33. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses: Methods and Protocols; Springer: New York, NY, USA, 2015; pp. 1–23. [Google Scholar]
- Larson, E. Guideline for use of topical antimicrobial agents. AJIC Am. J. Infect. Control 1988, 16, 253–266. [Google Scholar] [CrossRef]
- Larson, E.; Talbot, G.H. An approach for selection of health care personnel handwashing agents. Infect. Control 1986, 7, 419–424. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html (accessed on 8 May 2020).
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)–A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Anderson, R.L. Iodophor Antiseptics: Intrinsic Microbial Contamination with Resistant Bacteria. Infect. Control Hosp. Epidemiol. 1989, 10, 443–446. [Google Scholar] [CrossRef] [Green Version]
- Traoré, O.; Fayard, S.F.; Laveran, H. An in-vitro evaluation of the activity of povidone-iodine against nosocomial bacterial strains. J. Hosp. Infect. 1996, 34, 217–222. [Google Scholar] [CrossRef]
- Goldenheim, P.D. In vitro efficacy of povidone-iodine solution and cream against methicillin-resistant Staphylococcus aureus. Postgrad. Med. J. 1993, 69 (Suppl. 3), S62–S65. [Google Scholar]
- Davies, J.G.; Babb, J.R.; Bradley, C.R.; Ayliffe, G.A.J. Preliminary study of test methods to assess the virucidal activity of skin disinfectants using poliovirus and bacteriophages. J. Hosp. Infect. 1993, 25, 125–131. [Google Scholar] [CrossRef]
- Dexter, F.; Parra, M.C.; Brown, J.R.; Loftus, R.W. Perioperative COVID-19 Defense: An Evidence-Based Approach for Optimization of Infection Control and Operating Room Management. Anesth. Analg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.D.; Jampani, H.B.; Newman, J.L.; Lee, A.S. Triclosan: A review of effectiveness and safety in health care settings. Am. J. Infect. Control 2000, 28, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Fahimipour, A.K.; Ben Mamaar, S.; McFarland, A.G.; Blaustein, R.A.; Chen, J.; Glawe, A.J.; Kline, J.; Green, J.L.; Halden, R.U.; Van Den Wymelenberg, K.; et al. Antimicrobial Chemicals Associate with Microbial Function and Antibiotic Resistance Indoors. Am. Soc. Microbiol. 2018, 3, e00200-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honari, G.; Maibach, H. Skin Structure and Function. In Applied Dermatotoxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–10. [Google Scholar]
- Wickett, R.R.; Visscher, M.O. Structure and function of the epidermal barrier. Am. J. Infect. Control 2006, 34, S98–S110. [Google Scholar] [CrossRef]
- Feingold, K.R. Lamellar bodies: The key to cutaneous barrier function. J. Investig. Dermatol. 2012, 132, 1951–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans cells-programmed by the epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.; Mathew, S. Merkel Cells: A Collective Review of Current Concepts. Int. J. Appl. Basic Med. Res. 2019, 9, 9–13. [Google Scholar]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Nash, A.A.; Dalziel, R.G.; Fitzgerald, J.R. Attachment to and Entry of Microorganisms into the Body. In Mims’ Pathogenesis of Infectious Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 9–49. [Google Scholar]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19); StatPearls Publishing: St. Petersburg, FL, USA, 2020. [Google Scholar]
- Goldsmith, C.S.; Tatti, K.M.; Ksiazek, T.G.; Rollin, P.E.; Comer, J.A.; Lee, W.W.; Rota, P.A.; Bankamp, B.; Bellini, W.J.; Zaki, S.R. Ultrastructural Characterization of SARS Coronavirus. Emerg. Infect. Dis. 2004, 10, 320–326. [Google Scholar] [CrossRef] [PubMed]
- WHO. Annex 1 19th WHO Model List of Essential Medicines; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Siddharta, A.; Pfaender, S.; Vielle, N.J.; Dijkman, R.; Friesland, M.; Becker, B.; Yang, J.; Engelmann, M.; Todt, D.; Windisch, M.P.; et al. Virucidal Activity of World Health Organization-Recommended Formulations Against Enveloped Viruses, Including Zika, Ebola, and Emerging Coronaviruses. J. Infect. Dis. 2017, 215, 902–906. [Google Scholar] [CrossRef] [Green Version]
- Kampf, G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018, 98, 331–338. [Google Scholar] [CrossRef]
- Rabenau, H.F.; Kampf, G.; Cinatl, J.; Doerr, H.W. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005, 61, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, K.-P. Prevention of Surfactant-Induced Irritant Contact Dermatitis. In Current Problems in Dermatology; Karger Publishers: London, UK, 1996; Volume 25, pp. 78–85. [Google Scholar]
- Ale, I.S.; Maibach, H.I. Irritant contact dermatitis. Rev. Environ. Health 2014, 29, 195–206. [Google Scholar] [CrossRef]
- Misteli, H.; Weber, W.P.; Reck, S.; Rosenthal, R.; Zwahlen, M.; Fueglistaler, P.; Bolli, M.K.; Oertli, D.; Widmer, A.F.; Marti, W.R. Surgical glove perforation and the risk of surgical site infection. Arch. Surg. 2009, 144, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Larson, E.L.; Hughes, C.A.; Pyrek, J.D.; Sparks, S.M.; Cagatay, E.U.; Bartkus, J.M. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am. J. Infect. Control 1998, 26, 513–521. [Google Scholar] [CrossRef]
- Löffler, H.; Kampf, G.; Schmermund, D.; Maibach, H.I. How irritant is alcohol? Br. J. Dermatol. 2007, 157, 74–81. [Google Scholar] [CrossRef]
- Graham, M.; Nixon, R.; Burrell, L.J.; Bolger, C.; Johnson, P.D.R.; Grayson, M.L. Low rates of cutaneous adverse reactions to alcohol-based hand hygiene solution during prolonged use in a large teaching hospital. Antimicrob. Agents Chemother. 2005, 49, 4404–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelova-Fischer, I.; Dapic, I.; Hoek, A.K.; Jakasa, I.; Fischer, T.W.; Zillikens, D.; Kezic, S. Skin barrier integrity and natural moisturising factor levels after cumulative dermal exposure to alkaline agents in atopic dermatitis. Acta Derm. Venereol. 2014, 94, 640–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emilson, A.; Lindberg, M.; Forslind, B. The temperature effect of in vitro penetration of sodium lauryl sulfate and nickel chloride through human skin. Acta Derm. Venereol. 1993, 73, 203–207. [Google Scholar] [PubMed]
- Øhlenschlæger, J.; Friberg, J.; Ramsing, D.; Agner, T. Temperature dependency of skin susceptibility to water and detergents. Acta Derm. Venereol. 1996, 76, 274–276. [Google Scholar] [PubMed]
- Rosenberg, A.; Alatary, S.D.; Peterson, A.F. Safety and efficacy of the antiseptic chlorhexidine gluconate. Surg. Gynecol. Obstet. 1976, 143, 789–792. [Google Scholar]
- Ophaswongse, S.; Maibach, H.I. Alcohol dermatitis: Allergic contact dermatitis and contact urticaria syndrome: A review. Contact Dermat. 1994, 30, 1–6. [Google Scholar] [CrossRef]
- Cimiotti, J.P.; Marmur, E.S.; Nesin, M.; Hamlim-Cook, P.; Larson, E.L. Adverse reactions associated with an alcohol-based hand antiseptic among nurses in a neonatal intensive care unit. Am. J. Infect. Control 2003, 31, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Guin, J.D.; Goodman, J. Contact urticaria from benzyl alcohol presenting as intolerance to saline soaks. Contact Dermat. 2001, 45, 182–183. [Google Scholar] [CrossRef]
- De Groot, A.C. Contact allergy to cosmetics: Causative ingredients. Contact Dermat. 1987, 17, 26–34. [Google Scholar] [CrossRef]
- Podda, M.; Zollner, T.; Grundmann-Kollmann, M.; Kaufmann, R.; Boehncke, W.H. Allergic contact dermatitis from benzyl alcohol during topical antimycotic treatment. Contact Dermat. 1999, 41, 302–303. [Google Scholar] [CrossRef]
- Bissett, L. Skin care: An essential component of hand hygiene and infection control. Br. J. Nurs. 2007, 16, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.; Leyden, J.J.; McGinley, K.J.; Grove, G.L.; Talbot, G.H. Physiologic and microbiologic changes in skin related to frequent handwashing. Infect. Control 1986, 7, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.L.; Aiello, A.E.; Bastyr, J.; Lyle, C.; Stahl, J.; Cronquist, A.; Lai, L.; Della-Latta, P. Assessment of two hand hygiene regimens for intensive care unit personnel. Crit. Care Med. 2001, 29, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Kelliher, S.; Vallande, N. Skin Irritation and Dryness Associated With Two Hand-Hygiene Regimens: Soap-and-Water Hand Washing Versus Hand Antisepsis With an Alcoholic Hand Gel. Infect. Control Hosp. Epidemiol. 2000, 21, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Kantor, R.; Silverberg, J.I. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 15–26. [Google Scholar] [CrossRef]
- Quality Medical Care Section; Medical Development Division; Ministry of Health Malaysia. Policies and Procedures on Infection Control. 2009. Available online: https://www.moh.gov.my/moh/images/gallery/Polisi/infection_control.pdf (accessed on 23 March 2020).
- Hadaway, A. Handwashing: Clean Hands Save Lives. J. Consum. Health Internet 2020, 24, 43–49. [Google Scholar] [CrossRef]

| Chemical Group | Examples | Mechanism of Action |
|---|---|---|
| Alcohol |
| Denaturation of proteins in the plasma membrane |
| Chlorine compounds |
| Halogenation/oxidation of cellular proteins |
| Iodine compounds |
| Iodine can easily penetrate through the cell membranes of pathogens Followed by attacking vital proteins, nucleotides and fatty acids of cell |
| Quaternary ammonium compounds |
| Lower surface tension Inactivate enzymes Degrade cell-proteins |
| Peroxygens |
| Free-radical oxidation of essential cell components |
| (Bis) phenols | Triclosan | Penetrate cytoplasmic bilayer |
| Biguanide | Chlorhexidine | Ionic interaction Disrupt cell membrane |
| Active Ingredient | Patient Antiseptic Skin Preparations | Healthcare Personal Hand Wash | Healthcare Personal Hand Rub | Surgical Hand Scrub | Surgical Hand Scrub |
|---|---|---|---|---|---|
| Alcohol 60%–95% | Y | N | Y | N | Y |
| Benzalkonium chloride | Y | Y | Y | Y | N |
| Benzethonium chloride | Y | Y | N | Y | N |
| Chlorhexidine gluconate | N | N | N | N | N |
| Chloroxylenol | Y | Y | N | Y | N |
| Cloflucarban | Y | Y | N | Y | N |
| Fluorosalan | Y | Y | N | Y | N |
| Hexylresorcinol | Y | Y | N | Y | N |
| Iodine complex (ammonium ether sulfate and polyoxyethylene sorbitan monolaurate) | N | Y | N | Y | N |
| Iodine complex (phosphate ester of alkylaryloxy polyethylene glycol) | Y | Y | N | Y | N |
| Iodine tincture United States Pharmacopeia (USP) | Y | N | N | N | N |
| Iodine topical solution USP | Y | N | N | N | N |
| Nonylphenoxypoly (ethyleneoxy) ethanoliodine | Y | Y | N | Y | N |
| Poloxamer-iodine complex | Y | Y | N | Y | N |
| Povidone-iodine 5%–10% | Y | Y | N | Y | N |
| Undecoylium chloride iodine complex | Y | Y | N | Y | N |
| Isopropyl alcohol 70%–91.3% | Y | N | Y | N | Y |
| Mercufenol chloride | Y | N | N | N | N |
| Methylbenzethonium chloride | Y | Y | N | Y | N |
| Phenol (equal to or less than 1.5%) | Y | Y | N | Y | N |
| Phenol (greater than 1.5%) | Y | Y | N | Y | N |
| Secondary amyltricresols | Y | Y | N | Y | N |
| Sodium oxychlorosene | Y | Y | N | Y | N |
| Triclocarban | Y | Y | N | Y | N |
| Triclosan | Y | Y | N | Y | N |
| Combinations: Calomel, oxyquinoline benzoate, triethanolamine, and phenol derivative | Y | N | N | N | N |
| Combinations: Mercufenol chloride and secondary amyltricresols in 50% alcohol | Y | N | N | N | N |
| Combinations: Triple dye | Y | N | N | N | N |
| Components | Formulation 1: Ethanol Antiseptic 80% Topical Solution | Formulation 2: Isopropyl Alcohol Antiseptic 75% Topical Solution | Formulation 3: Isopropyl Alcohol Antiseptic 75% Topical Solution |
|---|---|---|---|
| Ethanol 96% | 833.3 mL | - | - |
| Isopropyl Alcohol 99% | - | 757.6 mL | - |
| Isopropyl Alcohol 91% | - | - | 824.2 mL |
| Hydrogen Peroxide 3% | 41.7 mL | 41.7 mL | 41.7 mL |
| Glycerol 98% | 14.5 mL | 7.5 mL | 7.5 mL |
| Water *, sufficient quantity to make | 1000 mL | 1000 mL | 1000 mL |
| Ingredient | Function | Remarks |
|---|---|---|
| Alcohol-Based | ||
| Alcohol | Denatures protein and lipid membrane of microorganisms. | Optimum concentration 60%–95%. |
| Hydrogen peroxide | Inactivates contaminating spores in the bulk solutions or excipients. |
|
| Non-Alcohol Based | ||
| Chlorhexidine gluconate | Inhibits the growth of microorganisms on living tissues. |
|
| Chloroxylenol |
| |
| Iodine/Iodophors |
| |
Quaternary ammonium compounds
|
| |
| Triclosan |
| |
| Excipients | ||
| Glycerol | Acts as a humectant that maintains the skin moisture. | A lower concentration is considered to reduce the stickiness of the formulation. |
| Essential oils | Antibacterial, antiviral, antimicrobial and antiseptic properties, Flavoring agent | |
| Xanthum gum, polyacrylic acid and polyethylene glycol | Thickening agents | To enhance the viscosity of products |
| Fragrance and colorant | Aesthetic
| May cause allergic reactions. |
| Formulation | Concentration | Exposure Time (s) | Efficacy Against SARS CoV | Ref |
|---|---|---|---|---|
| 45% propan-2-ol (w/w) 30% propan-1-ol (w/w) 0.2% mecetronium ethyl sulphate | Undiluted | 30 | RF: ≥4.25 | [75] |
| 80% ethanol (w/w) | Undiluted | 30 | RF: ≥4.25 | |
| 85% ethanol (w/w) | Undiluted | 30 | RF: ≥5.5 | |
| 95% ethanol (w/w) | Undiluted | 30 | RF: ≥5.5 | |
| 85% ethanol (v/v) 0.725% glycerol (v/v) 0.125% hydrogen peroxide (v/v) | 20% | 30 | Log10 of viral infection: 7 | [73] |
| 40%–80% | 30 | Log10 of viral infection: Undetectable level | ||
| 75% isopropanol (w/w) 0.725% glycerol (v/v) 0.125% hydrogen peroxide (v/v) | 20% | 30 | Log10 of viral infection: 6.8 | |
| 40%–80% | 30 | Log10 of viral infection: Undetectable level |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, J.L.J.; Pei Yi, T.; Bose, R.J.C.; McCarthy, J.R.; Tharmalingam, N.; Madheswaran, T. Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. Int. J. Environ. Res. Public Health 2020, 17, 3326. https://doi.org/10.3390/ijerph17093326
Jing JLJ, Pei Yi T, Bose RJC, McCarthy JR, Tharmalingam N, Madheswaran T. Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. International Journal of Environmental Research and Public Health. 2020; 17(9):3326. https://doi.org/10.3390/ijerph17093326
Chicago/Turabian StyleJing, Jane Lee Jia, Thong Pei Yi, Rajendran J. C. Bose, Jason R. McCarthy, Nagendran Tharmalingam, and Thiagarajan Madheswaran. 2020. "Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations" International Journal of Environmental Research and Public Health 17, no. 9: 3326. https://doi.org/10.3390/ijerph17093326
APA StyleJing, J. L. J., Pei Yi, T., Bose, R. J. C., McCarthy, J. R., Tharmalingam, N., & Madheswaran, T. (2020). Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. International Journal of Environmental Research and Public Health, 17(9), 3326. https://doi.org/10.3390/ijerph17093326






