Incidence of Thyroid Cancer in Italian Contaminated Sites
Abstract
:1. Introduction
2. Materials and Methods
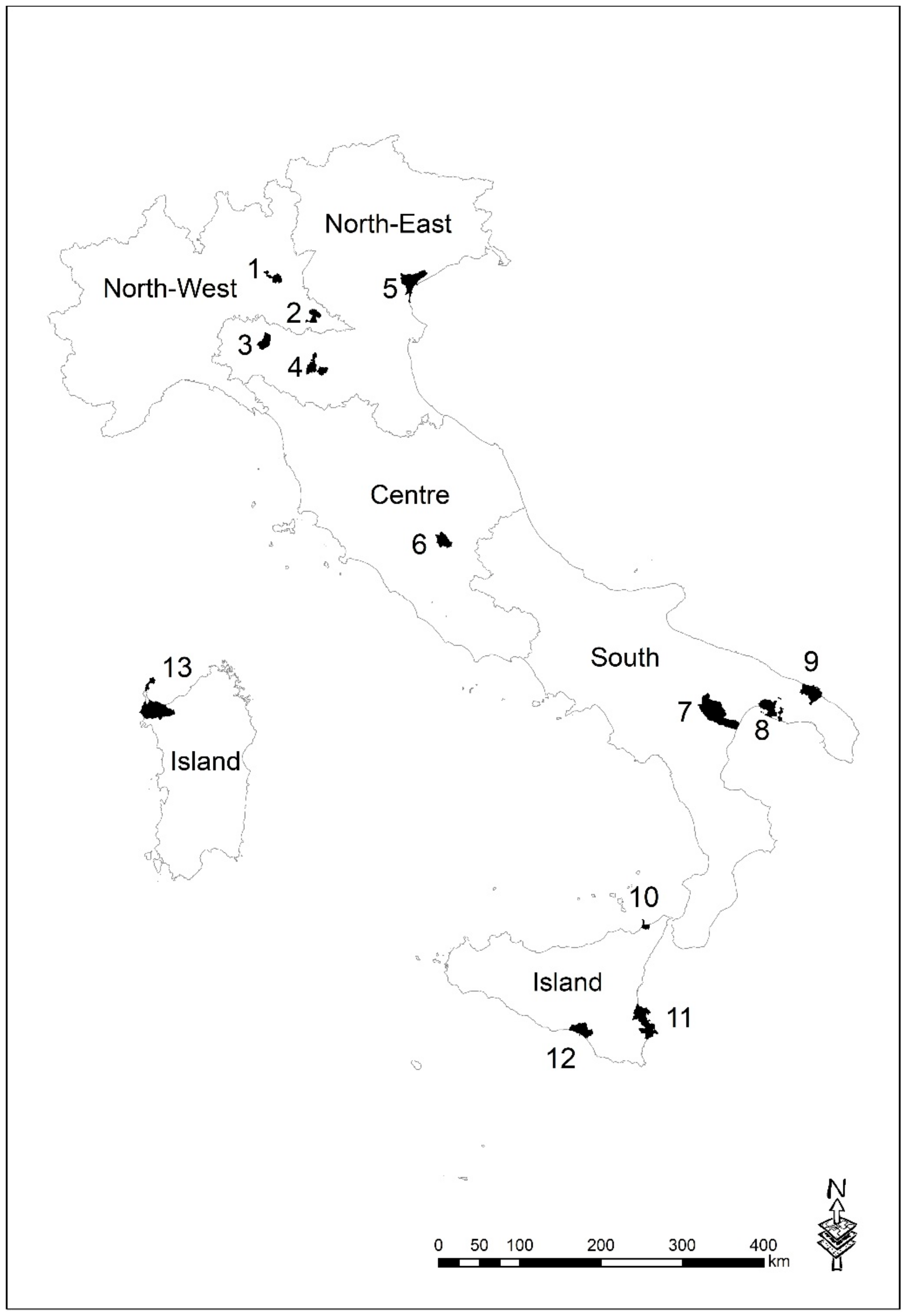
2.1. NPCSs Description
2.2. Study Design
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIRTUM | Italian Association of Cancer Registries |
| As | Arsenic |
| Cd | Cadmium |
| CI | Confidence Interval |
| COD | Chemical Oxygen Demand |
| BPA | Bisphenol A |
| EDs | Endocrine disruptors |
| IARC | International Agency for Research on cancer |
| HCB | Hexachlorobenzene |
| NPCSs | National Priority Contaminated Sites |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PBDEs | Polybrominated Diphenyl Ethers |
| PCBs | Polychlorinated Biphenyls |
| PCDDs | Polychlorinated Dibenzo-p-dioxins |
| PCDFs | Polychlorinated Dibenzofurans |
| Pb | Lead |
| SIRs | Standardized Incidence Ratios |
| TC | Thyroid cancer |
| TCDD | 2,3,7,8-Tetrachlorodibenzo-p-dioxin |
| WHO | World Health Organization |
Appendix A
| Name | Affiliation |
| Antonino Ardizzone | Registro tumori Brindisi |
| Adele Caldarella | Registro tumori toscano, Istituto per lo studio, la prevenzione e la rete oncologica (ISPRO), Firenze |
| Lorenza Boschetti | Registro tumori Pavia |
| Angelita Brustolin | Registro tumori Viterbo c/o Dipartimento prevenzione ASL Viterbo |
| Rossella Cavallo | Registro tumori Salerno |
| Giuseppina Candela | Registro tumori Trapani |
| Giuliano Carrozzi | Registro tumori Modena |
| Luca Cavalieri D’Oro | Registro tumori ATS della Brianza |
| Rosaria Cesareccio | Registro tumori Sassari |
| Giorgio Chiaranda | Registro tumori Piacenza |
| Maria Lia Contrino | Registro tumori integrato Catania-Messina-Siracusa-Enna |
| Antonella Dal Cin | Registro tumori Veneto |
| Fabio Falcini | Registro tumori Romagna |
| Anna Clara Fanetti | Registro tumori Sondrio |
| Stefano Ferretti | Registro tumori Ferrara |
| Rosa Filiberti | Registro tumori Genova |
| Rocco Galasso | Registro tumori Basilicata |
| Iolanda Grappasonni | Registro tumori infantili Marche c/o Università di Camerino |
| Silvia Iaccovacci | Registro tumori Latina |
| Michele Magoni | Registro tumori Brescia |
| Lucia Mangone | Registro tumori Reggio Emilia |
| Guido Mazzoleni | Registro tumori Alto Adige |
| Anna Melcarne | Registro tumori Lecce |
| Maria Michiara | Registro tumori Parma |
| Aldo Minerba | Registro tumori Taranto |
| Fernando Palma | Registro tumori Foggia |
| Silvano Piffer | Registro tumori della provincia di Trento c/o Azienda provinciale servizi sanitari, Provincia di Trento |
| Paolo Ricci | Registro tumori Mantova |
| Stefano Rosso | Registro tumori del Piemonte |
| Antonio Giampiero Russo | Registro tumori ATS della Città Metropolitana di Milano |
| Carlotta Sacerdote | Registro tumori infantili Piemonte |
| Giuseppe Sampietro | Registro tumori della ATS di Bergamo |
| Salvatore Sciacca | Registro tumori integrato Catania-Messina-Siracusa-Enna |
| Antonella Sutera | Registro tumori Catanzaro |
| Giovanna Tagliabue | Registro tumori di Varese, Fondazione IRCCS Istituto Nazionale dei Tumori |
| Rosario Tumino | Registro Tumori Ragusa-Caltanissetta, ASP 7 Ragusa |
| Mario Usala | Registro tumori Nuoro |
| Francesco Vitale | Registro tumori Palermo e provincia c/o UOC epidemiologia clinica, Azienda ospedaliero-universitaria Policlinico P. Giaccone, Palermo |
| Registro Tumori Barletta | Contributed by providing cancer registry data for the calculation of reference rates |
References
- Bernier, M.O.; Withrow, D.R.; Berrington de Gonzalez, A.; Lam, C.J.K.; Linet, M.S.; Kitahara, C.M.; Shiels, M.S. Trends in pediatric thyroid cancer incidence in the United States, 1998–2013. Cancer 2019, 125, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Araque, D.V.P.; Bleyer, A.; Brito, J.P. Thyroid cancer in adolescents and young adults. Future Oncol. 2017, 13, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L. The Current Histologic Classification of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 1–22. [Google Scholar] [CrossRef] [PubMed]
- James, B.C.; Mitchell, J.M.; Jeon, H.D.; Vasilottos, N.; Grogan, R.H.; Aschebrook-Kilfoy, B. An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control. 2018, 29, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dal Maso, L.; Vaccarella, S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020, 8, 468–470. [Google Scholar] [CrossRef]
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Maso, L.D. Worldwide thyroid-cancer epidemic? The increasing impact of Overdiagnosis. N. Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, R.; Vella, V.; Pellegriti, G.; Belfiore, A. Editorial: Clinical and Molecular Epidemiology of Thyroid Cancer of Follicular Origin. Front. Endocrinol. 2018, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanabria, A.; Kowalski, L.P.; Shah, J.P.; Nixon, I.J.; Angelos, P.; Williams, M.D.; Rinaldo, A.; Ferlito, A. Growing incidence of thyroid carcinoma in recent years: Factors underlying overdiagnosis. Head Neck 2018, 40, 855–866. [Google Scholar] [CrossRef]
- Paulson, V.A.; Rudzinski, E.R.; Hawkins, D.S. Thyroid Cancer in the Pediatric Population. Genes (Basel) 2019, 10, 723. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.Y.; Davies, L. Children and thyroid cancer: Interpreting troubling trends. Cancer 2019, 125, 2359–2361. [Google Scholar] [CrossRef]
- Lortet-Tieulent, J.; Franceschi, S.; Dal Maso, L.; Vaccarella, S. Thyroid cancer “epidemic” also occurs in low- and middle-income countries. Int. J. Cancer 2019, 144, 2082–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, M.; Oliveri Conti, G.; Caltabiano, R.; Buffone, A.; Zuccarello, P.; Cormaci, L.; Cannizzaro, M.A.; Ferrante, M. Role of Emerging Environmental Risk Factors in Thyroid Cancer: A Brief Review. Int. J. Environ. Res. Public Health 2019, 16, 1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, H.; Rafei, M.; Bahrami, M.; Haghdoost, A.; Shabani, Y. Attributable risk fraction of four lifestyle risk factors of thyroid cancer: A meta-analysis. J. Public Health 2018, 40, e91–e98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, K.J.; Chiamolera, M.I.; Giannocco, G.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. Thyroid Function Disruptors: From nature to chemicals. J. Mol. Endocrinol. 2019, 62, R1–R19. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Calsolaro, V.; Pasqualetti, G.; Niccolai, F.; Caraccio, N.; Monzani, F. Thyroid Disrupting Chemicals. Int. J. Mol. Sci. 2017, 18, 2583. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization/United Nations Environment Programme (WHO/UNEP). State of the Science of Endocrine Disrupting Chemicals 2012; Bergman, A., Jerrold, J., Heindel, J.J., Jobling, S., Karen, A., Kidd, K.A., Zoeller, R.T., Eds.; An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme and World Health Organization; WHO: Geneva, Switzerland; UNEP: Nerobi, Kenya, 2013; Available online: http://www.who.int/ceh/publications/endocrine/en/ (accessed on 10 September 2020).
- European Commission; Kortenkamp, A.; Evans, R.; Martin, O.; McKinlay, R.; Orton, F.; Rosivatz, E. State of the Art Assessment of Endocrine Disruptors, Final Report, Annex 1, Revised Version, 29 January 2012. Available online: http://ec.europa.eu/environment/chemicals/endocrine/pdf/annex1_summary_state_of_sciece.pdf (accessed on 10 September 2020).
- European Environment Agency. The Impact of Endocrine Disrupters on Wildlife, People and Their Environments-the Weybrige+15 (1996–2011) Technical Report N. 2/2012. Available online: http://www.eea.europa.eu/publications/the-impacts-of-endocrine-disrupters (accessed on 10 September 2020).
- Zhang, C.; Wu, H.B.; Cheng, M.X.; Wang, L.; Gao, C.B.; Huang, F. Association of exposure to multiple metals with papillary thyroid cancer risk in China. Environ. Sci. Pollut. Res. Int. 2019, 26, 20560–20572. [Google Scholar] [CrossRef]
- Stojsavljević, A.; Rovčanin, B.; Krstić, Đ.; Borković-Mitić, S.; Paunović, I.; Kodranov, I.; Gavrović-Jankulović, M.; Manojlović, D. Evaluation of trace metals in thyroid tissues: Comparative analysis with benign and malignant thyroid diseases. Ecotoxicol. Environ. Saf. 2019, 183, 109479. [Google Scholar] [CrossRef]
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Jiang, C.; Li, H.; Zhang, C.; Wu, H.; Huang, F. Effect of the Interaction Between Cadmium Exposure and CLOCK Gene Polymorphisms on Thyroid Cancer: A Case-Control Study in China. Biol. Trace Elem. Res. 2020, 196, 86–95. [Google Scholar] [CrossRef]
- Jancic, S.A.; Stosic, B.Z. Cadmium effects on the thyroid gland. Vitam. Horm. 2014, 94, 391–425. [Google Scholar] [PubMed]
- Le, K.T.; Sawicki, M.P.; Wang, M.B.; Hershman, J.M.; Leung, A.M. High prevalence of agent orange exposure among thyroid cancer patients in the National VA Healthcare System. Endocr. Pract. 2016, 22, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Iervasi, G.; Coi, A.; Pitto, L.; Bianchi, F. The Role of Polybrominated Diphenyl Ethers in Thyroid Carcinogenesis: Is It a Weak Hypothesis or a Hidden Reality? From Facts to New Perspectives. Int. J. Environ. Res. Public. Health 2018, 15, 1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, K.; Lorenzo, A.; Butt, C.M.; Hammel, S.C.; Henderson, B.B.; Roman, S.A.; Scheri, R.P.; Stapleton, H.M.; Sosa, J.A. Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: A case-control study. Environ. Int. 2017, 107, 235–242. [Google Scholar] [CrossRef]
- Mughal, B.B.; Demeneix, B.A. Endocrine disruptors: Flame retardants and increased risk of thyroid cancer. Nat. Rev. Endocrinol. 2017, 13, 627–628. [Google Scholar] [CrossRef]
- Deziel, N.C.; Yi, H.; Stapleton, H.M.; Huang, H.; Zhao, N.; Zhang, Y. A case-control study of exposure to organophosphate flame retardants and risk of thyroid cancer in women. BMC Cancer 2018, 18, 637. [Google Scholar] [CrossRef]
- Miao, H.; Liu, X.; Li, J.; Zhang, L.; Zhao, Y.; Liu, S.; Ni, S.; Wu, Y. Associations of urinary phthalate metabolites with risk of papillary thyroid cancer. Chemosphere 2020, 241, 125093. [Google Scholar] [CrossRef]
- Marotta, V.; Russo, G.; Gambardella, C.; Grasso, M.; La Sala, D.; Chiofalo, M.G.; D’Anna, R.; Puzziello, A.; Docimo, G.; Masone, S.; et al. Human exposure to bisphenol AF and diethylhexylphthalate increases susceptibility to develop differentiated thyroid cancer in patients with thyroid nodules. Chemosphere 2019, 218, 885–894. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, J.; Jiang, F.; Xie, Y.; Zhang, X.; Jiang, L. Higher urinary bisphenol A concentration and excessive iodine intake are associated with nodular goiter and papillary thyroid carcinoma. Biosci. Rep. 2017, 37, BSR20170678. [Google Scholar] [CrossRef] [Green Version]
- Malandrino, P.; Russo, M.; Gianì, F.; Pellegriti, G.; Vigneri, P.; Belfiore, A.; Rizzarelli, E.; Vigneri, R. Increased Thyroid Cancer Incidence in Volcanic Areas: A Role of Increased Heavy Metals in the Environment? Int. J. Mol. Sci. 2020, 21, 3425. [Google Scholar] [CrossRef]
- Uetani, M.; Kobayashi, E.; Suwazono, Y.; Honda, R.; Nishijo, M.; Nakagawa, H.; Kido, T.; Nogawa, K. Tissue cadmium (Cd) concentrations of people living in a Cd polluted area, Japan. Biometals 2006, 19, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Grimalt, J.O.; Sunyer, J.; Moreno, V.; Amaral, O.C.; Sala, M.; Rosell, A.; Anto, J.M.; Albaiges, J. Risk excess of soft-tissue sarcoma and thyroid cancer in a community exposed to airborne organochlorinated compound mixtures with a high hexachlorobenzene content. Int. J. Cancer 1994, 56, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Navarro, P.; García-Pérez, J.; Ramis, R.; Boldo, E.; López-Abente, G. Proximity to mining industry and cancer mortality. Sci. Total Environ. 2012, 435–436, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kloczko, N. Residential Proximity to Manufacturing Facilities and Risk of Thyroid Cancer. Public Health Theses. Master’s Thesis, School of Public Health, Yale University, New Haven, CT, USA, 1 January 2016. [Google Scholar]
- Benedetti, M.; Zona, A.; Beccaloni, E.; Carere, M.; Comba, P. Incidence of Breast, Prostate, Testicular, and Thyroid Cancer in Italian Contaminated Sites with Presence of Substances with Endocrine Disrupting Properties. Int. J. Environ. Res. Public Health 2017, 14, 355. [Google Scholar] [CrossRef] [Green Version]
- Fei, X.; Chen, W.; Zhang, S.; Liu, Q.; Zhang, Z.; Pei, Q. The spatio-temporal distribution and risk factors of thyroid cancer during rapid urbanization-A case study in China. Sci. Total Environ. 2018, 630, 1436–1445. [Google Scholar] [CrossRef]
- Arias-Ortiz, N.E.; Icaza-Noguerad, G.; Ruiz-Rudolphe, P. Thyroid cancer incidence in women and proximity to industrial air pollution sources: A spatial analysis in a middle size city in Colombia. Atmos. Pollut. Res. 2018, 9, 464–574. [Google Scholar] [CrossRef]
- Zona, A.; Pasetto, R.; Fazzo, L.; Iavarone, I.; Bruno, C.; Pirastu, R.; Comba, P. SENTIERI—Studio Epidemiologico Nazionale dei Territori e degli Insediamenti Esposti a Rischio da Inquinamento: Quinto Rapporto, e Gruppi di Lavoro SENTIERI, AIRTum SENTIERI e Malformazioni congenite. Epidemiol. Prev. 2019, 43 (Suppl. S1), 1–208. [Google Scholar]
- Reiners, C.; Demidchik, Y.E.; Drozd, V.M.; Biko, J. Thyroid cancer in infants and adolescents after Chernobyl. Minerva Endocrinol. 2008, 33, 381–395. [Google Scholar]
- Mahoney, M.C.; Lawvere, S.; Falkner, K.L.; Averkin, Y.I.; Ostapenko, V.A.; Michalek, A.M.; Moysich, K.B.; McCarthy, P.L. Thyroid cancer incidence trends in Belarus: Examining the impact of Chernobyl. Int. J. Epidemiol. 2004, 33, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Mitro, S.D.; Rozek, L.S.; Vatanasapt, P.; Suwanrungruang, K.; Chitapanarux, I.; Srisukho, S.; Sriplung, H.; Meza, R. Iodine deficiency and thyroid cancer trends in three regions of Thailand, 1990–2009. Cancer Epidemiol. 2016, 43, 92–99. [Google Scholar] [CrossRef]
- Zane, M.; Parello, C.; Pennelli, G.; Townsend, D.M.; Merigliano, S.; Boscaro, M.; Toniato, A.; Baggio, G.; Pelizzo, M.R.; Rubello, D.; et al. Estrogen and thyroid cancer is a stem affair: A preliminary study. Biomed. Pharmacother. 2017, 85, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldien, M.M.S.; Abdou, A.G.; Rageh, T.; Abdelrazek, E.; Elkholy, E. Immunohistochemical expression of ER-α and PR in papillary thyroid carcinoma. Ecancermedicalscience 2017, 11, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosé, V. Familial thyroid cancer: A review. Mod. Pathol. 2011, 24, S19–S33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitahara, C.M.; Sosa, J.A. Understanding the ever-changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2020, 16, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.G.; Guo, X.G.; Ba, C.X.; Wang, W.; Yang, Y.Y.; Wang, J.; Yang, Y.Y.; Wang, J.; Cao, H.Y. Overweight, obesity and thyroid cancer risk: A meta-analysis of cohort studies. J. Int. Med. Res. 2012, 40, 2041–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Heindel, J.J. History of the Obesogen Field: Looking Back to Look Forward. Front. Endocrinol. 2019, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Vita, R.; Ieni, A.; Tuccari, G.; Benvenga, S. The increasing prevalence of chronic lymphocytic thyroiditis in papillary microcarcinoma. Rev. Endocr. Metab. Disord. 2018, 19, 301–309. [Google Scholar] [CrossRef]
- Zaccarelli-Marino, M.A. Chronic autoimmune thyroiditis in industrial areas in Brazil: A 15-year survey. J. Clin. Immunol. 2012, 32, 1012–1018. [Google Scholar] [CrossRef]
- Musmeci, L.; Bellino, M.; Falleni, F.; Piccardi, A. Environmental characterization of the National Contaminated Sites in SENTIERI project. Epidemiol. Prev. 2011, 35, 20–23. [Google Scholar] [PubMed]
- Beccaloni, E.; Cicero, M.R.; Falleni, F.; Piccardi, A.; Scaini, F.; Soggiu, M.E.; Vanni, F.; Carere, M. Environmental characterization and exposure evaluation. Epidemiol. Prev. 2014, 38, 137–143. [Google Scholar] [PubMed]
- Miniero, R.; Ingelido, A.M.; Abballe, A.; di Domenico, A.; Valentinia, S.; Marra, V.; Barbieri, P.G.; Garattini, S.; Speziani, F.; De Felip, E. Occupational exposure to PCDDs, PCDFs, and DL-PCBs in metallurgical plants of the Brescia (Lombardy Region, northern Italy) area. Chemosphere 2017, 166, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Turrio-Baldassarri, L.; Abate, V.; Battistelli, C.L.; Carasi, C.; Casella, M.; Iacovella, N.; Indelicato, A.; La Rocca, C.; Scarcella, C.; Alivernini, S. PCDD/F and PCB in human serum of differently exposed population groups of an Italian city. Chemosphere 2008, 73, S228–S234. [Google Scholar] [CrossRef]
- Turrio-Baldassarri, L.; Alivernini, S.; Carasi, S.; Casella, M.; Fuselli, S.; Iacovella, N.; Iamiceli, A.L.; La Rocca, C.; Scarcella, C.; Battistelli, C.L. PCB, PCDD and PCDF contamination of food of animal origin as the effect of soil pollution and the cause of human exposure in Brescia. Chemosphere 2009, 76, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Bustaffa, E.; Cori, L.; Imbriani, M.; Minichilli, F.; Migliore, S.; Minoia, C.; Ronchi, A.; Turci, R. Human biomonitoring in the area around the petrochemical site of Gela, Sicily, Italy. Epidemiol. Prev. 2010, 34, 82. [Google Scholar]
- Sarcomi ed Esposizione a Sostanze Diossino-Simili in Mantova. Consensus Report [Sarcoma and Exposure to Dioxin-Like Chemicals in Mantua. Consensus Report]. ASL Provincia di Mantova 2007. Available online: http://cdca.it/wp-content/uploads/2017/05/mantova_ASL.pdf (accessed on 16 September 2010).
- Grassi, P.; Fattore, E.; Generoso, C.; Fanelli, R.; Arvati, M.; Zuccato, E. Polychlorobiphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in fruit and vegetables from an industrial area in northern Italy. Chemosphere 2010, 79, 292–298. [Google Scholar] [CrossRef]
- Interdonato, M.; Bitto, A.; Pizzino, G.; Irrera, N.; Pallio, G.; Mecchio, A.; Cuspilici, A.; Minutoli, L.; Altavilla, D.; Squadrito, F. Levels of heavy metals in adolescents living in the industrialised area of Milazzo-Valle del Mela (Northern Sicily). J. Environ. Public Health 2014, 2014, 326845. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.M.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy metals and PAHs in meat, milk, and seafood from Augusta Area (Southern Italy): Contamination levels, dietary intake, and human exposure assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Baldassarre, A.; Gatti, M.F.; Gagliardi, T.; Serinelli, M.; De Maria, L.; Caputi, A.; Dirodi, A.A.; Galise, I.; Cuccaro, F.; et al. Non-occupational exposure to heavy metals of the residents of an industrial area and biomonitoring. Environ. Monit. Assess. 2016, 188, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingelido, A.M.; Abate, V.; Abballe, A.; Albano, F.L.; Battista, T.; Carraro, V.; Conversano, M.; Corvetti, R.; De Luca, S.; Franchini, S.; et al. Concentrations of polychlorinated dibenzodioxins, polychlorodibenzofurans, and polychlorobiphenyls in women of reproductive age in Italy: A human biomonitoring study. Int. J. Hyg. Environ. Health 2017, 220, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Zianni, R.; Anzillotta, G.; Palma, A.; Vitacco, V.; Scrano, L.; Cataldi, T.R. Dibenzo-p-dioxins and dibenzofurans in human breast milk collected in the area of Taranto (Southern Italy): First case study. Anal. Bioanal. Chem. 2013, 405, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Cuccaro, F.; Serinelli, M.; Bisceglia, L.; Galise, I.; Conversano, M.; Minerba, S.; Mincuzzi, A.; Martino, T.; Storelli, M.A.; et al. Exposure assessment to heavy metals in general population in a polluted area through biological monitoring. In Proceedings of the 16th International Conference on Heavy Metals in the Environment, Rome, Italy, 23–27 September 2013. [Google Scholar]
- Iavarone, I.; De Felip, E.; Ingelido, A.M.; Iacovella, N.; Abballe, A.; Valentini, S.; Marra, V.; Violante, N.; D’Ilio, S.; Senofonte, O.; et al. Exploratory biomonitoring study among workers of livestock farms of the Taranto Province. Epidemiol. Prev. 2012, 36, 321–331. [Google Scholar] [PubMed]
- Giandomenico, S.; Spada, L.; Annicchiarico, C.; Assennato, G.; Cardellicchio, N.; Ungaro, N.; Di Leo, A. Chlorinated compounds and polybrominated diphenyl ethers (PBDEs) in mussels (Mytilus galloprovincialis) collected from Apulia Region coasts. Mar. Pollut. Bull. 2013, 73, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Cardellicchio, N.; Buccolieri, A.; Giandomenico, S.; Lopez, L.; Pizzulli, F.; Spada, L. Organic pollutants (PAHs, PCBs) in sediments from the Mar Piccolo in Taranto (Ionian Sea, Southern Italy). Mar. Pollut. Bull. 2007, 55, 451–458. [Google Scholar] [CrossRef]
- Matozzo, V.; Binelli, A.; Parolini, M.; Locatello, L.; Marina, M.G. Biomarker responses and contamination levels in the clam Ruditapes philippinarum for biomonitoring the Lagoon of Venice (Italy). J. Environ. Monit. 2010, 12, 776–786. [Google Scholar] [CrossRef]
- R Development Core Team R: A Language and Environment for Statistical Computing. 2007. Available online: http://www.r-project.org (accessed on 18 November 2019).
- World Health Organization. Contaminated Sites and Health; Pasetto, R., Martin Olmedo, P., Martuzzi, M., Eds.; Report of two WHO Workshops: Syracuse, Italy, 18 November 2011; Catania, Italy, 21–22 June 2012; WHO Regional Office for Europe: Copenhagen, Denmark, 2013; Available online: www.euro.who.int/__data/assets/pdf_file/0003/186240/e96843e.pdf (accessed on 10 September 2020).
| NPCS | Pollution Source | Start of First Industrial Activity (Year) | EDs of Interest Detected in Environmental Matrices [55,56] | Human Biomonitoring | EDs Detected in Food |
|---|---|---|---|---|---|
| Area industriale Val Basento (Southern Italy) | Chemical plant | 1961 | Cd, Hg, solvents | ||
| Brindisi (Southern Italy) | Chemical plant, petrochemical plant, oil refinery, electric power plant, harbor area, industrial waste landfill | 1959 | Cd, Pb, As, PCB, chlorobenzene, other solvents | ||
| Brescia Caffaro * (North West Italy) | Chemical plants, landfill | 1930 | As, PCBs, PCDDs/PCDFs, chlorobenzene | PCDDs/PCDFs, PCBs (serum) [57,58] | PCB (food of animal and vegetal origin);PCDDs/PCDFs, PCB (cattle’s meat, cow milk, forage) [59] |
| Fidenza * (North East Italy) | Chemical plants, urban and hazardous waste landfills | 1888 | As, PCBs, PCDDs, benzene, other solvents | ||
| Gela (Southern Island) | Chemical plant, Petrochemical plant, oil refinery, industrial waste landfill | 1965 | As, PCB, benzene, other solvents, | As, Hb, Pb (urine, serum); 59 PCBs congeners (serum) [60] | |
| Laghi Mantova * (North West Italy) | Metallurgy plants, paper plant, petrochemical plant, harbor area, industrial waste landfills, hazardous waste incinerator | 1953 | AS, Cd, PCDDs, ethylbenzene, other solvents | PCDDs, PCBs (serum) [61] | PCBs (fruit, vegetables) [62] |
| Milazzo * (Southern Italy) | Oil refinery, steel plant, thermal power plant, electrical equipment factories, illegal dumping site | 1961 | PCDDs, heavy metals. Benzo(a)pyrene | As, Cd, Hg (urine); Pb (serum) [63] | |
| Area industriale Porto Torres * (Southern Island) | Chemical plants, petrochemical plant, oil refinery, power plant, harbor area, illegal dumping site | 1962 | As, Cd, chlorobenzene, other solvents | ||
| Priolo * (Southern Island) | Chemical plants, petrochemical plant, refinery, harbor area, hazardous waste landfills | 1949 | PCB, hexachlorobenzene | As, Cd, Pb, Hg, PHSs organochlorine compounds (meat, milk, seafood) [64] | |
| Sassuolo–Scandiano * (North East Italy) | Ceramic industries, industrial waste landfills | 1920 | Heavy metals | ||
| Taranto * (Southern Italy) | Oil refinery, steel plant, harbor area, cement plant, controlled and illegal waste dumps | 1945 | As, Cd, PCDDs, PCBs, benzene, xylene | As, Cd (serum, urine): PCDDs, PCBs (serum, milk) [65,66,67,68,69] | PCDDs, PCBs (sheep and cow’s milk, clams); PCB, HCB, PAHs (clams) [70,71] |
| Terni–Papigno * (Central Italy) | Steel plant, hazardous waste landfills | 1935 | PCB | ||
| Venezia Porto Marghera * (North East Italy) | Chemical plants, petrochemical plant, oil refinery, harbor area, illegal dumping sites | 1924 | As, Cd, PCBs, PCDDs, solvents | As, Cd, PCDDs, PCDFs (shellfish) [72] |
| NPCS | Age Group of 15–39 Years | Age gGroup of 40 Years or Over | Referred to the Years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | ||||||||||
| Population | Obs. | SIRs (90% CI) | Population | Obs. | SIRs (90% CI) | Population | Obs. | SIRs (90% CI) | Population | Obs. | SIRs (90% CI) | ||
| Area industriale Val Basento (Southern Italy) | 41,080 | 0 | - | 39,899 | <3 | NA | 57,478 | 5 | 78 (37–163) | 64,456 | 17 | 62 (42–93) | 2006–2010 |
| Brindisi (Southern Italy) | 46,417 | 3 | 95(37–244) | 46,609 | 9 | 77 (44–132) | 62,798 | 8 | 94 (53–169) | 74,289 | 38 | 134 (102–175) | 2006–2008 |
| Brescia Caffaro * (North West Italy) | 92,518 | 7 | 146 (79–272) | 88,319 | 18 | 128 (87–189) | 156,289 | 30 | 170 (126–230) | 193,772 | 80 | 152 (126–182) | 2006–2008 |
| Fidenza * (North East Italy) | 52,711 | 3 | 86 (33–222) | 50,531 | 16 | 140 (93–211) | 97,675 | 20 | 132 (92–191) | 126,580 | 63 | 153 (124–188) | 2006–2013 |
| Gela (Southern Island) | 54,855 | 4 | 74 (33–169) | 55,971 | 21 | 105 (73–150) | 67,558 | 8 | 59 (32–104) | 73,461 | 45 | 104 (81–133) | 2007–2012 |
| Laghi di Mantova * (North West Italy) | 42,121 | 0 | - | 39,984 | 6 | 94 (48–184) | 77,992 | 16 | 196 (130–295) | 100,511 | 41 | 161 (124–208) | 2006–2010 |
| Milazzo * (Southern Island)) | 54,544 | 6 | 173 (88–339) | 53,104 | 10 | 81 (48–136) | 79,576 | 16 | 168 (111–254) | 91,377 | 36 | 119 (90–157) | 2006–2011 |
| Area industriale Porto Torres * (Southern Island) | 153,285 | 13 | 119 (75–188) | 149,155 | 31 | 78 (58–104) | 226,528 | 35 | 114 (87–151) | 264,859 | 106 | 103 (88–121) | 2006–2011 |
| Priolo * (Southern Italy) | 221,991 | 11 | 68 (42–112) | 215,146 | 43 | 75 (59–97) | 309,143 | 33 | 76 (57–101) | 345,748 | 105 | 77 (66–91) | 2006–2012 |
| Sassuolo–Scandiano * (North East Italy) | 136,530 | 18 | 202 (137–297) | 129,176 | 30 | 104 (77–141) | 201,585 | 54 | 175 (140–219) | 217,998 | 125 | 153 (132–177) | 2006–2012 |
| Taranto * (Southern Italy) | 244,733 | 22 | 135 (95–191) | 241,659 | 68 | 113 (92–138) | 349,441 | 87 | 193 (162–230) | 424,559 | 215 | 139 (125–156) | 2006–2012 |
| Terni–Papigno * (Central Italy) | 124,927 | 14 | 159 (102–247) | 126,072 | 17 | 54 (36–80) | 238,796 | 49 | 149 (117–188) | 290,818 | 112 | 119 (102–139) | 2006–2013 |
| Venezia * (North East Italy) | 147,945 | 9 | 91 (53–157) | 138,601 | 11 | 34 (21–56) | 298,547 | 35 | 75 (57–99) | 369,088 | 80 | 60 (50–72) | 2006–2009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, M.; Zona, A.; Contiero, P.; D’Armiento, E.; Iavarone, I.; AIRTUM Working Group. Incidence of Thyroid Cancer in Italian Contaminated Sites. Int. J. Environ. Res. Public Health 2021, 18, 191. https://doi.org/10.3390/ijerph18010191
Benedetti M, Zona A, Contiero P, D’Armiento E, Iavarone I, AIRTUM Working Group. Incidence of Thyroid Cancer in Italian Contaminated Sites. International Journal of Environmental Research and Public Health. 2021; 18(1):191. https://doi.org/10.3390/ijerph18010191
Chicago/Turabian StyleBenedetti, Marta, Amerigo Zona, Paolo Contiero, Eleonora D’Armiento, Ivano Iavarone, and AIRTUM Working Group. 2021. "Incidence of Thyroid Cancer in Italian Contaminated Sites" International Journal of Environmental Research and Public Health 18, no. 1: 191. https://doi.org/10.3390/ijerph18010191
APA StyleBenedetti, M., Zona, A., Contiero, P., D’Armiento, E., Iavarone, I., & AIRTUM Working Group. (2021). Incidence of Thyroid Cancer in Italian Contaminated Sites. International Journal of Environmental Research and Public Health, 18(1), 191. https://doi.org/10.3390/ijerph18010191





